Abstract
Macrophages play a central role in inflammation and host defence against microorganisms, but they also participate actively in the resolution of inflammation after alternative activation. However, it is not known whether the resolution of inflammation requires alternative activation of new resting monocytes/macrophages or if proinflammatory activated macrophages have the capacity to switch their activation towards anti-inflammation. In order to answer this question, we first characterized differential human macrophage activation phenotypes. We found that CD163 and CD206 exhibited mutually exclusive induction patterns after stimulation by a panel of anti-inflammatory molecules, whereas CCL18 showed a third, overlapping, pattern. Hence, alternative activation is not a single process, but provides a variety of different cell populations. The capacity of macrophages to switch from one activation state to another was then assessed by determining the reversibility of CD163 and CD206 expression and of CCL18 and CCL3 production. We found that every activation state was rapidly and fully reversible, suggesting that a given cell may participate sequentially in both the induction and the resolution of inflammation. These findings may provide new insight into the inflammatory process as well as new fields of investigation for immunotherapy in the fields of chronic inflammatory diseases and cancer.
Keywords: activation, inflammation, macrophage, versatility
Introduction
Macrophages play a crucial role in the innate and adaptive immune responses to pathogens, and are critical mediators of inflammatory processes. During infection, inflammatory processes are critical to pathogen removal. However, inflammation is associated with deleterious effects for the tissue environment, and must thus be repressed to allow complete healing. Macrophages also play a major role in the resolution of inflammation by producing anti-inflammatory cytokines and chemokines and eliminating tissue debris. Macrophages can thus exhibit pro- and anti-inflammatory properties, depending on the disease stage and the signals they receive, i.e. the inflammatory balance in the microenvironment. Since the introduction of the concept of alternative activation of macrophages in 1992 [1], these cells have been assigned to two groups: classically activated or type I macrophages (CAMΦ), which are proinflammatory effectors, and alternatively activated or type II macrophages (AAMΦ) that exhibit anti-inflammatory properties. AAMΦ express a molecular repertoire leading to tolerance and the resolution of inflammation, and display a high phagocytotic capacity [1]. Such cells have been observed during the healing phase of acute inflammation [2], in chronic inflammatory diseases such as rheumatoid arthritis [3] and psoriasis [4], and in wound healing [5]. AAMΦ are also abundant in human term placenta [6], where they contribute to protection of the fetus, and in the lung [7], where they prevent undesirable inflammatory reactions to non-pathogenic microorganisms. These data suggest that alternatively activated macrophages may participate in the three phases of healing: the down-regulation of inflammation, angiogenesis and the elimination of tissue debris and apoptotic bodies [8,9].
In the inflamed tissue, it is not known whether the AAMΦ that appear during the healing phase [9] originate in newly attracted monocytes or arise from a switch in the activation state of previously proinflammatory macrophages. Indeed, true activation plasticity would permit the involvement, in the healing phase, of the same cells that participated in inflammation, with an obvious advantage for healing efficiency and speed.
In this study, we aimed to answer this question in a single model of mature, differentiated, human monocyte-derived macrophages (MDM) to mimic resident macrophages. In this model we first determined the effect of a panel of pro- and anti-inflammatory agents on the expression of classical and alternative activation markers. We chose to assess the expression of CD163, CD206 and CCL-18 as markers of anti-inflammation. CD206, the macrophage mannose receptor, is up-regulated following interleukin (IL)-4 stimulation, which led to the advent of the concept of alternative activation of macrophages [1]. Later the haptoglobin–haemoglobin scavenger receptor CD163 [10] was suggested, with CD206, as another marker of alternatively activated macrophages [9, 11, 12]. In 1998, Kodelja and colleagues [13] cloned CCL18, a chemokine that is also specifically overexpressed by AAMΦ (for review see [9,12]). We chose CCL3, the proinflammatory counterpart of CCL18 (for review [14]) as a type I marker as in combination with CCL18 expression, it may exclusively discriminate classical from alternative activation. Our experiments pointed out the diversity of macrophage activation phenotypes among the type II subpopulation, and suggested that the CAMφ/AAMφ dichotomy rather represents a network of different cell populations. We thus chose to evaluate the versatility of differentially activated monocyte-derived macrophages, instead of classically or alternatively activated macrophages, terms that do not represent the diversity and the complexity of macrophage activation. The versatility of macrophages was determined by measuring the expression of the different markers after stimulation by a panel of pro- or anti-inflammatory agents, and after stimulation arrest or a further counterstimulation. We found that macrophages stimulated toward a specific phenotype have the ability to return to a quiescent state after signal arrest, or to switch their activation phenotype rapidly upon counterstimulation.
Materials and methods
Human monocyte isolation and differentiation
Human peripheral blood mononuclear cells (PBMC) were isolated from healthy donors by Ficoll-Hypaque density gradient centrifugation. Monocytes were separated from PBMC by immunomagnetic depletion of T cells, natural killer (NK) cells, B cells, dendritic cells and basophils, using a monocyte isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocyte preparations were more than 95% pure, as shown by flow cytometry.
Monocytes were added to 75 cm2 culture flasks containing Dulbecco's minimum essential medium (DMEM)–glutamax (Invitrogen, San Diego, CA, USA) supplemented with 10% heat-inactivated (+ 56°C for 30 min) fetal calf serum (Bio West, Nuaillé, France) and 1% antibiotic mixture (penicillin, streptomycin, neomycin, Invitrogen). Macrophage-colony stimulating factor (M-CSF) and granulocyte macrophage (GM)-CSF (10 ng/ml and 1 ng/ml, respectively, R&D Systems, Minneapolis, MN, USA) were included in the medium from day 0 to day 6. These relative concentrations of cytokines maintained a neutral environment with respect to activation marker CD163 and CD206 expression levels, similar to those observed with MDM cultured in medium alone (data not shown). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Blood monocytes adhered to plastic after 1 h, detached spontaneously after 48 h and retained a monocyte-like appearance for 5 days. After 3 days, monocytes were washed with phosphate-buffered saline (PBS), counted using trypan blue and dispensed into 48-well plates (3 × 105 cells/well) containing medium supplemented with M-CSF and GM-CSF. On day 6, cells were washed and fresh medium was added. This model gives rise to postmitotic, fully differentiated macrophages by day 8. On day 8, mature cells were stimulated with pro- or anti-inflammatory molecules.
Recombinant cytokines and biologically active substances
Recombinant human M-CSF, GM-CSF, IL-4, IL-10, IL-13, interferon (IFN)-γ, transforming growth factor (TGF)-β1 and tumour necrosis factor (TNF)-α were purchased from R&D Systems and used at a concentration of 10 ng/ml for stimulation. Dexamethasone (40 ng/ml) was purchased from Qualimed Laboratories (Paris, France) and prostaglandin E2 (PGE2) (10 ng/ml), from Cayman Biochemicals (Ann Arbor, MI, USA). All substances except dexamethasone contained less than 0·1 ng endotoxin per microgram of product, leading to endotoxin concentrations of less than 1 pg per ml during the stimulations. Dexamethasone was from a sterile, apyrogenic batch suitable for injection in humans. This ensured that the observed results did not arise from endotoxin contamination. Lipopolysaccharide (LPS) (10 ng/ml) was purchased from Sigma (St Louis, MO, USA). We chose to apply these concentrations for the different stimuli based on those used within the literature to activate macrophages [1, 13, 15–17].
Flow cytometric analysis of cell surface molecule expression
Adherent cells were washed with PBS and detached from the plastic by thorough scraping with a rubber policeman. Non-specific sites were saturated by incubation at +4°C with 2% Tégéline™ (LFB, Les Ulis, France) in PBS. The cells were then incubated for 30 min at +4°C with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) against human leucocyte antigen (HLA)-DR (Immunotech, Marseilles, France), CD163 (BD Biosciences Mountain View, CA, USA), CD206 (BD Biosciences) or irrelevant isotype-matched controls (BD Biosciences) in PBS. The cells were then washed twice with cold PBS, fixed in 200 µl CellFix™ (BD Biosciences) and their fluorescence was assessed with an LSR flow cytometer (BD Biosciences). Viable cells were gated using forward and side light-scatter patterns. The mean equivalent fluorochrome bound per cell (MEF) was determined with the Dako Fluorosphere kit (Dako, Glostrup, Denmark). This made it possible to eliminate variation due to cytometer settings and day-to-day performance variability, as well as differences in stimulation dependent autofluorescence levels.
Viability assay
Stimulated cell viability was measured by the tetrazolium salt (MTT) assay (Sigma). Briefly, cells were washed twice in PBS and incubated in the presence of 50 µl of MTT solution (5 mg/ml) for 2 h at 37°C. We then added 0·3 ml of extraction buffer (8% HCl in isopropanol). The solution was transferred to a 96-well plate, and optical density was measured at 540–630 nm. The OD of blank wells (lacking cells, but manipulated in the same way) was subtracted from the values obtained for the test wells. We checked that MTT results were representative of the number of viable cells by counting the number of cells in five microscope fields per well by trypan blue exclusion. The results obtained were similar to those obtained with the MTT assay (data not shown). We therefore used the MTT assay in subsequent experiments to normalize data to a fixed number of cells. MTT assays were performed for every treatment on two additional replicates, and in the same cells as the triplicate wells used for markers assessment.
Cytokine determination
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to determine the concentration of macrophage inflammatory protein (MIP)-1α/CCL3 (R&D Systems). PARC/CCL18 ELISA was performed using a specific antibody pair according to the manufacturer's instructions (R&D Systems). To eliminate variation due to differences in viability, concentrations were expressed with respect to the number of untreated cells, according to MTT results, as follows: normalized pg/ml = measured pg/ml × (control cells MTT OD/treated cells MTT OD). Cytokine content was measured in supernatants harvested 24 h after culture passage and the addition of fresh stimulation medium.
Phagocytotic activity assay
The macrophages were tested for their ability to ingest fluorescein isothiocyanate-labelled Escherichia coli using the Vybrant Phagocytosis Assay Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions. The fluorescence was determined using a microplate fluorescence reader (FL600, Bio-Tek). We ensured that the fluorescence measured accounted exclusively for ingested particles, as the signal potentially generated from any non-internalized bioparticles was quenched by the addition of trypan blue, as supplied by the manufacturer. To eliminate variation due to differences in viability, concentrations were expressed with respect to the number of untreated cells, according to MTT results, as follows: normalized activity = measured activity × (control cells MTT OD/treated cells MTT OD).
Results
Effect of stimulation on macrophage morphology
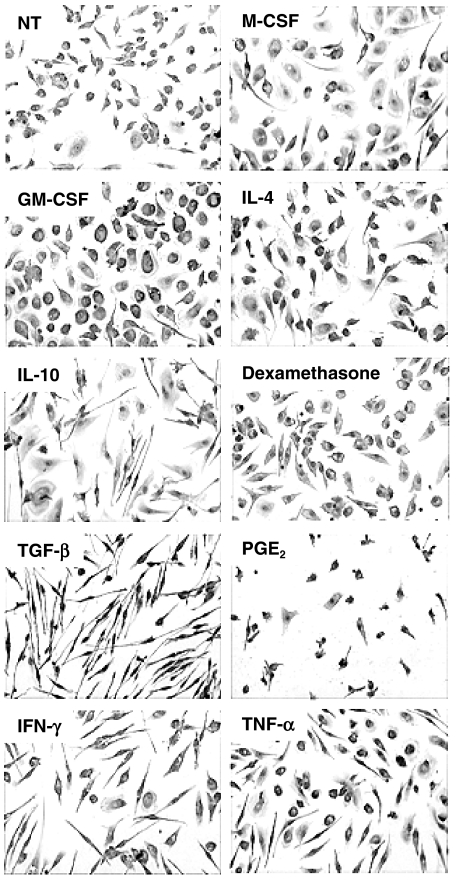
MDM were treated from day 8 to day 12 with a panel of pro- and anti-inflammatory molecules. MDM morphology after stimulation differed according to the molecule used (Fig. 1). Treatments with M-CSF and GM-CSF resulted in macrophages adopting a fully differentiated amoeboid morphology. IL-10, IL-4 and TNF-α induced a mixed morphology, with both fibroblastoid and round cells. Stimulation with IFN-γ or TGF-β resulted in cells with clear fibroblastoid shapes, whereas dexamethasone- and PGE2-treated cells adopted a condensed morphology, suggestive of cell damage. Indeed, these macrophages had a shorter life in our culture model. As measured by MTT assay after a 4-day stimulation, viability of dexamethasone and PGE2-treated macrophages was decreased by 39% and 53%, respectively. Other treatments also induced a decrease in cell survival compared with control, although the effect was less pronounced: IL-10 (25%), TGF-β (18%), IFN-γ (24%) and LPS (13%). By contrast, M-CSF and GM-CSF induced a better survival, by approximately 31% and 45%, respectively, whereas IL-4 and TNF-α had nearly no effect. Of note, none of these treated macrophages were actively proliferating, as assessed by BrdU incorporation (less than 5% positive cells, data not shown). Cell morphology was not found associated with the expression of any of the tested markers; CAMΦ and AAMΦ thus do not exhibit a particular morphology. Specific markers for each activation are thus needed for activation studies.
Fig. 1.
Morphology of differentially stimulated macrophages. Eight-day differentiated monocyte-derived macrophages (MDM) were cultured for 5 days in the presence of medium alone (NT) or the specified stimulation. Cells were then stained with May–Grünwald–Giemsa stain. Original magnification: × 200.
CD163 and CD206 exhibit mutually exclusive induction patterns
Because of the highly variable basal expression of the assayed markers among donors, we expressed these data as percentage of induction compared with controls. Basal expression levels of CD163 and CD206 by unstimulated macrophages were 180 320 ± 63 750 MEF (mean ± s.e.m., n = 8, range: 26 000–240 000) and 390 380 ± 41 520 MEF (mean ± s.e.m., n = 8, range: 200 000–550 000), respectively. All cell suspensions exhibited unimodal expression of the tested membrane markers, and there was no evidence for any distinct subpopulation. This suggests strongly that our MDM represent a unique cell population.
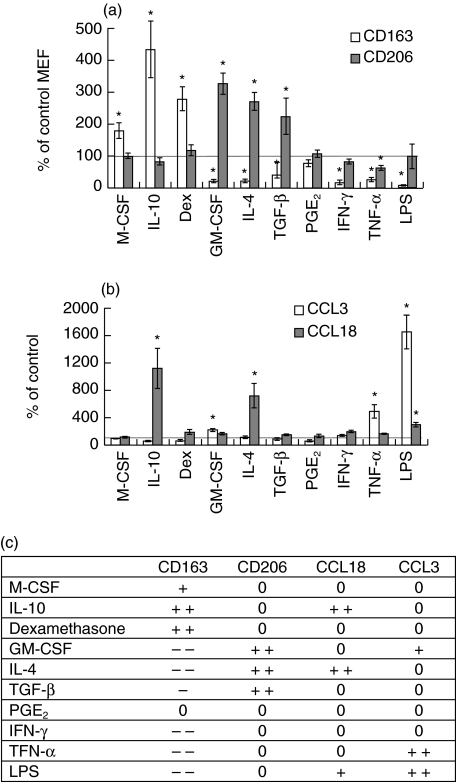
CD163, which displays high basal levels of expression, was overexpressed following treatment with IL-10, dexamethasone and, to a lesser extent, M-CSF (Fig. 2a). In contrast, GM-CSF, IL-4, TGF-β, IFN-γ, TNF-α and LPS strongly reduced CD163 expression, whereas PGE2 had no effect (Fig. 2a). As far as anti-inflammatory stimuli are concerned, CD206 showed the opposite pattern as it was strongly induced by GM-CSF, IL-4 and TGF-β, whereas it was not affected by M-CSF, IL-10, dexamethasone, PGE2, IFN-γ and LPS and slightly repressed by TNF-α (Fig. 2a). Thus, anti-inflammatory molecules may have opposite effects on the CD163 and CD206 expression. This suggests the existence of at least two different macrophage populations as well as a proinflammatory stimulated one.
Fig. 2.
(a) Effect of pro- and anti-inflammatory stimuli on CD163 and CD206 expression. Mature macrophages were treated from day 8 to day 12 with a panel of pro- and anti-inflammatory molecules. Levels of CD163 and CD206 expression at the membrane were then assessed by quantitative flow cytometry. Results are expressed as a percentage of untreated cells mean equivalent fluorochrome (MEF) ± s.e.m. for five independent donors. *P < 0·05, Student's unpaired t-test. (b) Effect of pro- and anti-inflammatory stimuli on the production of CCL3 and CCL18. Mature macrophages were treated from day 8 to day 12 with a panel of pro- and anti-inflammatory molecules, and CCL3 and CCL18 production was assessed by enzyme-linked immunosorbent assay (ELISA). Concentration values were normalized on the basis of tetrazolium salt (MTT) assay results, to account for variations in cell viability. Results are expressed as a percentage of untreated cells cytokine production ± s.e.m. for five independent donors. *P < 0·05, Student's unpaired t-test. (c) Summary of the effect of pro- and anti-inflammatory stimuli on tested markers showing the data displayed in (a) and (b). The effect of the different stimuli on the four markers tested are indicated as follows: 0: no effect; + and ++ : slight and strong increase, respectively; – and –: slight and strong decrease, respectively. Only significant effects are considered. The chosen cut-off for a strong versus slight effect are twofold modulations for flow cytometry and threefold for cytokine expression.
CCL18 and CCL3 production by stimulated macrophages
Because of the highly variable basal expression of these markers among donors macrophages, we expressed these data as a percentage of induction compared with controls. Basal secretion level (untreated cells) of CCL3 and CCL18 were 410 ± 125 pg/ml 106 cells (mean ± s.e.m., n = 9, range: 15–1025) and 1210 ± 265 pg/ml 106 cells (mean ± s.e.m., n = 10, range: 205–2685), respectively.
IL-4 and IL-10 strongly increased CCL18 production by macrophages, by about 10- and sevenfold, respectively, whereas other stimuli had almost no effect (Fig. 2b). Thus, CCL18 displays a third expression pattern, clearly different from those of CD163 and CD206. Although previous reports have already suggested this finding [1, 7, 13, 15, 17, 18], it has never been shown in a model unique to macrophages, leading to controversial data particularly with respect to the effect of IL-4 on CD163 expression (for reviews see [9, 12, 14]). In contrast, CCL3 was strongly up-regulated by LPS and TNF-α, by about 15- and fivefold, respectively, whereas the other cytokines had little or no effect. The expression patterns of CCL3 and CCL18 suggest active and different chemoattraction capacity properties for both CAMΦ and AAMΦ, mediated by CCL3 and/or CCL18, depending on the cytokines in the microenvironment.
Anti-inflammatory stimulations do not deactivate mature macrophages with respect to HLA-DR expression and phagocytotic capacities
Because of the highly variable basal expression of these markers among donors macrophages, we expressed HLA-DR expression as percentage of induction compared with controls. Basal expression level (untreated cells) was 20 500 ± 9800 MEF (mean ± s.e.m., n = 6, range: 11 700–41 500).
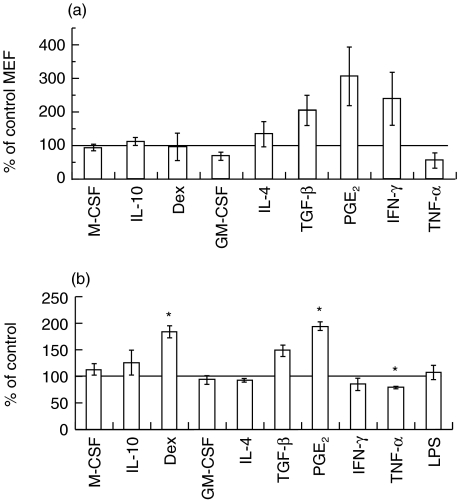
The level of HLA-DR expression and macrophage capacity to phagocytose were assessed to evaluate the activation level of differentially stimulated MDM. TGF-β, PGE2 and IFN-γ tended to increase HLA-DR expression but these effects were not statistically significant, due probably to its broad range of expression (Fig. 3a). The other stimulations had little or no effect. Interestingly, neither IL-10 nor dexamethasone decreased HLA-DR antigen density. In order to evaluate the ability of differentially activated macrophages to react against infection and/or to remove tissue debris to allow healing, their phagocytotic capacity was assessed. As shown in Fig. 3b, the different populations had similar capacity, except those induced by dexamethasone and PGE2, which exhibited improved phagocytotic ability. TGF–β stimulation led to a slight enhancement of phagocytotic ability that was not significant (Fig. 3b). Taken together, these results show that neither IL-10, dexamethasone or TGF-β particularly deactivate macrophages.
Fig. 3.
(a) Effect of pro- and anti-inflammatory stimuli on human leucocyte antigen (HLA)-DR expression. Mature macrophages were treated from day 8 to day 12 with a panel of pro- and anti-inflammatory molecules. Level of HLA-DR expression at the membrane was then assessed by quantitative flow cytometry. Results are expressed as a percentage of untreated cells mean equivalent fluorochrome (MEF) ± s.e.m. for five independent donors. *P < 0·05, Student's unpaired t-test. (b) Effect of pro- and anti-inflammatory stimuli on phagocytotic activity. Mature macrophages were treated from day 8 to day 12 with a panel of pro- and anti-inflammatory molecules. Phagocytotic activity was assessed by the capacity of macrophage to engulf fluorescein-labelled Escherichia coli. Results are expressed as a percentage of untreated cells fluorescence ± s.e.m. for three independent donors. *P < 0·05, Student's unpaired t-test.
Versatility of AAMφ and CAMφ
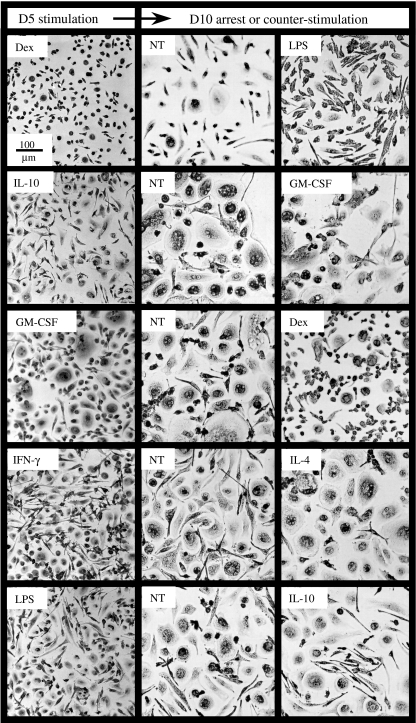
Mature macrophages were stimulated for 5 days with various pro- or anti-inflammatory molecules. They were then cultured for another 5 days in medium alone, or treated with a counterstimulatory cytokine. As shown in Fig. 4, treatment arrest (NT) partly or totally reverted macrophage morphology to the one of unstimulated cells. Moreover, although the effect was not always complete, counterstimulation led macrophages to adopt the same shapes as they had before activation (compare Figs 1 and 4, right-hand panels).
Fig. 4.
Plasticity of macrophage morphology. Mature macrophages were treated for 5 days by different pro- or anti-inflammatory stimuli (first round, left-hand panels). Cells from one well per condition were then stained with May–Grünwald–Giemsa stain. The remaining wells were then washed and cultured for another 5 days in medium alone (central panels, NT) or treated with molecules different from that used during the first round (right-hand panels). Cells were then stained with May–Grünwald–Giemsa stain.
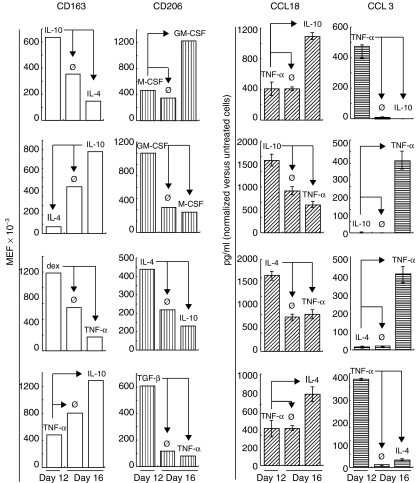
We quantified CD163 and CD206 expression and CCL18 and CCL3 release before and after the second stimulation. When the treatment was removed, or when counterstimulation was applied, the cells reversed the effects of the initial stimulation for CD163, CD206, CCL18 and CCL3 (Fig. 5). We also evaluated the capacity of fully activated macrophages to change CD163 quantitative expression in a very short period of time. After 15 days of treatment by M-CSF + dex (CD163 MEF = 620 000), macrophages were able to fully reverse their phenotype towards inflammation in only 2 days by using a cocktail of GM-CSF + TNF-α + IFN-γ (MEF = 0).
Fig. 5.
Reversibility of macrophage activation phenotype. Macrophages were treated with a cytokine or dexamethasone from day 8 until day 12 or from day 8 to day 11. They were then washed and cultured for another 3 or 4 days in medium alone (→) or were counterstimulated with another cytokine. Levels of CD163 and CD206 expression at the membrane were assessed by quantitative flow cytometry on days 12 and 16. CCL3 and CCL18 concentrations in the supernatant were quantified by enzyme-linked immunosorbent assay (ELISA) on days 11 and 14. Data are expressed as mean equivalent fluorochrome bound per cell (membrane expression, left-hand panel), or as pg/ml normalized according to the tetrazolium salt (MTT) test (chemokine secretion, right-hand panel). Data are representative of three independent experiments.
Similarly, after 15 days of treatment by GM-CSF (CD163 MEF = 4000), macrophages reversed their phenotype towards anti-inflammation in 2 days by using a cocktail of M-CSF + dex (MEF = 65 000)(data not shown).
These data suggest that differentially activated macrophages remain highly susceptible to changes in the microenvironment.
Discussion
In this study, we looked at the capacity of macrophages to sequentially exhibit pro- and anti-inflammatory properties. To do that, we first needed a precise characterization of different macrophage activation phenotypes. Indeed, the concept of alternative macrophage activation, which usually suggests a dichotomy between pro- and anti-inflammatory activation, should be reconsidered, keeping in mind the lack of unity that arise from diverse studies performed in different models and species. In this context, Siamon Gordon [12] proposed to redefine alternative macrophage activation: he limited this term to activation by IL-4 and IL-13, suggesting that other anti-inflammatory molecules should be regarded as deactivating macrophages. In line with this view, we found that CD163 and CD206 display mutually exclusive induction patterns: M-CSF, IL-10 and dexamethasone induced the overexpression of CD163, whereas GM-CSF, IL-4 and TGF-β up-regulated CD206 expression and repressed that of CD163. These results are consistent with most published data on CD163 and CD206 expression [1, 7, 15, 17, 18]. CD206 and CD163 thus define two categories for AAMΦ, the first being sensitive to endogenous stimuli such as the haptoglobin–haemoglobin complex through CD163 [10], and the second with a high ability to respond to mannose-rich microorganisms through CD206. Differing from Gordon's view, our data include TGF-β into the IL-4 group as far as scavenger receptor expression is concerned.
Consistent with the reverse transcription-polymerase chain reaction (RT-PCR) and Northern blot data published by Kodelja et al. [13], CCL18 production was increased by IL-4 and IL-10. This induction pattern is clearly different from those of CD163 and CD206, as it clusters IL-4 and IL-10 together. The anti-inflammatory molecules thus induce heterogeneous and partially overlapping expression patterns for CD163, CD206 and CCL18. These results, summarized in Fig. 2c, highlight the diversity of activation/deactivation phenotypes that can be obtained in vitro, probably reflecting a similar level of diversity in vivo, where different activation signals may interact intricately. Our data are thus in agreement with Gordon's view that a strong distinction should be made between IL-4 and IL-10 and other anti-inflammatory molecules [12]. Nevertheless, the idea that IL-10, corticoids, M-CSF and TGF-β should be regarded as deactivating macrophages remains open to debate. First, IL-10, like IL-4, strongly induced CCL18 expression in our cellular model, conferring to macrophages a highly selective chemoattraction for naive lymphocytes [19,20]. Moreover, neither IL-10 nor M-CSF, dexamethasone or TGF-β repressed HLA-DR expression level or decreased the phagocytotic ability of macrophages. Taken together, our results suggest strongly that these molecules, or at least some of them, orientate macrophages toward specific and exclusive activation pathways rather than deactivate them. This is illustrated by the fact that IL-10 renders macrophages unresponsive to IL-13 [21] and, in association with CCL17, inhibits the generation of CAMΦ [22]. IL-10 thus actively excludes the setting of other activation pathways rather than deactivates macrophages. Erwig et al. [23] generalized this concept to stimulation other than IL-10, such as IL-4, TGF-β, IFN-γ and TNF-α and demonstrated that a first-round stimulation renders macrophages refractory to subsequent stimulation with an opposite effect.
All these observations show that macrophage activation depends on which stimulation is provided first, but it is not clear whether activation to pro- or anti-inflammation is reversible or not when stimulation ends and/or is replaced by another one. The fate of these macrophages is, however, of importance for understanding how inflammation is resolved in tissue and whether or not the recruitment of new monocytes is required. From this viewpoint, the data of Palucka et al. [24], showing that the removal of GM-CSF and IL-4 allows monocyte-derived immature dendritic cells to revert to a macrophage phenotype, argue for a great versatility of macrophages and the other monocyte-derived cell types. Moreover, a recent review by Stout and Suttles [25] raised the hypothesis that macrophages could reverse their phenotype and functions depending on their microenvironment. These authors also point out that the demonstration of such a plasticity would be of great interest for therapy in chronic inflammation. We designed our study from a similar perspective, enquiring whether or not macrophages differentiate under specific stimuli. To address this question in the context of pro- and anti-inflammation, we assessed whether macrophages could switch from one phenotype to another when stimulation is removed and/or replaced by another cytokine with an opposite effect. Our results show that macrophage activation is plastic, rapid and fully reversible, as shown by the expression of CD163 and CD206, and the production of CCL3 and CCL18. Human MDM are postmitotic cells without detectable proliferation but in a minor subpopulation, and phenotype variation may thus not be due to differential cell growth as it would necessitate a complete renewal of the cell population within 4 days (and even 2 days in the CD163 reversion by pooled treatments experiment), which is not possible given the very scarse replicative capacity of MDM. Moreover, CD163 and CD206 expression patterns were homogeneous within each donor replicates (although with interindividual variations), and the modulations observed in MEF values affected the whole cell population. This excludes the possibility that the variations observed arise from selective cell death. Together, our data show clearly, at least for the membrane markers, that a single macrophage may switch its phenotype actively. Thus, the cytokine stimulations we tested do not lead to an irreversible differentiation and respect macrophage versatility. Accordingly, our data suggest strongly that a macrophage population may first take part in inflammation and then participate actively in its resolution. Our results also suggest that the relative loss in cell viability that we observed with dexamethasone and PGE2 may participate in the final resolution of inflammatory processes, i.e. the reduction of the number of macrophages. These features are consistent with the view of McGrath [26], who suggested a balance in macrophage activation, and that of Hume et al. [27], that monocyte recruitment would not be needed. This leads to a dynamic view of macrophage activation, that integrates macrophage capacity to switch from an activated state to another, being either classical or alternative, upon a specific signal. This versatility of macrophage activation may greatly enhance tissue macrophage ability to resolve inflammation quickly without new monocyte/macrophage recruitment and may provide new insight into the inflammatory process as well as new fields of investigation for cell trafficking. These findings may help to improve therapeutic approaches that target tissue inflammation and could be considered for immunotherapy in the fields of chronic inflammatory diseases and cancer.
Acknowledgments
This work was supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS) and Ensemble Contre le SIDA (Sidaction), and recurrent funds from the Commissariat à l'Energie Atomique (CEA). Fabrice Porcheray, Cathie Léone and Boubekeur Samah hold doctoral fellowships from the ANRS. Fabrice Porcheray is a recipient of a fellowship from the Fondation pour la Recherche Médicale (FRM).
References
- 1.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topoll HH, Zwadlo G, Lange DE, Sorg C. Phenotypic dynamics of macrophage subpopulations during human experimental gingivitis. J Periodont Res. 1989;24:106–12. doi: 10.1111/j.1600-0765.1989.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 3.Szekanecz Z, Haines GK, Lin TR, et al. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 1994;37:221–31. doi: 10.1002/art.1780370211. [DOI] [PubMed] [Google Scholar]
- 4.Djemadji-Oudjiel N, Goerdt S, Kodelja V, et al. Immunohistochemical identification of type II alternatively activated dendritic macrophages (RM 3/1+3, MS-1+/-, 25F9-) in psoriatic dermis. Arch Dermatol Res. 1996;288:757–64. doi: 10.1007/BF02505293. [DOI] [PubMed] [Google Scholar]
- 5.Goerdt S, Bhardwaj R, Sorg C. Inducible expression of MS-1 high-molecular-weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. Am J Pathol. 1993;142:1409–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Mues B, Langer D, Zwadlo G, Sorg C. Phenotypic characterization of macrophages in human term placenta. Immunology. 1989;67:303–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Heuvel MM, Tensen CP, van As JH, et al. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–66. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen- presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 11.Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible membrane of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–90. [PubMed] [Google Scholar]
- 12.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 13.Kodelja V, Muller C, Politz O, et al. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J Immunol. 1998;160:1411–18. [PubMed] [Google Scholar]
- 14.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Sulahian TH, Hogger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 16.Montaner LJ, da Silva RP, Sun J, et al. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-gamma or IL-10. J Immunol. 1999;162:4606–13. [PubMed] [Google Scholar]
- 17.Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 18.Schaer DJ, Boretti FS, Schoedon G, Schaffner A. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Br J Haematol. 2002;119:239–43. doi: 10.1007/s002510100304. [DOI] [PubMed] [Google Scholar]
- 19.Hieshima K, Imai T, Baba M, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–9. [PubMed] [Google Scholar]
- 20.Adema GJ, Hartgers F, Verstraten R, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–17. [Google Scholar]
- 21.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 22.Katakura T, Miyazaki M, Kobayashi M, et al. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 23.Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–8. [PubMed] [Google Scholar]
- 24.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. [PubMed] [Google Scholar]
- 25.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath MS, Kodelja V. Balanced macrophage activation hypothesis: a biological model for development of drugs targeted at macrophage functional states. Pathobiology. 1999;67:277–81. doi: 10.1159/000028079. [DOI] [PubMed] [Google Scholar]
- 27.Hume DA, Ross IL, Himes SR, et al. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–7. [PubMed] [Google Scholar]