Abstract
The functional differentiation of immune cells at early age plays a central role in immune physiology, e.g. for the sufficient eradication of pathogens. However, imbalances in effector cell responses may also have an impact in the pathophysiology of childhood diseases such as atopy and autoimmune disorders. As information on immune cell responses in infancy and early childhood is scarce, we conducted an observational, cross-sectional study in healthy newborns (n = 18), infants and young children (n = 54) aged 1–96 months and adult controls (n = 19) to assess cytokine mRNA and protein expression upon phorbol 12-myristate 13-actate/ionomycin stimulation and LPS-induced IL-12 expression in monocytes. The intracellular expression of interferon (IFN)-γ, tumour necrosis factor (TNF)-α (R = 0·748, P < 0·0001; R = 0·784, P < 0·0001, respectively) and interleukin (IL)-2 protein expression (R = 0·384, P = 0·008) was demonstrated to increase progressively with age. While a correlation between IL-4 protein expression and age was noted (R = 0·342, P = 0·007), the levels of IL-5 and IL-10 protein expression tended to be regulated on an individual basis during infancy and early childhood. An age correlation was also observed for intracellular IL-12 expression (R = 0·331, P = 0·009) in monocytes. These findings are valuable for further assessment of normal variations and maturation processes in immune cell responses and for the clinical–therapeutic monitoring of immunological status in various childhood diseases.
Keywords: age correlation, childhood, cytokine, infancy, T lymphocyte
Introduction
The assessment of T cell cytokine levels has provided an extraordinary insight into the state of activation of the human immune system. Two subsets of T helper (Th) cells, referred to as Th1 and Th2, have been identified initially in the mouse and later in humans. Th1 cells mainly produce the cytokines interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-2 and are essential to mount a protective response against infectious agents. Th2 cells secrete IL-4, IL-5, and IL-10, and are involved in allergic responses and humoral immunity [1]. Previous studies have demonstrated a deficient T cell cytokine response in newborns, which explains in part the physiological immaturity of the neonatal immune system [2,3]. During infancy and early childhood, the development of the immune repertoire requires exposure to many formerly unknown antigens, which together stimulate a battery of activation and maturation processes. These processes may continue until sufficient levels of specific immune surveillance and memory function have been acquired. Most information on maturation of the immune system relies on immunophenotyping data of blood lymphocytes. Significant phenotypic differences between T cells of neonates, infants and children were reported, suggesting a gradual development of cell-mediated immunological defence mechanisms [4,5]. Although considerable evidence that cytokines are involved in immunological responses and disease pathogenesis [6,7] has been accumulated, data on normal cytokine production profiles from the neonatal period to later childhood are limited. In all previous studies, cytokine production was assessed using intracellular staining and flow cytometry. Chipeta et al. [8] noted that IFN-γ-producing T cell populations increase progressively with age, while cells capable of producing IL-4 are minimal across all age groups. In a different study with infants aged 2–12 months, the number and percentage of T lymphocytes producing IFN-γ and IL-4 was shown to be increased over the first year of life but remained significantly lower than in adults [9]. Gasperoni et al. [3] observed lower percentages of IFN-γ- and IL-4-producing T cells in neonates compared to children and adults while the number of IL-2 producing cells was higher in neonates. A better knowledge of the ontogeny of the immune system during the first years of life may be useful to develop new strategies for the prevention and therapy of childhood disease in which gross imbalances of T cell cytokine production are crucial [10]. To characterize the age-related immune cell responses, we conducted an observational, cross-sectional study in healthy neonates, infants and young children and assessed the cytokine response on mRNA [quantitative polymerase chain reaction (PCR)] and protein levels after T cell stimulation (intracellular staining and flow cytometry, protein expression and flow cytometry). In addition, we investigated the IL-12 response of monocytes after lipopolysaccharide (LPS) stimulation, as IL-12 has been demonstrated to be a powerful regulator of various T cell functions [11].
Methods
Study population
The study was approved by the medical ethics review board of the University of Lübeck Medical School.
Blood samples were taken after informed consent from healthy adult blood donors (n = 19) and from cord blood of healthy term newborns (n = 18). For intracellular assessment of IFN-γ and TNF-α expression, we also included venous cord blood samples of preterm infants with a mean gestational age of 33·6 weeks (n = 30, range 31·0–36·6) and 34·1 weeks (n = 15, range 32·0–36·6), respectively. No infants included had either signs of infection or history of intra-amniotic infection.
For ethical reasons, we restricted our study population of infants and young children (aged between 1 month and 96 months, n = 54) to those individuals who had to undergo a medically indicated peripheral venous blood sampling before elective surgical intervention or within the scope of elective diagnostic procedures. To be eligible, informed consent had to be obtained from the parent. Furthermore, the individual could not have an acute or chronic infectious disease, any clinically significant disorder, any medication with known influence on immunological factors (e.g. corticosteroids) or other findings in the medical history that might compromise the study measures. Patients’ histories on breast feeding, vaccinations, previous infectious diseases and allergy were documented but not evaluated as covariates in this study, because subgroup analysis would require a larger population size.
Whole blood assay
Heparinized whole blood samples were suspended in RPMI-1640 supplemented with 1% penicillin/streptomycin, 2 mM glutamine, 1 mM pyruvate and non-essential amino acids (Seromed Biochrome, Berlin, Germany) at a concentration of 5 × 106 leucocytes/ml. To induce cytokine production in lymphocytes, preincubated whole blood cultures were stimulated with 3 µg/ml phorbol myristate acetate (PMA) and 3 µM ionomycin for 5 h (intracellular production of IFN-γ, TNF-α) or 24 h [intracellular production of transforming growth factor (TGF)-β, cytokine mRNA expression, cytokine protein production]. For intracellular assessment of IL-12 in monocytes, whole blood cultures were stimulated with 10 ng/ml LPS for 5, 10, 18 or 24 h as indicated. For the induction of intracellular IL-12 expression, whole blood cultures were preincubated for 2 h with 10 ng/ml recombinant IFN-γ (Pharmingen, Heidelberg, Germany).
Intracellular staining of cytokines
Cells were exposed to 3 µM monensin (Sigma, Deisenhofen, Germany) during the whole stimulation period, followed by fixation with 4% paraformaldehyde (Riedel de Haen, Seelze, Germany), as described previously [12]. Stimulated whole blood cells were washed in Hanks's balanced salt solution (HBSS) and resuspended in a buffer consisting of HBSS, 0·1% saponin (Riedel de Haen) and 0·01 M HEPES buffer (Seromed Biochrome). Two hundred-litre aliquots of cells were added to tubes containing 0·5 µg/10 µl of monoclonal antibodies against CD3 (17A2, cychrome-conjugated), IFN-γ[4S.B3, phycoerythrin (PE)-conjugated], TNF-α[MAb11, fluoroscein isothiocyanate (FITC)-conjugated], IL-12 (11·5, p40/70, FITC-conjugated) and TGF-β (TB21; PE-conjugated; IQ Products, Munich, Germany). Preincubation with a surplus of unconjugated anticytokine monoclonal antibodies (mAbs) (5 µg/10 µl; Pharmingen) served as a negative control for intracellular staining to each sample [13]. Isotype-specific antibodies were used to detect irrelevant specificity for surface molecule staining. Units for assessment: percentage of stimulated whole blood cells (CD3+ T cells or CD14+ monocytes) producing the individual cytokine.
Assessment of cytokine protein production
To assess the production of IL-2, IL-4, IL-5, IL-10 and TNF-α in supernatants of stimulated whole blood cultures, we applied the technique of simultaneous quantification of cytokines by a multiplexed flow cytometric assay [cytometric bead array (CBA); BD human Th1/Th2 cytokine CBA kit, BD Biosciences, San Diego, USA]. In principle, six bead populations with distinct fluorescence intensities have been coated simultaneously with capture antibodies specific for the cytokines indicated above, and mixed together to form the CBA. After addition of PE-conjugated detection antibodies, the CBA was incubated with a single set of recombinant standards or test samples to form sandwich complexes according to the manufacturer's instructions [13]. Following acquisition of sample data using flow cytometric analysis, BD CBA Analysis Software was used to generate results in tabular format. Units for assessment were protein expression in supernatants of stimulated whole blood cultures (pg/ml).
Determination of cytokine mRNA expression
After 24-h culture, whole blood leucocytes were prepared as described previously [14] and total RNA was isolated from stimulated cells using the Purescript RNA isolation kit (Gentra Systems, Minneapolis, USA) according to the manufacturer's protocol. The resulting RNA was resuspended in 300 µl and used for quantitative detection of IL-2, IL-4 and TNF-α mRNA expression using TaqMan reverse transcriptase PCR, as described previously [14,15]. Standardized cytokine mRNA quantities (cytokine copies/106β-actin copies) were determined by dividing the interpolation-derived values from the cytokine standard curve by the normalization factor (β-actin content in test samples). Units for assessment were cytokine mRNA expression in stimulated whole blood cells (cytokine mRNA copies/106β-actin mRNA copies).
Statistical analysis
We analysed data by estimating the non-parametric correlation between age and cytokine expression using Spearman's rho test (R, rank order correlation coefficient; two-sided level of significance is indicated as P-value; SPSS version 11·0). A two-sided P-level < 0·05 was regarded as statistically significant.
Results
The percentage of T cells capable of producing IFN-γ and TNF-α is strongly age-correlated
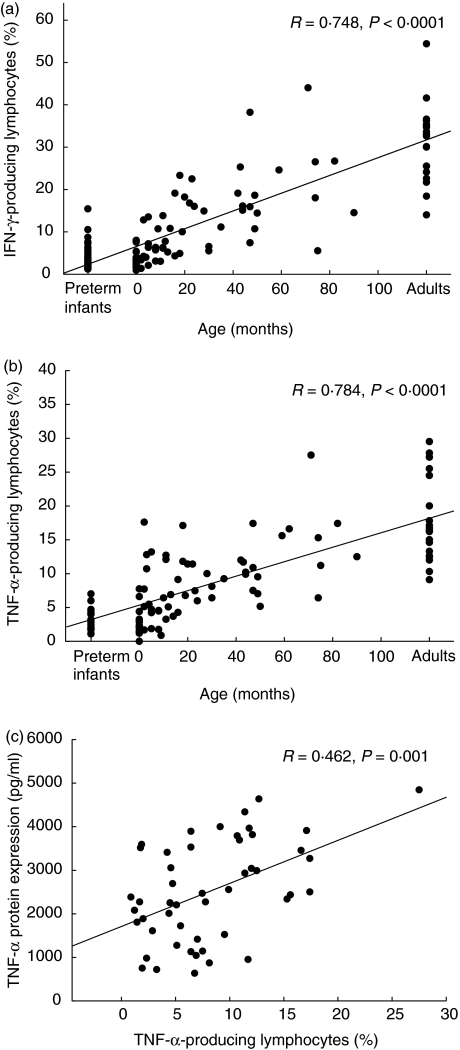
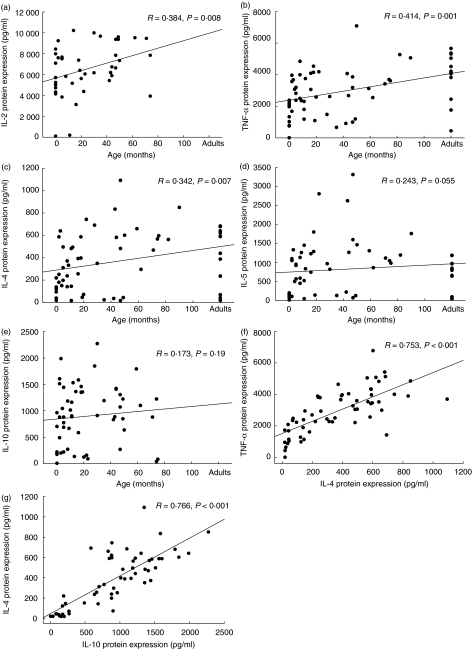
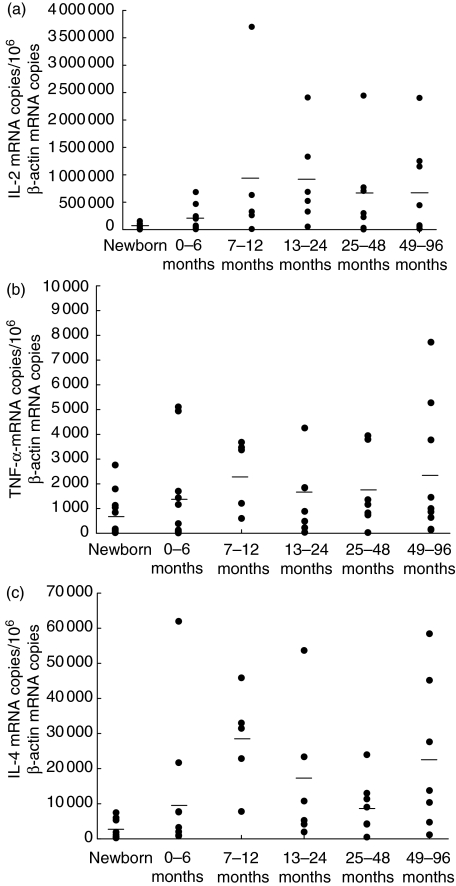
As described in Fig. 1, neonatal T cells in both preterm infants and healthy newborns failed to produce a significant amount of Th1 cytokines such as IFN-γ and TNF-α. Within the first years of life, however, a strong age correlation was demonstrated for the percentage of stimulated cells capable of intracellular IFN-γ and TNF-α expression (R = 0·748, P < 0·0001; R = 0·784, P < 0·0001, respectively, Fig. 1a, b) after stimulation with 3 µg/ml PMA and 3 µM ionomycin. After 40 months of age, the percentages of IFN-γ- and TNF-α-producing cells were almost comparable to the percentage of cytokine positive cells in healthy adults. In line with this, the protein production of IL-2 and TNF-α determined by cytokine bead array in supernatants of stimulated whole blood cultures increases with age (R = 0·384, P = 0·008; R = 0·414, P = 0·001, respectively, Fig. 2a, b). The IL-2 and TNF-α mRNA expression levels in whole blood cells tended to be lower in newborn infants compared to infants at age 1–6 months, but were comparable in infants and young children of different age groups (Fig. 3a, b). When different methods for the assessment of TNF-α expression were compared, a significant correlation between the percentage of TNF-α-producing cells (intracellular staining) and TNF-α expression in supernatants of stimulated whole blood cultures was found (R = 0·462, P = 0·001, Fig. 1c), while TNF-α mRNA data did not correlate with data from other assessment techniques. The protein expression of IL-4 in supernatants of whole blood cultures was demonstrated to be increased in early childhood (R = 0·342, P = 0·007; Fig. 2c). While the IL-4 mRNA expression levels were diminished in neonates, IL-4 mRNA levels tended to be increased after birth and remain relatively stable in infancy and childhood (R = 0·29, P = 0·05; Fig. 3c). No correlation was observed between IL-4 protein expression and IL-4 mRNA levels (data not shown).
Fig. 1.
Strong age correlation of intracellular interferon production in infancy and early childhood. Whole blood cultures were stimulated for 5 h with 3 µg/ml phorbol myristate acetate (PMA) and 3 µM ionomycin to induce IFN-γ (a: preterm infants, n = 30; newborns, n = 26; infants and children n = 54; adults, n = 18) and TNF-α synthesis (b: preterm infants, n = 14; newborns, n = 13, infants and children, n = 54; adults, n = 19). Data are expressed as percentage of cytokine-positive cells and plotted as individual points on the graph. All data were included to estimate the non-parametric correlation between age and cytokine expression using Spearman's rho test (R, rank order correlation coefficient; two-sided level of significance is indicated as P-value; SPSS version 11·0); (c: correlation of assessment methods for investigation of TNF-α expression (cytokine production in supernatants vs. percentage of TNF-α producing T cells; Spearman's rho test).
Fig. 2.
Th1 and Th2 cytokine production are correlated differentially with age in infancy and early childhood. Whole blood cultures were stimulated for 24 h with 3 µ g/ml phorbol myristate acetate (PMA) and 3 µM ionomycin to induce cytokine protein secretion. Data are expressed as protein secretion (pg/ml) and plotted as individual points on the graph (newborns, n = 10, infants and children, n = 41; adults, n = 12). All data were included to estimate the non-parametric correlation between age and cytokine production (a–e) or cytokine/cytokine production (f, g) using the Spearman's rho test (R, rank order correlation coefficient; two-sided level of significance is indicated as P-value, P < 0·05 was regarded as significant; SPSS version 11·0).
Fig. 3.
Cytokine mRNA expression levels increase with age in early infancy. Whole blood cultures were stimulated for 24 h with 3 µg/ml phorbol myristate acetate (PMA) and 3 µM ionomycin to induce cytokine mRNA expression. mRNA levels were determined in duplicate by real-time reverse transcription-polymerase chain reaction (RT-PCR) and normalized with respect to β-actin mRNA levels (cytokine mRNA expression/106β-actin mRNA copies). Following age strata were applied to this experiment: newborns (1–28 days old; n = 10), 0–6 months (29–182 days old; n = 10), 7–12 months (183–364 days old; n = 5), 13–24 months old (n = 6), 25–48 months old (n = 7), 49–96 months old (n = 9), and data are plotted as individual points on the graph with mean levels indicated as horizontal lines.
In contrast, no significant correlation was observed between age and protein expression of IL-5 and IL-10 in supernatants of stimulated whole blood cultures. More interestingly, individual neonates and infants were demonstrated to have an IL-4, IL-5 and IL-10 cytokine expression which is above adult levels (Fig. 2c–e). Furthermore, no age correlation was found for the percentage of cells capable of TGF-β expression in lymphocytes (R = 0·257; n.s., data not shown). Independently of age, the quantitative extent of cytokine expression is supposed to be regulated on an individual level, representing low/intermediate and high producers of both, Th1 and Th2 cytokines. In our experimental setting, the expression of IL-4 correlated significantly with the expression of TNF-α (Fig. 2f; R = 0·753; P < 0·001) and IL-2 (R = 0·579; P < 0·001). Significant correlations were also noted for IL-4 expression and the expression of IL-5 (R = 0·845; P < 0·001) and IL-10 (Fig. 2g; R = 0·766; P < 0·001). Furthermore, the expression of IL-10 was demonstrated to have a significant relationship with TNF-α (R = 0·736; P < 0·001), IL-2 (R = 0·519; P = 0·001) and IL-5 expression (R = 0·756; P < 0·001), while the IL-2 expression correlated clearly with TNF-α expression (R = 0·744; P < 0·001, data not shown).
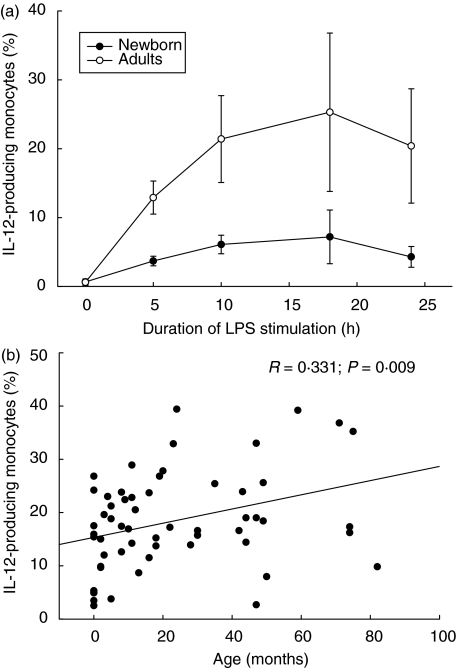
The percentage of IL-12 producing monocytes increases with age
As we could demonstrate a diminished proportion of monocytes capable of IL-12 production in newborns compared to adults at various time-points during LPS stimulation kinetics (Fig. 4a), we further investigated the percentage of IL-12-producing monocytes in whole blood cultures of infants and children stimulated with 10 ng/ml LPS, and found an age correlation (Fig. 4b; R = 0·331, P = 0·009). As the production of IL-12 is known to influence the Th1-directed immune response, we further found a correlation to intracellular IFN-γ (R = 0·329; P = 0·01) but not TNF-α expression.
Fig. 4.
Age correlation of monocytic interleukin (IL)-12 expression in early childhood. (a) Intracellular IL-12 expression kinetics after lipopolysaccharide (LPS) stimulation. Whole blood cultures were preincubated with 10 ng/ml interferon (IFN)-γ for 2 h and stimulated with 10 ng/ml LPS to induce IL-12 expression as indicated. The IL-12 expression in newborns (n = 5; mean ± s.d.) was lower compared to adults (n = 5; mean ± s.d.) throughout the LPS-stimulation kinetics. (b) Whole blood cultures were preincubated with 10 ng/ml IFN-γ for 2 h stimulated for 24 h with 10 ng/ml LPS to induce IL-12 expression in newborns, infants and children (n = 61). Data are expressed as a percentage of cytokine-positive cells and plotted as individual points on the graph. All data were included to estimate the non-parametric correlation between age and cytokine expression using Spearman's rho test (R, rank order correlation coefficient; two-sided level of significance is indicated as P-value; SPSS version 11·0).
Discussion
In the present study, we investigated T cell cytokine responses after PMA/ionomycin stimulation in neonates, infants and children. The results not only confirm previous reports that T cells capable of IFN-γ production increase progressively with age [3,8], but also demonstrate the linear relationship between intracellular TNF-α expression and age.
In line with this, protein expression of IL-2, IL-4 and TNF-α was significantly related to age, while mRNA levels of these cytokines achieve a physiological increase from neonatal period to infancy. These data confirm the previous report by Smart and Kemp [16], who observed a significant age correlation of IFN-γ, IL-4 and IL-5 release in blood samples of atopic and non-atopic children after stimulation with staphylococcal enterotoxin B (SEB). Although we noted a trend for the age-relation of IL-5 expression, the extent of IL-5 and IL-10 expression is supposed to be more regulated on an individual basis.
In addition, the expression of IL-12 in monocytes which is known to support the T cell immune response was also demonstrated to be age-correlated.
At neonatal age, the prevalence of suppression factors during fetal life and antigenic naivety may account for a physiological immaturity of the immune system at birth [17]. Besides this, the increased propensity of newborns to infections has been attributed to a deficient secretion of IFN-γ [18,19]. In this study, the low intracellular IFN-γ expression levels in newborns were numerically comparative with data previously published [3, 8, 9]. In preterm infants, the number of T cells capable of producing IFN-γ and TNF-α was also markedly reduced compared to infants beyond the neonatal period, although no significant difference between term and preterm infants was noted. While previous reports have documented the ability of neonatal T cells to raise immune responses competently upon polyclonal activation [20], our data substantiated the aspect of physiological deficiency for IFN-γ production in neonates. The observed progressive increase of TNF-α and IFN-γ may represent a gradual development of the immune repertoire.
Interestingly, we also observed a positive age correlation of IL-2 production, as previous studies had reported an age-related decrease of T cell subsets capable of IL-2 expression [3,8]. Because we used a whole blood environment which better preserves the original white blood cell distribution compared to separation of mononuclear cells, these contrary data may be explained by differences in the assay system and in the source of whole blood, i.e. cord blood was used in our study to assess neonatal immune responses while other groups used peripheral blood of neonates [3]. Furthermore, we provide novel data on cytokine mRNA expression in infancy and childhood. Confirming the data on the protein level, the expression of the IL-2 and TNF-α transcript tended to be increased in infancy compared to the neonatal period. To our best knowledge, no comparative data on physiological cytokine transcript expression during infancy and childhood are available, and age groups of larger size need to be investigated before firm conclusions can be drawn.
When different methods for the assessment of TNF-α expression were compared, a significant correlation between the percentage of TNF-α producing cells (intracellular staining) and TNF-α expression in supernatants of stimulated whole blood cultures was found, while TNF-α mRNA data did not correlate with data from other assessment techniques. Furthermore, no correlation was found for the comparison of IL-4 protein expression and IL-4 mRNA and IL-2 protein expression and IL-2 mRNA. The differences in protein and mRNA data indicate that post-transcriptional processes (including mRNA stability) may play a significant role for individual cytokine protein expression.
While previous reports had noted a low-level intracellular IL-4 expression which remained unchanged during infancy and childhood [3,8], we were not able to detect T cells capable of IL-4 expression reliably by flow cytometry in our experimental setting. However, the extracellular detection of IL-4 protein by cytometric bead assay revealed an age-correlation of IL-4 protein response, while a trend was observed for the assessment of IL-4 mRNA levels. This observation may reflect physiological maturation of the immune repertoire as maternal antibodies diminish within the first 6 months of life, and de novo synthesis of immunoglobulins is essential. In line with this, IL-4 is regarded as a driving force for the induction of Th2 responses [21], thus promoting humoral and cellular immune responses. The production of IL-5 and IL-10 was not demonstrated to be age correlated, although we previously noted a significantly diminished ability to produce IL-10 in neonates compared to adults [22]. While IL-4 may regulate Th subset development and further IL-4 production either directly via an autocrine pathway or indirectly by controlling the function of antigen-presenting cells in whole blood, IL-5 and IL-10 are supposed to be regulated on an individual level. Independently of age, however, the quantitative extent of TNF-α was demonstrated to correlate significantly with the expression of IL-4. This aspect will merit further study, as low/high producers of cytokines during maturation of the immune repertoire may have a different susceptibility to infections, allergy and autoimmune disease [23]. Interestingly, there are some subjects who even at older ages fail to produce IL-4, IL-2 or TNF-α. Large-scale studies are essential to investigate whether a general low- or high-producing cytokine profile may have clinical implications or should be regarded as physiological variation.
To our best knowledge, this is the first report describing an age-correlated IL-12 expression in monocytes. As IL-12 is known to influence T cell immune response in a proinflammatory mode, we further found a correlation to intracellular IFN-γ but not TNF-α expression. Additional studies are necessary to elucidate the impact of this observation on immune physiology.
Because so few data on immune responses in infancy and childhood are available, our data on cytokine production might help to formulate hypotheses on the normal ontogeny of immune cells from birth to childhood. The whole blood system used offers several advantages including straightforward, sensitive and up-to-date technology, with no necessity for cell separation procedures and small blood sample volumes [12, 13, 15]. On the other hand, our experimental approach is limited by the fact that T cell sources of cytokine production (CD4+ T helper versus CD8+ cytotoxic T cells) are not clearly distinguishable by CD3 phenotyping, and co-stimulation of antigen-presenting cells (e.g. monocytes) in the surrounding whole blood may lead to additional cytokine production (IFN-γ, TNF-α). One should be aware that the ontogeny of T cell cytokine levels should be interpreted within a complex network of immunological effective cells and molecules, and changes of cytokine responses may be induced by several causes, such as infections and other environmental stresses.
Finally, this study provides the basis for the necessity of age-matched reference values of cytokine production which are essentially valuable in the diagnosis and clinical–therapeutic monitoring of infants and young children with severe infections and other immune-mediated disorders.
Acknowledgments
We would like to thank Nina Klapproth, Jessica Richter and Brigitte Ebel for excellent technical assistence, and all children and their parents for participation in this study. The study was supported in part by ‘Lübeck-Hilfe für krebskranke Kinder e.V.’ and Bluhme-Jebsen-Stiftung.
References
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B. T cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 3.Gasparoni A, Caildelli L, Avanzino A, et al. Age-related changes in intracellular Th1/Th2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 4.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. J Pediatr. 1997;130:388–93. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 5.Shearer W, Rosenblatt H, Gelman R, et al. for the Pediatric AIDS Clinical Trials Group. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–35. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 7.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–61. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 8.Chipeta J, Komada Y, Zhang XL, et al. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Imunol. 1998;183:149–52. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 9.Buck RH, Cordle CT, Thomas DJ, Winship TR, Schaller JP, Dugle JE. Longitudinal study of intracellular T cell cytokine production in infants compared to adults. Clin Exp Immunol. 2002;128:490–7. doi: 10.1046/j.1365-2249.2002.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delespesse G, Yang LP, Ohshima Y, et al. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into TH1/TH2 effectors. Vaccine. 1998;16:1415–9. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 11.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–21. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 12.Schultz C. Intracytoplasmic detection of proinflammatory cytokines and chemokines in monocytes by flow cytometry. In: Körholz D, Kiess W, editors. Cytokines and colony stimulating factors, methods and protocols. Vol. 215. New Jersey, USA: Humana Press; 2002. pp. 29–39. (Part II. Detection assays for cytokines and growth factors. Methods in molecular biology). [DOI] [PubMed] [Google Scholar]
- 13.Carson R, Vignali D. Simultaneous quantitation of fifteen cytokines using a multiplexed flow cytometric assay. J Immunol Meth. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 14.Härtel C, Bein G, Kirchner H, Klüter H. A human whole-blood assay for analysis of T-cell function by quantification of cytokine mRNA. Scand J Immunol. 1999;49:649–54. doi: 10.1046/j.1365-3083.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Härtel C, Bein G, Müller-Steinhardt M, Klüter H. Ex vivo induction of cytokine mRNA expression in human blood samples. J Immunol Meth. 2001;249:63–71. doi: 10.1016/s0022-1759(00)00334-3. [DOI] [PubMed] [Google Scholar]
- 16.Smart JM, Kemp AS. Ontogeny of T-helper 1 and T-helper 2 cytokine production in childhood. Pediatr Allergy Immunol. 2001;12:181–7. doi: 10.1034/j.1399-3038.2001.012004181.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CB. The ontogeny of T lymphocyte maturation and function. J Pediatr. 1991;118:S5–9. doi: 10.1016/s0022-3476(05)82182-1. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CB, Westall J, Johnston L, Lewis DB, Dower SK, Alpert AR. Decreased production of interferon-gamma by human neonatal cells: intrinsic and regulatory deficiencies. J Clin Invest. 1986;77:860–7. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotiranta-Ainamo A, Rautonen J, Rautonen N. Imbalanced cytokine secretion in newborns. Biol Neonate. 2004;85:55–60. doi: 10.1159/000074959. [DOI] [PubMed] [Google Scholar]
- 20.Chipeta J, Komada Y, Zhang XL, Azuma E, Yamamoto H, Sakurai M. Neonatal (cord blood) T cells can competently raise type 1 and type 2 immune responses upon polyclonal activation. Cell Imunol. 2000;205:110–9. doi: 10.1006/cimm.2000.1718. [DOI] [PubMed] [Google Scholar]
- 21.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–57. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 22.Schultz C, Temming P, Bucsky P, Göpel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–6. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pretolani M, Goldman M. IL 10: a potential therapy allergic inflammation. Immunol Today. 1997;18:277–80. doi: 10.1016/s0167-5699(97)80023-0. [DOI] [PubMed] [Google Scholar]