Abstract
Periodontitis, a chronic inflammatory disease, is characterized by increased expression of interleukin (IL)-1 and other inflammatory mediators resulting in extensive osteoclast formation and bone loss. Expression of receptor activator of nuclear factor kappa B ligand (RANKL) and its decoy receptor, osteoprotegerin (OPG), by osteoblasts is important to regulate osteoclast differentiation. The aim of the present study was to investigate the regulatory effects of IL-1 on RANKL and OPG production by mesenchymal fibroblasts in periodontal tissue. Human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL) were stimulated with IL-1α with or without protein synthesis inhibitor cycloheximide (CHX), protein kinase A (PKA) inhibitors, protein kinase C (PKC) inhibitors and prostaglandin E2 (PGE2) inhibitor. In some experiments, the cultured cells were directly stimulated with either PKA or PKC activators. In HGF, IL-1α-stimulated OPG mRNA expression was high and could be reduced by CHX. PKA inhibitor completely abrogated IL-1α-induced OPG mRNA expression and OPG production. Endogenous PGE2 further enhanced IL-1α-induced OPG production in HGF. In PDL, RANKL mRNA expression was greatly augmented by IL-1α. IL-1α induced OPG mRNA expression and protein production. PKC inhibitor partially reduced IL-1α-induced OPG production and PKC activator enhanced OPG production in PDL. The IL-1α-stimulated OPG mRNA expression in HGF was greater than PDL. These results provide new evidence for the possible osteoclastogenesis-inhibitory function of HGF through PKA activity pathway. PDL utilized PKC for OPG production. Thus, we emphasize that HGF and PDL have different characteristics of host defence mechanism against inflammatory process.
Keywords: IL-1, OPG, osteoclast, periodontitis, RANKL
Introduction
Osteoclasts are monocyte/macrophage lineage cells derived from haematopoietic stem cells [1,2]. The receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL) is expressed on osteoblasts and T cells. Mice with disrupted RANKL gene lack osteoclasts and show severe osteopetrosis [3]. Osteoprotegerin (OPG), a secreted glycoprotein, is a decoy receptor for RANKL [4]. OPG transgenic mice showed severe osteopetrosis, suggesting that OPG is crucial for in vivo suppression of osteoclastogenesis. Osteoclast differentiation is regulated by RANKL and OPG expression in the local milieu [4–9].
Periodontitis, a chronic inflammatory disease, is characterized by the increased expression of inflammatory cytokines and accelerated osteoclast differentiation. RANKL expression is increased in periodontitis tissue compared with healthy periodontal tissue [10,11]. Activated T cells reside in the periodontitis tissue [11,12] and are actively involved in bone resorption [13]. Among the inflammatory cytokines in periodontitis tissue, interleukin (IL)-1 is one of the most potent cytokines associated with inflammatory bone resorption in periodontitis [14–16]. IL-1 increases RANKL expression by osteoblasts [17]. The expression of RANKL and OPG by osteoblasts stimulated with IL-1 might be responsible for the inflammatory bone resorption.
Human periodontal tissue has three fibroblastic cells of mesenchymal origin, human gingival fibroblasts (HGF), human periodontal ligament fibroblasts (PDL) and osteoblasts. The HGF are members of the connective tissue cells and constitute 65% of the cellular population of the gingiva [18]. These cells constantly remodel different components of the connective tissue in response to different cytokines [19]. We have reported that the production of OPG in HGF stimulated with bacterial lipopolysaccharide (LPS) and culture supernatants from HGF suppressed osteoclast differentiation, suggesting that the suppression was mediated by OPG [11]. PDL occupy the space between the roots of teeth and alveolar bone and exhibit some characteristics of osteoblasts, as they support new bone formation in vivo, and PDL cultured with 1α, 25-dihydroxyvitamin D3 have the ability to produce RANKL [20]. Because of these findings not only osteoblasts, but also HGF and PDL are now believed to be involved in the regulation of bone metabolism in alveolar bone [11,20]. This study was conducted to investigate the RANKL and OPG expression in PDL and HGF stimulated with IL-1. The differential roles of connective tissue fibroblasts and osteoblastic cells in inflammatory bone diseases are also discussed.
Materials and methods
Reagents
Cycloheximide (CHX), human recombinant IL-1α, phorbol 12,13-didecanoate (PDD), forskolin (For) and Staurosporine were purchased from Sigma Chemicals (St Louis, MO, USA). Indomethacin was purchased from Wako (Tokyo, Japan). Myristoylated protein kinase C peptide inhibitor (Myr), cAMP-dependent protein kinase peptide inhibitor (c-AMP Inh), phorbol-12-myristate 13-acetate (PMA) and dibutyryl cyclic AMP (c-AMP) were purchased from Promega (WI, USA). Protein kinase inhibitor N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulphonamide (H89) was purchased from Seikagaku Corporation (Tokyo, Japan). Monoclonal anti-human osteoprotegerin antibody, biotinylated anti-human osteoprotegerin antibody and recombinant human OPG were purchased from Techne (NJ, USA). Superscript II RNase H- reverse transcriptase, Oligo (dT) 12–18 primer, AccuPrime Pfx DNA polymerase and pCRII-TOPO vector were purchased from Invitrogen (CA, USA). QIAEXII was purchased from Qiagen (Germany) LightCycler Faststart DNA Master SYBR Green I was purchased from Roche Molecular Biochemical (Indianapolis, IN, USA). The quantitative real-time polymerase chain reaction (PCR) reagents were purchased from Gibco BRL (Rockville, MD, USA). RNAzol B solution was purchased from Biotech Laboratory (Houston, TX, USA). Dako TMB + substrate-chromogen one-step substrate system (substrate) was purchased from the Dako Corporation (Carpentaria, CA, USA).
Cell isolation and culture
HGF were isolated from 13 systemically healthy patients, aged 25–48 years (eight females and five males, mean age 37·1 ± 7·3). Healthy gingiva were collected from 11 patients during routine crown lengthening or distal wedge surgical procedures and inflamed gingiva were collected from two subjects who had at least one pocket site with probing depth more than 5 mm in the surgical area. The characteristics of the subjects are shown in Table 1. The washed explants were placed in a sterile dish and minced into smaller pieces with a sterile scalpel blade. Attempts were made to remove epithelium and leave only connective tissue. PDL were isolated from 11 systemically healthy patients aged 18–31 years (five females and six males, mean age 22·5 ± 3·9) who had clinical healthy premolar teeth extracted for orthodontic treatment. The mid-root surfaces of the teeth were scraped lightly with a sterile scalpel blade. All cell lines were prepared as described elsewhere [15]. The cell lines growing from the explanted tissue were subcultured. Cell lines from passage levels 4–7 were used in this study. Informed consent had been obtained from all 24 subjects, after verbal and written explanation regarding the nature of the study. Prior to commencement, the study protocol was approved by Ethics Committee of Tokyo Medical and Dental University.
Table 1. Sources of tissues and patients' characteristics.
| Cell line no. | Gender | Age | Operation type | Type of tissue |
|---|---|---|---|---|
| HGF 1 | Female | 25 | Distal wedge of lower left molar | Gingiva (healthy) |
| HGF 2 | Female | 29 | Upper left first premolar crown lengthening | Gingiva (healthy) |
| HGF 3 | Male | 47 | Upper right first premolar crown lengthening | Gingiva (healthy) |
| HGF 4 | Female | 40 | Distal wedge of lower right molar | Gingiva (healthy) |
| HGF 5 | Male | 43 | Distal wedge of lower left molar | Gingiva (healthy) |
| HGF 6 | Female | 40 | Upper right first premolar crown lengthening | Gingiva (healthy) |
| HGF 7 | Female | 38 | Lower right first molar crown lengthening | Gingiva (healthy) |
| HGF 8 | Female | 39 | Upper anterior teeth crown lengthening | Gingiva (healthy) |
| HGF 9 | Male | 33 | Upper left first premolar crown lengthening | Gingiva (healthy) |
| HGF 10 | Female | 29 | Distal wedge of lower right molar | Gingiva (healthy) |
| HGF 11 | Male | 30 | Upper left first premolar crown lengthening | Gingiva (healthy) |
| HGF 12 | Female | 42 | Upper left posterior open flap surgery | Gingiva (periodontitis) |
| HGF 13 | Male | 48 | Upper right posterior open flap surgery | Gingiva (periodontitis) |
| PDL 1 | Female | 18 | Lower left premolar extraction | Periodontal ligament |
| PDL 2 | Male | 21 | Lower right premolar extraction | Periodontal ligament |
| PDL 3 | Male | 24 | Lower left premolar extraction | Periodontal ligament |
| PDL 4 | Male | 27 | Upper left premolar extraction | Periodontal ligament |
| PDL 5 | Female | 31 | Upper right premolar extraction | Periodontal ligament |
| PDL 6 | Female | 19 | Lower left premolar extraction | Periodontal ligament |
| PDL 7 | Male | 23 | Lower right premolar extraction | Periodontal ligament |
| PDL 8 | Female | 19 | Lower right premolar extraction | Periodontal ligament |
| PDL 9 | Male | 20 | Upper right premolar extraction | Periodontal ligament |
| PDL 10 | Female | 21 | Lower left premolar extraction | Periodontal ligament |
| PDL 11 | Male | 24 | Lower left premolar extraction | Periodontal ligament |
HGF, human gingival fibroblasts; PDL, periodontal ligament fibroblasts.
HGF or PDL were seeded in 96-well flat-bottomed culture plates at 1 × 105 cells per well, and were grown to confluence. Once confluent, the cells were stimulated with various additives and supernatants were harvested for 24 h. Each cell was pretreated with specific protein kinase A (PKA) inhibitor (5 mM c-AMP Inh or 10 µM H89), specific protein kinase C (PKC) inhibitor (10 µM myristorylated PKC peptide inhibitor or 40 nM Staurosporine) or 1 µg/ml indomethacin for 30 min before washing. Then medium was changed to α-minimum essential medium (α-MEM) containing 10 ng/ml IL-1α and the same concentration of that inhibitor. The effect of PGE2, PKA and PKC activation on OPG production was determined using PGE2, PKA and PKC activators. Each cell line was stimulated with 1 µM PGE2, either 30 µM forskolin or 10 µM c-AMP for testing the PKA pathway and 60 µM PMA or 120 nM PDD for testing the PKC pathway. After 24 h of stimulation, OPG production in the culture supernatants was measured using enzyme-linked immunosorbent assay (ELISA).
RNA extraction and first-strand synthesis system
Primary HGF and PDL (1 × 107 cells) were cultured separately or in combination with or without 1% CHX or 10 ng/ml IL-1α in six-well culture plates for 12 h. Total RNA was isolated by using RNAzol, as described previously [11]. The precipitated RNA was redissolved in 0·1% diethylpyrocarbonate-treated distilled water and complementary DNA (cDNA) was synthesized using 2 µg of RNA through a reverse transcription reaction, first-strand cDNA Superscript II, according to the manufacturer's recommendations. After all reactions were terminated, 1 µl of RNase H− was added to each tube and incubated before amplifying the target DNA.
Real-time PCR
Real-time PCR quantitative mRNA analyses were performed in a LightCycler II Detection Machine using the SYBR-green fluorescence quantification system. Analysis of the results was carried out using the LightCycler Software supplied with the machine. The sequences of human primers were designed using the PrimerExpress software (Applied Biosystems) using nucleotide sequences present in the GenBank database. The following nucleotides primers were used (forward/reverse): OPG (GCAGCGGCACATTGGAC; 482–502)/(CCCGGTAAGCTTTCCATCAA; 533–554), RANKL (CGTTGGATCACAGCACATCAG; 905–921)/(GCTCCTCT TGGCCAGATCTAAC; 954–973) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) (GCTCTCCAGAACA TCATCC; 679–697)/(GTGTCGCTGTTGAAGTC AG; 926–944).
The nucleotide sequences were derived from the following GenBank Accession no: RANKL (AB064269), OPG (AK223155) and GAPDH (NM_002046). Amplification profile was 95/0; 55/7; 72/20 [temperature (°C)/time (s)] and 35 cycles. Positive control was prepared from IL-1-stimulated PDL cells. Total RNA was isolated from cultured cells and cDNA was synthesized from 2 µg of total RNA each using oligo(dT)11 NN primer with Superscript II reverse transcriptase, according to the manufacturer's instructions. PCR amplification was performed with AccuPrime Pfx DNA polymerase. The PCR product was separated by electrophoresis on a 1% agarose gel. The band of interest was excised and DNA was eluted by QIAEXII and cloned into the pCRII-TOPO vector, according to the manufacturer's instructions. Three independent clones were sequenced and diluted three- to 256-fold to generate relative standard curves to which sample cDNA was compared.
ELISA
Human OPG ELISA was performed as described previously [11] using monoclonal anti-human osteoprotegerin antibody and biotinylated anti-human osteoprotegerin antibody, according to the manufacturer's instructions. Recombinant human OPG was used as standard. Streptavidin HRP was added and the plate was incubated, followed with substrate solution and stop solution. All samples were tested in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation (s.d.). Data were subjected to one-way analysis of variance (anova) and Fisher's protected least-significant difference (LSD). The statistical analysis was performed with the SPSS program for Windows version 10.
Results
Effect of IL-1α on RANKL and OPG mRNA expression
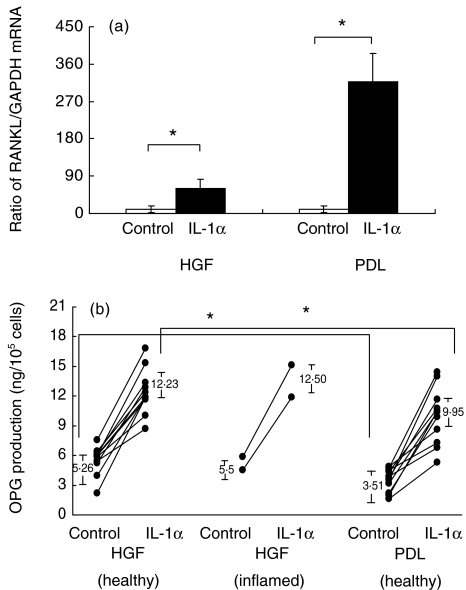
To elucidate the effect of IL-1α on RANKL and OPG expression by HGF and PDL, we measured RANKL, OPG and GAPDH mRNA expression. The ratio between the levels of RANKL and GAPDH mRNA expression from four HGF and four PDL cell lines is shown in Fig. 1a, and the ratio between levels of OPG and GAPDH mRNA expression from the same cell lines is shown in Fig. 2. Without stimulation, very little RANKL/GAPDH mRNA expression was observed from both kinds of cells, whereas OPG/GAPDH mRNA expression from HGF was almost three times greater than that from PDL. With IL-1α stimulation, the RANKL/GAPDH mRNA expression ratio was increased significantly in PDL and HGF (P < 0·05). We observed that RANKL/GAPDH mRNA expression ratio from PDL was five times greater than that by HGF. The OPG/GAPDH mRNA expression ratio was increased in both PDL and HGF, but the ratio in IL-1α-stimulated PDL was 2·5 times lower than that in HGF.
Fig. 1.
(a) Ratio between receptor activator of nuclear factor kappa B ligand (RANKL) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA expression in four cell lines of human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL) stimulated with 10 ng/ml interleukin (IL)-1α. RNA was extracted from HGF and PDL, first-strand cDNA synthesis and real-time polymerase chain reaction (PCR) analysis for osteoprotegerin (OPG) and GAPDH was carried out as described in Materials and methods. Data are expressed as a ratio between the amount of RANKL and GAPDH mRNA expression ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05). (b) All the primary HGF (11 cell lines from clinically healthy gingiva and two cell lines from clinically periodontitis gingiva) and PDL (11 cell lines from clinically healthy premolar) cells lines were stimulated with 10 ng/ml IL-1α for 24 h. Production of OPG in cultured supernatant was determined using enzyme-linked immunosorbent assay (ELISA). Data are expressed as concentration of OPG product per cell.
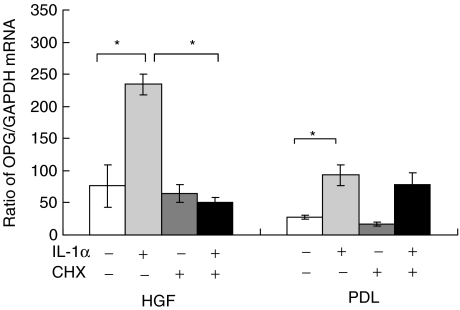
Fig. 2.
Ratio between osteoprotegerin (OPG) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA expression in four cell lines of human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL) stimulated with 10 ng/ml interleukin (IL)-1α with or without 1% cycloheximide (CHX). RNA was extracted from both HGF and PDL, first-strand cDNA synthesis and real-time polymerase chain reaction (PCR) analysis for osteoprotegerin (OPG) and GAPDH was carried out as described in Materials and methods. Data are expressed as a ratio between the amount of OPG and GAPDH mRNA expression ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05).
Effect of IL-1α on OPG production in HGF and PDL
All primary HGF and PDL cell lines were used to determine the effect of IL-1α on OPG production in HGF and PDL, as shown in Fig. 1b. Mean OPG production by healthy HGF and PDL cultured in control medium was 5·26 ± 1·71 and 3·51 ± 1·14 ng/105 cells, and with IL-1α stimulation they were increased to 12·23 ± 2·03 and 9·95 ± 2·84 ng/105 cells. Mean OPG production by clinically inflamed HGF cultured in control medium was 5·49 ± 0·42 ng/105 cells and with IL-1α stimulation it was increased to 12·50 ± 0·98 ng/105 cells. The amount of OPG production by IL-1α-stimulated HGF was significantly greater than that by PDL (P < 0·05). HGF without IL-1α stimulation also showed significantly greater OPG production than PDL (P < 0·05).
Effect of IL-1α on OPG mRNA expression in the presence of CHX
Either HGF or PDL, four cell lines each were stimulated with IL-1α in the presence or absence of CHX. The OPG and GAPDH mRNA expression ratio is shown in Fig. 2. With IL-1α stimulation, the OPG/GAPDH mRNA expression ratio was increased significantly (P < 0·01) in both HGF and PDL. CHX alone could not change OPG mRNA expression from a baseline level in both HGF and PDL. OPG/GAPDH mRNA expression ratio in HGF stimulated with IL-1α was suppressed significantly by CHX. In PDL, CHX did not affect OPG/GAPDH mRNA expression ratio.
Effect of PKA inhibitors on OPG production in HGF and PDL
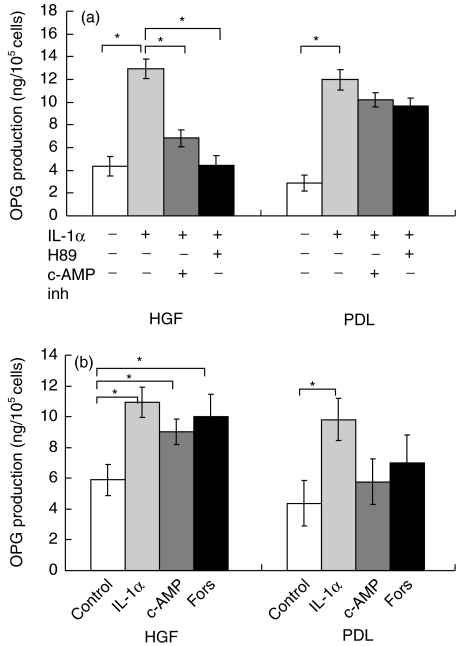
Effects of PKA inhibitors on IL-1α-induced OPG production in four HGF and four PDL cell lines were investigated with specific PKA inhibitors, c-AMP inh and H89. The c-AMP inh significantly suppressed IL-1α-induced OPG production in HGF (P < 0·05), as shown in Fig. 3a. However, there was no significant suppression in PDL. Another PKA inhibitor, H89, also significantly suppressed IL-1α-induced OPG production in HGF to the baseline level, but we could not observe this phenomenon in PDL.
Fig. 3.
(a) Effect of c-AMP inhibitor and H89 on osteoprotegerin (OPG) production in cultures of human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL). Conditioned media were collected and analysed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05). (b) Effect of c-AMP and forskolin (Fors) on OPG production in cultures of HGF and PDL. Conditioned media were collected and analysed by ELISA. Data are expressed as mean ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05).
Effect of PKA activator on OPG production in HGF and PDL
The effects of PKA activation on OPG production, using four HGF and four PDL cell lines, were examined with specific PKA activators, c-AMP and forskolin. In HGF, c-AMP significantly enhanced OPG production (Fig. 3b, P < 0·05). The amount of OPG production by HGF stimulated with c-AMP was 94·11% of that induced by IL-1α. In contrast, c-AMP did not affect OPG production by PDL. Another PKA activator, forskolin, also significantly stimulated HGF to produce OPG (P < 0·05). The amount of OPG production by HGF stimulated with forskolin was almost the same amount as that induced by IL-1α. OPG production from PDL did not significantly increase after stimulation with forskolin.
Effect of PKC inhibitors on OPG production in HGF and PDL
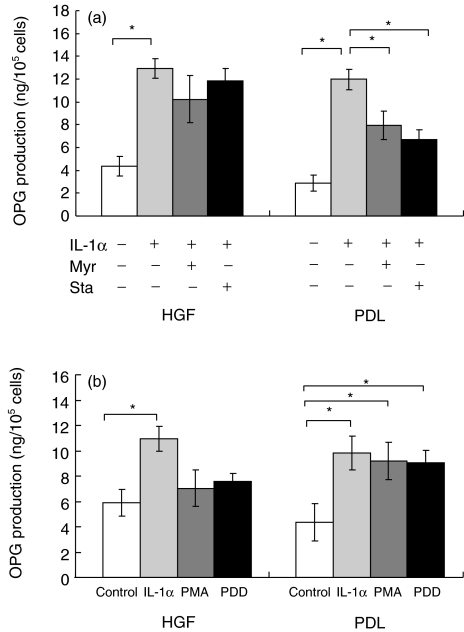
The effects of PKC inhibitors on IL-1α-induced production of OPG, using four HGF and four PDL cell lines, were investigated with specific PKC inhibitors, myristorylated PKC peptide inhibitor and Staurosporine. Myristorylated PKC peptide inhibitor significantly inhibited IL-1α-induced OPG production from PDL (P < 0·05), as shown in Fig. 4a. However, we could not find this significance in HGF. Treatment with another PKC inhibitor, Staurosporine, also significantly reduced IL-1α-induced OPG production only in PDL (P < 0·05). No statistically significant reduction by Staurosporine was observed in IL-1α-induced OPG production in HGF.
Fig. 4.
(a) Effect of myristorylated protein kinase C (PKC) peptide inhibitor (Myr) and Staurosporine on osteoprotegerin (OPG) production in cultures of human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL). Conditioned media were collected and analysed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05). (b) Effect of phorbol-12-myristate 13-acetate (PMA) and phorbol 12,13-didecanoate (PDD) on OPG production in cultures of HGF and PDL. Conditioned media were collected and analysed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± s.d. of four experiments. An asterisk (*) represents statistical significance (P < 0·05).
Effect of PKC activator on OPG production by PDL and HGF
In the PDL cell lines, PMA significantly enhanced OPG production (Fig. 4b, P < 0·05). OPG production by PMA-stimulated-PDL was 94% of that by IL-1α. In contrast, PMA did not affect OPG production by HGF. PDD significantly activated OPG production by PDL (P < 0·05). The amount of OPG production by PDL stimulated with PDD was 92% of that induced by IL-1α. OPG production from HGF did not significantly increase after stimulation with PDD.
Effect of indomethacin on IL-1α-induced OPG production in PDL and HGFs
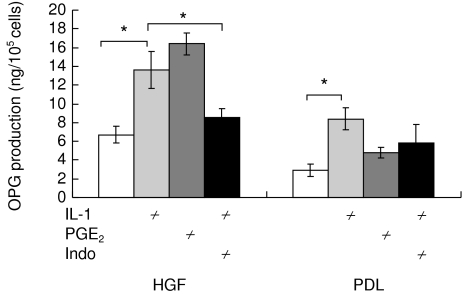
To determine whether the involvement of endogenous PGE2 mediates the effect of IL-1α-induced OPG production from PDLs and HGFs, we pretreated cells with indomethacin for 30 min before IL-1α stimulation. In the presence of indomethacin, indomethacin suppressed IL-1α-induced OPG production in all the four HGF cell lines (P< 0·05), but tended to enhance OPG production in the four PDL cell lines. Data are shown in Fig. 5.
Fig. 5.
Effect of indomethacin (Indo) on osteoprotegerin (OPG) production in cultures of human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDL). Conditioned media were collected and analysed by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± s.d. of three experiments. An asterisk (*) represents statistical significance (P < 0·05).
Discussion
The present findings demonstrate that IL-1-stimulated PDL have many similarities with osteoblasts, whereas HGF were distinct from osteoblastic cells. Both PDL and HGF produced OPG in response to IL-1 stimulation, and HGF produced significantly more OPG than PDL. Furthermore, IL-1-induced OPG production by HGF cell lines obtained from inflamed gingiva did not differ from that produced by HGF cell lines obtained from clinically healthy gingiva. IL-1α-induced OPG mRNA expression in HGF was 2·5 times greater than PDL. In addition, IL-1α-induced RANKL mRNA expression in HGF was five times lower than PDL. The discovery of OPG as a powerful inhibitor of osteoclastogenesis has generated great interest in its regulation by physiological agents that regulate bone resorption [4, 6, 7]. There is ample evidence in vitro and in vivo [6, 7, 21] that increased OPG expression may prevent bone resorption by affecting osteoclasts. Up-regulation of OPG mRNA was suppressed by CHX in IL-1-stimulated HGF, but not in PDL, suggesting that protein synthesis-dependent and independent pathways might have an effect on OPG mRNA expression in these cells. Although previous studies reported that PKC pathway was involved in OPG production and secretion by osteoblastic cells [22–24] and PGE2 suppressed OPG production by osteoblasts [25], the present study demonstrated that OPG production by HGF occurred predominantly through the PKA pathway, and that PGE2 induction by IL-1α further enhanced IL-1α-induced OPG production.
OPG and RANKL mRNA expression in marrow stromal cells stimulated with PTH is regulated by PKA and PKC activation [22] and IL-1 signalling can implicate directly both PKA and PKC, depending on cell types [26,27], suggesting that IL-1α-induced OPG mRNA expression might be regulated by PKA and PKC activity in these cells. IL-1-induced cAMP production has been reported in human and mouse fibroblasts [28–30] and some immune cells [28,31–33]. These reports suggested that IL-1 might augment intracellular cAMP level in HGF and PDL as well. Pretreatment with c-AMP inh suppressed IL-1α-induced OPG production by HGF to baseline level. The same inhibition was observed when using the PKA inhibitor H89, suggesting that IL-1-induced OPG production in HGF was dependent on PKA activation. HGF, but not PDL, produced OPG in response to cAMP or forskolin, indicating that the direct activation of PKA stimulates HGF to produce OPG. The PKA-dependent OPG production might be characteristic of HGF, as PKA activation in osteoblasts augments RANKL expression but does not affect OPG [34]. Halladay et al. reported that PTH regulates OPG transcription via activation of the cAMP/PKA signal transduction in rat osteoblastic cell lines [8]. They showed that the transcription was biphasic and inhibitory effects of PTH on OPG were mediated at the transcriptional level through cis elements in the proximal promoter. Fu et al. reported that dominant-negative forms of c-fos reduced the suppression of OPG by PTH [35]. To elucidate these molecular mechanisms, we are currently investigating the role of AP-1 in OPG expression of HGF and PDL.
Pretreatment with the myristorylated PKC peptide inhibitor suppressed IL-1α-induced OPG production by PDL, but did not affect IL-1α-induced OPG production by HGF (Fig. 4a,b). The same inhibition was observed when using the PKC inhibitor Staurosporine, suggesting that IL-1α-induced OPG production in PDL was dependent on PKC activation. In addition, PDL, but not HGF, produced OPG in response to PMA or PDD, indicating that the direct activation of PKC stimulates PDL to produce OPG. PKC stimulator up-regulated OPG mRNA expression by osteoblastic stromal cells, and direct PKC activation may negatively regulate osteoclastogenesis of PTH by inducing expression of OPG [22].
Synthesis of PGE2 suppressed the IL-1-induced OPG mRNA expression in PDL [36] and osteoblasts [37]. Production of PGE2 by PDL is dependent on cyclooxygenase-2 (COX2) synthesis [38]. In this study, OPG mRNA expression in HGF stimulated with IL-1α was partially inhibited by CHX, and pretreatment with indomethacin inhibited IL-1α-induced OPG production in HGF, but not in PDL. These results suggest that protein synthesis-dependent OPG mRNA expression pathways might involve synthesis of COX2, and that PGE2 activates OPG expression in an autocrine manner.
As we have reported previously, LPS-stimulated HGF produced OPG to inhibit the differentiation of monocytes into osteoclasts [11]. Recently, Suda et al. reported that the suppression of OPG expression by PGE2 was crucially involved in LPS-induced osteoclast formation [37]. Retarding bone resorption by inhibition of osteoclastogenesis requires the presence of the OPG molecule [4, 6, 7, 11, 14, 39]. HGF have the ability to suppress osteoclastogenesis induced by inflammatory mediators, including IL-1α and PGE2, but extensive bone resorption would occur if these mediators enter PDL and osteoblasts. This in vitro observation is in accordance with previous histological studies, which showed that influx of inflammatory infiltrate into the periodontal ligament or alveolar bone augmented osteoclast formation [40]. Further studies are necessary to clarify the precise mechanisms of OPG and RANKL expression in different mesenchymal cells. Novel treatment modalities would be possible if the regulatory mechanisms of OPG and RANKL expression in inflammatory bone diseases are elucidated.
Acknowledgments
This work was supported by the Japan Society for Promotion of Science nos. 15390642, 15659497, 15209071, Grants-in-Aid for Scientific Research of the Ministry of Education, Science, Sports and Culture of Japan Nos 14571977, 13470459 and Center of Excellence (COE) Program for Frontier Research on Molecular Destruction and Reconstruction of Tooth and Bone, Graduate School, Tokyo Medical and Dental University.
References
- 1.Ash P, Loutit JF, Townsend KM. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669–70. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 2.Scheven BA, Visser JW, Nijweide PJ. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986;321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 3.Odgren PR, Kim N, MacKay CA, et al. The role of RANKL (TRANCE/TNFSF11), a tumor necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res. 2003;44(Suppl. 1):264–71. [PubMed] [Google Scholar]
- 4.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuda E, Goto M, Mochizuki S, et al. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–42. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda H, Shima N, Nakagawa N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:132–7. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 8.Halladay DL, Miles RR, Thirunavukkarasu K, et al. Identification of signal transduction pathways and promoter sequences that mediate parathyroid hormone 1–38 inhibition of osteoprotegerin gene expression. J Cell Biochem. 2001;84:1–11. doi: 10.1002/jcb.1273. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Shima N, Nakagawa N, et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–13. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Xu JK, Figliomeni L, et al. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int J Mol Med. 2003;11:17–21. doi: 10.3892/ijmm.11.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Nagasawa T, Kobayashi H, Kiji M, et al. LPS-stimulated human gingival fibroblasts inhibit the differentiation of monocytes into osteoclasts through the production of osteoprotegerin. Clin Exp Immunol. 2002;130:338–44. doi: 10.1046/j.1365-2249.2002.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanamaru F, Iwai H, Ikeda T, et al. Expression of membrane-bound and soluble receptor activator of NF-kappaB ligand (RANKL) in human T cells. Immunol Lett. 2004;94:239–46. doi: 10.1016/j.imlet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Eisen-Lev R, Seki M, et al. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 14.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–11. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi J, Saito I, Ishikawa I, et al. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–12. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boch JA, Wara-aswapati N, Auron PE. Interleukin 1 signal transduction − current concepts and relevance to periodontitis. J Dent Res. 2001;80:400–7. doi: 10.1177/00220345010800020101. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Lacey DL, Dunstan CR, et al. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–9. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 18.Lindhe J, Karring T, Lang PN. 4. Copenhagen: Blackwell Munksgaard; 2003. Clinical periodontology and implant dentistry. [Google Scholar]
- 19.Alberts B, Johnson A, Lewis J, Raff M, Robert K, Walter P. 4. New York: Galand Science; 2002. Molecular biology of the cell. [Google Scholar]
- 20.Zhang D, Yang YQ, Li XT, et al. The expression of osteoprotegerin and the receptor activator of nuclear factor kappa B ligand in human periodontal ligament cells cultured with and without 1alpha,25-dihydroxyvitamin D3. Arch Oral Biol. 2004;49:71–6. doi: 10.1016/s0003-9969(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 21.Kanzaki H, Chiba M, Takahashi I, et al. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83:920–5. doi: 10.1177/154405910408301206. [DOI] [PubMed] [Google Scholar]
- 22.Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17:1667–79. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 23.Brandstrom H, Bjorkman T, Ljunggren O. Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem Biophys Res Commun. 2001;280:831–5. doi: 10.1006/bbrc.2000.4223. [DOI] [PubMed] [Google Scholar]
- 24.Wise GE, Ren Y, Yao S. Regulation of osteoprotegerin gene expression in dental follicle cells. J Dent Res. 2003;82:298–302. doi: 10.1177/154405910308200411. [DOI] [PubMed] [Google Scholar]
- 25.Liu XH, Kirschenbaum A, Yao S, et al. Cross-talk between the IL-6 and prostaglandin E2 signalling systems results in enhancement of osteoclastogenesis through effects on the OPG/ RANKL/RANK system. Endocrinology. 2005;146:1991–8. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–5. [PubMed] [Google Scholar]
- 27.Munoz Blanco E. IL-1 signal transduction. Immunol Today. 1991;12:287–8. doi: 10.1016/0167-5699(91)90129-H. [DOI] [PubMed] [Google Scholar]
- 28.Shirakawa F, Yamashita U, Chedid M, et al. Cyclic AMP − an intracellular second messenger for interleukin 1. Proc Natl Acad Sci USA. 1988;85:8201–5. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YH, Lin JX, Yip YK, et al. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc Natl Acad Sci USA. 1988;85:6802–5. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burch RM, Connor JR. Elevated cAMP is required for stimulation of eicosanoid synthesis by interleukin 1 and bradykinin in BALB/c 3T3 fibroblasts. J Cell Physiol. 1992;151:512–8. doi: 10.1002/jcp.1041510310. [DOI] [PubMed] [Google Scholar]
- 31.Weitzmann MN, Savage N. Cyclic adenosine 3′,5′-monophosphate, a second messenger in interleukin-1 mediated K562 cytostasis. Biochem Biophys Res Commun. 1993;190:564–70. doi: 10.1006/bbrc.1993.1085. [DOI] [PubMed] [Google Scholar]
- 32.Munoz E, Beutner U, Zubiaga A, et al. IL-1 activates two separate signal transduction pathways in T helper type II cells. J Immunol. 1990;144:964–9. [PubMed] [Google Scholar]
- 33.Chedid M, Mizel SB. Involvement of cyclic AMP-dependent protein kinases in the signal transduction pathway for interleukin-1. Mol Cell Biol. 1990;10:3824–7. doi: 10.1128/mcb.10.7.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone. 2002;31:252–9. doi: 10.1016/s8756-3282(02)00804-9. [DOI] [PubMed] [Google Scholar]
- 35.Fu Q, Jilka RL, Manolagas SC, et al. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277:48868–5. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 36.Sakata M, Shiba H, Komatsuzawa H, et al. Osteoprotegerin levels increased by interleukin-1beta in human periodontal ligament cells are suppressed through prostaglandin E(2) synthesized de novo. Cytokine. 2002;18:133–9. doi: 10.1006/cyto.2002.1026. [DOI] [PubMed] [Google Scholar]
- 37.Suda K, Udagawa N, Sato N, et al. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol. 2004;172:2504–10. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi K, Shitashige M, Ishikawa I. Involvement of cyclooxygenase-2 in interleukin-1alpha-induced prostaglandin production by human periodontal ligament cells. J Periodontol. 1999;70:902–8. doi: 10.1902/jop.1999.70.8.902. [DOI] [PubMed] [Google Scholar]
- 39.Kostenuik PJ, Shalhoub V. Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des. 2001;7:613–35. doi: 10.2174/1381612013397807. [DOI] [PubMed] [Google Scholar]
- 40.Soames JV, Entwisle DN, Davies RM. The progression of gingivitis to periodontitis in the beagle dog: a histological and morphometric investigation. J Periodontol. 1976;47:435–9. doi: 10.1902/jop.1976.47.8.435. [DOI] [PubMed] [Google Scholar]