Abstract
Anti-phospholipid antibodies (aPL) are autoantibodies associated with both infections and the pathogenesis of certain pregnancy complications. In the latter, but not the former, aPL are dependent on a co-factor, β2 glycoprotein I (β2GPI), which can also be used as an antigen for detection of such aPL in pregnancy. A cross-sectional study was carried out on serum samples from Kumasi, Ghana, to determine the occurrence and β2GPI-dependence of aPL in placental malaria. Anti-cardiolipin, anti-phosphatidylserine and anti-β2GPI enzyme-linked immunosorbent assays (ELISAs) were performed on sera from 103 HIV-non-infected gravid women. Placental malaria, both active and past infection, was diagnosed in 33/103 (32%) based on placental histology. In multiparae, β2GPI-independent IgM antibodies to cardiolipin (P = 0·018) and phosphatidylserine (P = 0·009) were observed, which were most pronounced in past placental malaria infection. In primiparae, no association emerged between aPL and placental malaria. Trends for improved clinical parameters were identified in infected women with levels of anti-cardiolipin beyond the 99th multiple of the median for a healthy, non-malarious population. This study in placental malaria reports parity associations of β2GPI-independent aPL profiles, and does not support a role for β2GPI-dependent aPL. It is of significance in the context of the known parity differences in pregnancy malaria immunity.
Keywords: anti-phospholipid antibody, β2 glycoprotein I, cardiolipin, malaria, placenta
Introduction
There is a high prevalence of serum antibodies reactive with negatively charged phospholipids (PL) (anti-phospholipid antibodies; aPL) in uncomplicated and severe non-pregnant malaria patients, as well as asymptomatic parasitaemic individuals [1,2]. Anti-phospholipid antibodies may be divided into two families, co-factor-dependent and co-factor-independent aPL. The first family are autoantibodies that bind to a complex antigen comprised of a negatively charged phospholipid and a phospholipid-binding protein co-factor [3]. The most studied of these phospholipid-binding proteins is the plasma apolipoprotein β2 glycoprotein I (β2GPI). These aPL are referred to as co-factor- or β2GPI-dependent aPL and are associated with thrombotic disease and pregnancy complications, e.g. 5–20% of women with recurrent pregnancy loss [4,5]. These antibodies may promote pathology by inducing local platelet aggregation and a subsequent decidual vasculopathy, as well as by binding to fetal trophoblast and causing defective placentation [6]. It is now recognized that β2GPI can also be used as an antigen for detection of such pathogenic aPL in pregnancy [7]. The second family (co-factor-independent aPL) are not uncommon in association with infections, often appearing transiently, and these antibodies bind directly to negatively charged phospholipids such as cardiolipin or phosphatidylserine [3,4]; syphilitic reagins are archetypal of this family. Co-factor-independent aPL are thought not to cause thrombosis or pregnancy complications.
In malaria, aPL are thought to bind phospholipid independently of β2GPI [1–3]. However, there are no published data on either the prevalence or β2GPI-dependency of aPL in pregnancy-associated malaria. Circulating aPL that are dependent on β2GPI might interact with mechanisms of sequestration of parasitized red cells within the placenta, the most characteristic pathological feature of malarial pregnancy [8,9], or contribute to the inflammatory vasculopathy observed in this infection. Conversely, β2GPI-independent aPL might comprise a component of the protective immune response against placental malaria, characteristic of infected multiparae. Primiparae are at greater risk from malaria than multiparae, and the mechanism of protection appears to reflect an anti-adhesion response to parasite subtypes binding chondroitin sulphate A (CSA) and other glycosaminoglycans at the syncytiotrophoblast surface [10]. Primiparae also tend to experience a more severe and protracted course of infection, associated with an exaggerated type-1 T helper (Th1) cell-mediated response and high levels of tumour necrosis factor-α (TNF-α) [11]. PLs induce TNF-α expression in macrophages, and animal models have demonstrated that aPL can block TNF-α induction in malaria and ameliorate the clinical course of the disease [12]. The same principle has been used to explain the results of studies on aPL in non-pregnant adults and children with malaria [13].
We have carried out a study in an urban West African setting, an area of high malaria transmission. The aim was to describe the occurrence and β2GPI co-factor-dependence of serum aPL in HIV–non-infected malarial pregnancy using anti-cardiolipin (aCL), anti-phosphatidylserine (aPS) and anti-β2GPI (aß2GPI) enzyme-linked immunosorbent assays (ELISAs).
Materials and methods
Study design
A cross-sectional study was performed at the Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana, from April to June 2003. The target population was perinatal women and their babies. This study was nested inside an analysis of transplacental transfer of measles antibody in pregnancy (Owens et al. unpublished data). Sample size was thus calculated separately.
Enrolment of subjects
Women who delivered vaginally were recruited consecutively at delivery in the labour unit. Those with blood pressure ≥ 90 mmHg diastolic or 140 mmHg systolic, multiple births and those who had received a blood transfusion ≥ 24 h before delivery were excluded. At enrolment, basic demographic data and antenatal care were documented on a preprepared questionnaire. Information was obtained from each patient's antenatal health card; patients without a card were questioned directly.
Only mothers whose babies were delivered alive after 24 weeks' gestation, and who gave consent, were recruited. Shortly after delivery, each baby was weighed and the heel–crown length measured. The placenta was also weighed after removing blood clots and cutting the cord close to its insertion (2–3 cm). Weights were recorded to the nearest 0·05 kg; lengths to the nearest 0·5 cm.
Collection of specimens
Maternal blood (5 ml) was obtained from a peripheral vein within 4 h of delivery, and cord blood (8 ml) from a large vein on the fetal side of the placenta immediately after delivery. Sera were separated and stored at −70°C within 8 h. Cubic placental villous tissue biopsy samples (1 cm3) were obtained from an off-centre position and stored in 20 ml of 10% formaldehyde in phosphate buffer until processed for histological examination. Thick and thin Giemsa-stained films were prepared with blood obtained from the cord.
Malaria diagnosis
Paraffin-embedded sections (5 µm) of placental tissue were stained with haematoxylin–eosin and examined under both light microscopy and polarized light ( × 40). Histology was reported blinded to numerical data. Placental malaria infection was defined and classified according to the presence of parasites and/or malaria pigment as non-infected, acute infection, chronic infection and past infection, as described previously [8,14]; in subsequent analyses, active infection included both acute and chronic infection. Films of cord blood were read under light microscopy ( × 100), and the number and species of parasites measured against 200 white cells. One hundred fields from each blood film were examined before a negative count was recorded.
Assessment of total serum immunoglobulin G (IgG) levels
Total serum IgG was assayed by laser nephelometry using an Array Protein System (Beckman Coulter, High Wycombe, UK).
aPL assays
The PLs, phosphatidylserine (PS) and cardiolipin (CL), were obtained from Sigma (Sydney, Australia). Antibody screening was conducted using our published methods [15]. Briefly, the relevant PL was diluted to 50 µg/ml in ethanol and 50 µl used to coat a 96-well ELISA plate (Corning, Amsterdam, the Netherlands) by evaporation at 4°C overnight. Plates were exposed to blocking solution, 10% newborn calf serum in phosphate-buffered isotonic saline (PBS), pH 7·4, for 1 h at room temperature. The blocking solution was discarded and plates washed three times with PBS, pH 7·4. Serum samples, diluted 1 : 100 in blocking solution, were incubated on the plates for 1 h at room temperature. Plates were then washed three times with PBS, pH 7·4 and horseradish peroxidase (HRP)-conjugated goat anti-human γ-chain or µ-chain anti-serum (Jackson Laboratories, West Grove, PA, USA), diluted 1 : 5000 in blocking solution, added for 1 h at room temperature. Plates were again washed three times with PBS, pH 7·4, and the assay developed by addition of 1 mg/ml o-phenylaminediamine dihydrochloride (Sigma) in 0·1 M citrate buffer, pH 5·5, containing freshly prepared 0·005% H2O2. The reaction was stopped by the addition of 10% HCl and the optical density determined at 490 nm. Multiples of the median (MOM) values were calculated in reference to 284 healthy individuals from a nonmalarious area, as published previously [15].
Solid phase aβ2GPI ELISA
β2GPI was purified from normal human plasma as described previously [16]. ELISA plates were coated overnight at 4°C with 50 µl β2GPI (10 µg/ml in 0·1 M carbonate buffer, pH 9·0). The antigen solution was discarded and plates blocked with 5% non-fat milk powder (NFM) dissolved in PBS containing 0·05% Tween 20 (PBST), pH 7·4, for 1 h at room temperature. Plates were washed three times with PBST before serum samples diluted 1 : 200 in blocking solution were added and incubated for 1 h at room temperature. Thereafter, the method was the same as for the aPL assays.
HIV testing
Anonymous HIV testing was carried out on the maternal samples using a non-quantitative ELISA technique (Genscreen® HIV 1/2 version 2; Bio-Rad, Marnes-la-Coquette, France).
Haemoglobin estimation
Maternal and cord haemoglobin concentrations were measured with the HemoCue® (Angelholm, Sweden).
Statistical analyses
Data entry and analyses were carried out in SPSS version 12 (Apache Software Foundation, Forest Hill, MD, USA). Variables associated commonly with malarial pregnancy were investigated under univariate analysis. Because aPL levels were not normally distributed, non-parametric analyses were performed using Mann–Whitney U and Kruskal–Wallis tests. Parametric analyses used independent t-tests, and categorical analyses used a χ2 test.
Ethical considerations
This study was approved by the Ethics Committees of the Liverpool School of Tropical Medicine and Kumasi University prior to commencing fieldwork. All laboratory data, with the exception of bedside haemoglobin measurements, were anonymous. Codes linking results to individual patients were erased prior to data entry. Informed consent was obtained from each mother, and anonymous HIV testing was discussed specifically.
Results
Patient cohort
Sera and histological data were available for 103 HIV-non-infected women. Mean maternal age (standard deviation, s.d.) was 26·5 years (6·6 years) and 38·8% were primiparae; multiparity refers to all parities greater than one. Mean infant gestational age (s.d.) was 38·5 weeks (3·2 weeks), and mean birth weight (s.d.) was 3022 g (442 g). Most women (93·1%) had documented attendance for at least one antenatal clinic.
Evidence of malaria infection was present in 33/103 placentae (32·0%) and there were no cases of cord parasitaemia. Active infection was found in 18 (17·5%) and past infection in 15 (14·6%). The prevalence of placental malaria among primigravidae was 20/40 (50·0%) and 13/63 (20·6%) among multiparae (relative risk 2·42; 1·36–4·31). Over half the women (58·3%) reported a history of using anti-malarials during pregnancy.
Table 1 summarises the characteristics of the sample population according to placental histology. There was a higher proportion of primiparae among women with placental infection than those with no evidence of infection (P = 0·002). Infants born to infected primiparae were smaller than those born to non-infected primiparae (2685 versus 2935 g; P = 0·088); infants born to multiparae were similar regardless of infection (3115 versus 3168 g; P = 0·653). The birth weight of infants born to infected primiparae was reduced compared with infants born to infected multiparae (2685 versus 3115 g; P = 0·015). Reduced maternal haemoglobin (P < 0·001) and birth weight (P = 0·007), and raised maternal total serum IgG (P < 0·001), were associated with placental malaria. There was no association between placental infection and a history of using anti-malarials during pregnancy (P = 0·327).
Table 1. Study population parameters according to placental malaria histology.
| Placental group | Number of primiparae* (%) | Mean maternal haemoglobin** g/l (s.d.) | Mean cord haemoglobin g/l (s.d.) | Geometric mean maternal total serum IgG** g/l (95% CI) | Mean birth weight† g (s.d.) | Mean neonatal length cm (s.d.) | Mean fetal– placental weight ratio (s.d.) | Mean gestation (weeks) (s.d.) | Neonatal sex ratio M : F |
|---|---|---|---|---|---|---|---|---|---|
| Non-infected | 20 | 118 | 129 | 25·2 | 3101 | 49·6 | 6·13 | 38·8 | 1·06 |
| (n = 70) | (29) | (15) | (22) | (23·9–26·7) | (385) | (2·9) | (1·25) | (2·7) | |
| Active infection | 11 | 104 | 130 | 31·6 | 2911 | 49·6 | 5·94 | 39·6 | 1·57 |
| (n = 18) | (61) | (15) | (24) | (27·7–36·0) | (327) | (1·8) | (1·03) | (2·2) | |
| Past infection | 9 | 104 | 130 | 29·1 | 2787 | 48·1 | 5·91 | 36·4 | 0·67 |
| (n = 15) | (60) | (19) | (16) | (25·5–33·2) | (673) | (2·2) | (1·62) | (4·9) |
P (infected versus non-infected) < 0·01, χ2 test;
P (infected versus non-infected) < 0·001, independent t-test;
P (infected versus non-infected) < 0·01, independent t-test.
aPL levels
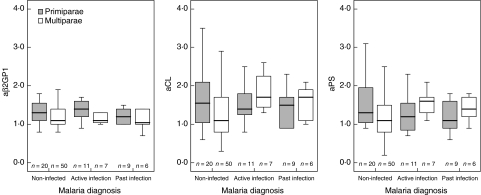
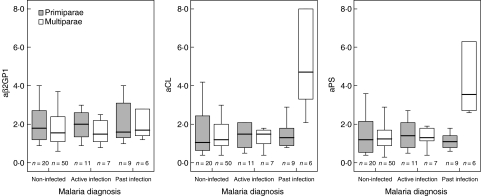
Figures 1 and 2 illustrate the relationships between placental malaria infection and aPL levels according to parity. Multiparae with placental malaria, both active and past infection, had significantly elevated concentrations of IgG aCL (P = 0·006), IgM aCL (P = 0·018), IgG aPS (P = 0·035) and IgM aPS (P = 0·009) compared with non-infected multiparae. This was most pronounced for IgM aPL in multiparae with past infection (both P = 0·003) (Fig. 2). No significant differences were found for aβ2GPI. There were no significant differences in aPL levels between infected primiparae compared to non-infected primiparae, as well as no significant parity differences in aPL levels in active placental infection.
Fig. 1.
IgG anti-phospholipid antibody (aPL) responses to placental malaria infection according to parity. Concentrations expressed as multiples of the median (MOM). Bar and box represent median and interquartile range. Whisker represents range of values within 1·5 box lengths of median.
Fig. 2.
IgM anti-phospholipid antibody (aPL) responses to placental malaria infection according to parity. Concentrations expressed as multiples of the median (MOM). Bar and box represent median and interquartile range. Whisker represents range of values within 1·5 box lengths of median.
Among non-infected women, primiparae had significantly higher concentrations of IgG aCL (P = 0·036) and aPS (P = 0·039) compared with multiparae (Fig. 1); elevated concentrations of IgG aβ2GPI were also observed (P = 0·060). However, in these women the absolute MOM levels for aPLs were rarely elevated markedly (above 3·0 MOM) and hence these observations may be of limited clinical significance. Also, among women with no evidence of placental malaria, those who took anti-malarial drugs had lower levels of IgG aβ2GPI than those who denied having taken such drugs (1·3 versus 1·1 MOM; P = 0·024).
In women with placental malaria, both active and past infection, IgM aCL levels in excess of the 99th MOM were associated with non-significant trends for increased maternal age, higher cord and maternal haemoglobin, heavier babies and increased fetal–placental weight ratio (Table 2). Infected women with high IgM aPS levels tended similarly to be older and delivered heavier babies with an increased fetal–placental weight ratio. Those with high IgM aβ2GPI tended to be younger, more anaemic and delivered shorter babies with lower fetal–placental weight ratios. Infected women with high levels of aβ2GPI (n = 5) had smaller placentae than women with lower levels (n = 28) (440 g versus 505 g; P = 0·035); other parameters were not significant. The number of infected women with high levels of IgG aCL and aPS was too small to undergo statistical analyses.
Table 2. Malaria parameters in relation to IgM anti-phospholipid antibodies (aPL) responses in women with placental infection.
| Antibody profile | Mean maternal age years (s.d.) | Mean maternal haemoglobin g/l (s.d.) | Mean cord haemoglobin g/l (s.d.) | Geometric mean maternal total serum IgG g/l (95% CI) | Mean birth weight g (s.d.) | Mean neonatal length cm (s.d.) | Mean fetal– placental weight ratio (s.d.) | Mean gestation weeks (s.d.) |
|---|---|---|---|---|---|---|---|---|
| aβ2GP1 | ||||||||
| Low | 25·7 | 106 | 129 | 30·2 | 2870 | 49·4* | 6·02 | 38·1 |
| (n = 23) | (7·3) | (22) | (25) | (27·1–33·6) | (512) | (1·8) | (1·37) | (3·8) |
| High | 22·2 | 98 | 132 | 31·0 | 2820 | 47·9* | 5·70 | 37·5 |
| (n = 10) | (4·7) | (18) | (11) | (25·5–37·6) | (527) | (2·4) | (1·20) | (4·8) |
| aCL | ||||||||
| Low | 23·8 | 101 | 127 | 30·4 | 2813 | 49·1 | 5·91 | 38·5 |
| (n = 24) | (6·7) | (18) | (23) | (27·1–34·1) | (457) | (1·8) | (133) | (3·9) |
| High | 26·9 | 112 | 136 | 30·4 | 2967 | 48·5 | 5·97 | 36·4 |
| (n = 9) | (6·5) | (27) | (11) | (26·0–35·5) | (644) | (2·7) | (1·33) | (4·2) |
| aPS | ||||||||
| Low | 23·9 | 105 | 130 | 30·5 | 2817 | 49·0 | 5·82 | 38·1 |
| (n = 29) | (6·4) | (22) | (22) | (27·6–33·7) | (511) | (2·1) | (1·34) | (4·3) |
| High | 29·8 | 98 | 130 | 29·7 | 3125 | 48·6 | 6·66 | 37·2 |
| (n = 4) | (7·9) | (16) | (13) | (22·7–39·1) | (457) | (1·9) | (0·91) | (1·8) |
P (high versus low aβ2GPI) = 0·045, independent t-test (‘high = values above 99th centile; ‘low' = values below 99th centile).
Discussion
This study reports a significant association between parity and specific aPL in malarial pregnancy. Multiparous women demonstrated IgM aCL and aPS responses in placental malaria, which were β2GPI co-factor-independent. Among these, the most vigorous responses were associated with resolving malaria infections, when parasites had been cleared from the placenta. In contrast, no association emerged for the aPL profile in primiparae with malaria infection, who have a greater prevalence of placental parasitaemia and more severe consequences of the disease. Primiparae with no evidence of malaria infection at delivery presented elevated levels of IgG aCL, aPS and aβ2GPI compared with non-infected multiparae; it is probable that many of these were infected earlier in pregnancy but had recovered [17]. It is thought that aPL are produced in a T cell-independent manner in response to malarial PL-containing antigens, and the immunoglobulin isotype of aPL in malarial pregnancy appears unlikely to represent the classical class switching of primary and secondary responses to infectious agents within this hypergammaglobulinaemic sample population [1].
Understanding the β2GPI co-factor dependency of aPL in malarial pregnancy is important because of potential the pathological effects of aβ2GPI on the placenta [6,18] and that these antibodies might play a role in parasite sequestration through endothelial activation and up-regulation of adhesion molecules [19]. This could be particularly important given that aPL may bind to endothelial cells only following an independent triggering event, and malaria infection might be such an event [20]. The consensus in the literature is that aPL in non-pregnancy malaria are β2GPI-independent [2]. Our data also do not support a significant association between aβ2GPI levels and placental malaria infection although, in women who reported using anti-malarial drugs during pregnancy, there was a significant decrease in the median level of IgG aβ2GPI but the absolute MOM levels suggested that this may be of limited clinical significance.
We hypothesize that parity-specific changes in the aPL profile may represent a component of the parity-dependent protective immune response to placental malaria [10]. In the absence of anti-adhesion antibodies acquired in later pregnancies, primiparae may develop an extensive intervillous mononuclear infiltrate in the placenta [20], generating significantly elevated levels of the proinflammatory cytokines interferon (IFN)-γ, interleukin (IL)-2 and TNF-α [11,21]. Although inflammatory cytokines are beneficial in controlling parasitaemia [22], excessive concentrations exacerbate fever and other symptoms [23] and have been implicated in the pathogenesis of cerebral malaria in children [24]. This exaggerated Th1-type response in placental malaria is associated with low birth weight [21], itself an adverse risk factor for infant morbidity and mortality [25].
Our results show that babies born to multiparae maintain birth weight despite placental malaria infection. This reflects the reduced disease severity described in multiparae, a consequence of acquisition of specific immunity limiting inflammatory placental infiltrates [20]. Among infected women with high levels of aCL, trends were observed for improved maternal and cord haemoglobin concentrations as well as increased birth weight, despite shorter gestation and similar total immunoglobulin concentrations. Women in the high aCL group were older, as were infected women with high aPS who also delivered heavier babies. In contrast, those with high aß2GPI were younger, more anaemic, with shorter babies and reduced fetal–placental weight ratios. Most of these differences did not reach statistical significance as subgroups were small, but the trends were consistent. Of particular interest is the potential role of aCL in reducing the severity of fetal anaemia associated with malarial pregnancy [25,26]. Cord haemoglobin levels in the present study were considerably lower than values from non-malarious areas and, in malarious areas, fetal anaemia has been associated with placental parasite density [27].
Highest levels of IgM aCL and aPS were noted in multiparae with past infection, when haemozoin trapped within intervillous fibrin was evident but parasites had been cleared. Together with the improved clinical parameters described above, these data indicate that high aPL levels may represent a protective response to placental parasitaemia. Such aPL may ameliorate the proinflammatory cytokine cascade exacerbated by free PL because Plasmodium falciparum glycosylphosphatidylinositol anchors are a major parasitic component inducing TNF-α production by macrophages in malaria [12]. Although a recent study [28] found no association between antibodies to P. falciparum glycosylphosphatidylinositol anchors and placental TNF-α levels or the presence of acute or chronic placental malaria in term and preterm deliveries, several other studies have proposed a protective role for aPL in non-pregnant adults and children with malaria [1,2]. A brisk aPL response might block TNF-α induction and improve morbidity. In primiparae, there was no association between placental malaria at delivery and aPL; conversely, uninfected primiparae had significantly elevated IgG aCL and aPS levels compared with uninfected multiparae. This may reflect prior malaria exposure earlier in pregnancy in women who had cleared their parasitaemia by term.
Our data demonstrated all the expected associations between placental malaria, hypergammaglobulinaemia, maternal anaemia, birth weight and parity [9], and therefore the other associations which emerged warrant careful consideration. Because the sample size was small and the study lacked sufficient power to fully investigate morbidity, a longitudinal study throughout pregnancy is now required, correlating episodes of peripheral parasitaemia with aPL profile, placental histology and pregnancy outcome.
Acknowledgments
The authors wish to thank Dr K. A. Danso, staff and patients of the Labour Unit, KATH, for their generous cooperation and assistance; also Mr John Amuasi and Mr Gordon Offei-Larbi, medical students at KATH, for gathering questionnaire data. We are grateful both to Mr Gregory Harper, laboratory supervisor, Child and Reproductive Health, Liverpool School of Tropical Medicine, and Ms Andrea Moore, Department of Obstetrics and Gynaecology, University of Auckland, for technical assistance. The fieldwork was completed in part fulfilment of the Master in Tropical Paediatrics degree, Liverpool School of Tropical Medicine. Financial support was derived from the European Commission Research Directorates General Fifth Framework grant (contract PREMA-EU-ICA4CT-2001–1110012), as well as the University of Auckland Staff Research Fund (9303 3603061) and the Auckland Medical Research Foundation (3601299).
References
- 1.Jakobsen PH, Morris-Jones SD, Hviid L, et al. Anti-phospholipid antibodies in patients with Plasmodium falciparum malaria. Immunology. 1993;79:653–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Consigny PH, Cauquelin B, Agnamey P, et al. High prevalence of co-factor independent anticardiolipin antibodies in malaria exposed individuals. Clin Exp Immunol. 2002;127:158–64. doi: 10.1046/j.1365-2249.2002.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt JE, McNeil HP, Morgan GJ, Crameri RM, Krilis SA. A phospholipid-beta 2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 4.Chamley LW. Antiphospholipid antibodies: biological basis and prospects for treatment. J Reprod Immunol. 2002;57:185–202. doi: 10.1016/s0165-0378(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 5.Kutteh WH, Rote NS, Silver R. Antiphospholipid antibodies and reproduction: the antiphospholipid antibody syndrome. Am J Reprod Immunol. 1999;41:133–52. doi: 10.1111/j.1600-0897.1999.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Chamley LW, Duncalf AM, Mitchell MD, Johnson PM. Action of anticardiolipin and antibodies to beta2-glycoprotein-I on trophoblast proliferation as a mechanism for fetal death. Lancet. 1998;352:1037–8. doi: 10.1016/s0140-6736(05)60080-3. [DOI] [PubMed] [Google Scholar]
- 7.Cockerill KA, Linnik MD, Iverson GM. Detection and characterization of B cell epitopes on beta2-glycoprotein I. Clin Immunol. 2004;112:129–35. doi: 10.1016/j.clim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Ismail MR, Ordi J, Menendez C, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 9.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 10.Brabin BJ, Romagosa C, Abdelgalil S, et al. The sick placenta – the role of malaria. Placenta. 2004;25:359–78. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–30. [PubMed] [Google Scholar]
- 12.Bate CA, Taverne J, Kwiatkowski D, Playfair JH. Phospholipids coupled to a carrier induce IgG antibody that blocks tumour necrosis factor induction by toxic malaria antigens. Immunology. 1993;79:138–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel-Ribeiro CT, Zanini G. Autoimmunity and malaria: what are they doing together? Acta Trop. 2000;76:205–21. doi: 10.1016/s0001-706x(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 14.Romagosa C, Menendez C, Ismail MR, et al. Polarisation microscopy increases the sensitivity of hemozoin and Plasmodium detection in the histological assessment of placental malaria. Acta Trop. 2004;90:277–84. doi: 10.1016/j.actatropica.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Stern C, Chamley L, Hale L, Kloss M, Speirs A, Baker HW. Antibodies to beta2 glycoprotein I are associated with in vitro fertilization implantation failure as well as recurrent miscarriage: results of a prevalence study. Fertil Steril. 1998;70:938–44. doi: 10.1016/s0015-0282(98)00312-4. [DOI] [PubMed] [Google Scholar]
- 16.Chamley LW, Allen JL, Johnson PM. Synthesis of beta2 glycoprotein 1 by the human placenta. Placenta. 1997;18:403–10. doi: 10.1016/s0143-4004(97)80040-9. [DOI] [PubMed] [Google Scholar]
- 17.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Org. 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 18.Bas de Laat H, Derksen RH, de Groot PG. Beta2-glycoprotein I, the playmaker of the antiphospholipid syndrome. Clin Immunol. 2004;112:161–8. doi: 10.1016/j.clim.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112::169–74. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Ordi J, Ismail MR, Ventura PJ, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Brown HC, Pollina E, et al. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun. 2003;71:267–70. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards AL. Tumour necrosis factor and associated cytokines in the host's response to malaria. Int J Parasitol. 1997;27:1251–63. doi: 10.1016/s0020-7519(97)00122-7. [DOI] [PubMed] [Google Scholar]
- 23.McGuire W, D'Alessandro U, Stephens S, et al. Levels of tumour necrosis factor and soluble TNF receptors during malaria fever episodes in the community. Trans R Soc Trop Med Hyg. 1998;92:50–3. doi: 10.1016/s0035-9203(98)90951-8. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowski D, Hill AV, Sambou I, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–4. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 25.Verhoeff FH, Le Cessie S, Kalanda BF, Kazembe PN, Broadhead RL, Brabin BJ. Post-neonatal infant mortality in Malawi: the importance of maternal health. Ann Trop Paediatr. 2004;24:161–9. doi: 10.1179/027249304225013448. [DOI] [PubMed] [Google Scholar]
- 26.le Cessie S, Verhoeff FH, Mengistie G, Kazembe P, Broadhead R, Brabin BJ. Changes in haemoglobin levels in infants in Malawi: effect of low birth weight and fetal anaemia. Arch Dis Child Fetal Neonatal Ed. 2002;86:F182–7. doi: 10.1136/fn.86.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brabin BJ, Kalanda BF, Verhoeff FH, Chimsuku LH, Broadhead R. Risk factors for fetal anaemia in a malarious area of Malawi. Ann Trop Paed. 2004;24:311–21. doi: 10.1179/027249304225019136. [DOI] [PubMed] [Google Scholar]
- 28.Suguitan AL, Jr, Gowda DC, Fouda G, et al. Lack of an association between antibodies to Plasmodium falciparum glycosylphosphatidylinositols and malaria-associated placental changes in Cameroonian women with preterm and full-term deliveries. Infect Immun. 2004;72:5267–73. doi: 10.1128/IAI.72.9.5267-5273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]