Abstract
Herpes simplex virus (HSV) represents a smart pathogen, which displays both lytic and latent modes of interaction with its natural human host. In order to be optimally equipped for immune evasion and to reply to any attacks of the host during reactivation, HSV has developed a multitude of cleverly devised defence strategies. Dendritic cells (DC) as antigen-presenting cells located at the border zones of the body and the environment have been shown to play a crucial role as one of the first cells interacting with HSV beside epithelial cells, on one hand, and as important controllers of the viral spreading on the other hand. Here, we provide a research update about the interaction of HSV with DC and summarize the latest proceedings in this field.
Keywords: dendritic cells, herpes simplex virus, interferon, Toll-like receptors
Introduction
The term ‘herpes’ is derived from the Greek word herpein, which means creeping, and stands for the characteristic creeping of the eruptions caused by the virus. Herpes simplex virus (HSV) belongs to the Alphaherpesviridae and is a member of the double-stranded DNA virus family (Fig. 1). The most striking feature of HSV is its capability to establish latency after primary infection. Thereby HSV becomes able to persist unperceived in the host in order to periodically reactivate and cause outbreaks of HSV infections during the whole lifetime. During its evolution, HSV has developed a multitude of strategies to hide for immune evasion and counterattacks against the host cell during the reactivation phases. These mechanisms comprise (i) viral escape, which can be achieved for instance by the reduction of the viral gene expression during the latency phase, (ii) viral resistance such as the sequential induction of pro- and anti-apoptotic effects on its defender cells and (iii) viral counterattacks to which the inhibition of the maturation of dendritic cells (DC) by HSV belongs.
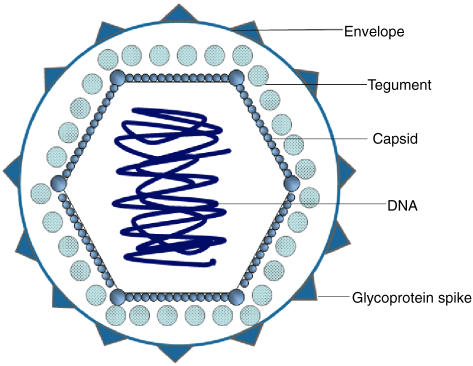
Fig. 1.
Structural characteristics of herpes simplex virus (HSV). Structural characteristics of HSV are summarized in this schematic figure. HSV belongs to the Alphaherpesviridae and is a member of the double-stranded DNA virus family. HSV consists of an envelope with different glycoproteins (gG) and spikes (gD, gB). The tegument contacts both the envelope and the capsid.
HSV infection affects more than one-third of the population worldwide and is responsible for a wide array of human diseases, ranging from mild localized HSV infections to disseminated forms known as Eczema herpeticatum in patients with atopic eczema or severe life-threatening variants which occur preferentially in individuals with immunodeficiencies or under immunosuppression. The seroprevalence of HSV-1 and HSV-2 varies, but increases with age and reaches about 88% of individuals at the age of 40 years for HSV-1, while seroprevalence of HSV-2 averages about 12–15% [1]. HSV enters the human body through lesional skin or mucous membranes, to reach epithelial cells which represent the primary targets of HSV. Infection with HSV induces innate defence mechanisms, which are aimed at limiting virus propagation and initial spreading from cell to cell, followed by the activation of acquired antigen-specific immune responses, which are aimed at clearing the infection effectively. As contributors to the clinical picture of the disease, HSV-infected cells undergo apoptosis and release liquid material into the intradermal space, which causes the typical vesicles going along with HSV. On the other hand, infected cells build multi-nucleated clusters with other cells and form the so-called giant cells which are characteristic for HSV and used as a diagnostic tool. The primary HSV infection is usually mild or occurs subclinically, and in most of the cases takes place in early childhood. The reactivation of HSV by triggers such as stress, UV-light, trauma or any kind of immunosuppression at later time-points induces the transport of the virus by the neurone back to the peripheral epithelium. Within the peripheral epithelial tissue more HSV replication occurs, which is the basis for the next outbreaks of mild to severe HSV infections.
What happens when HSV meets DC?
DC populations throughout the body, which are often found at the interface to the environment such as the skin, the airways and the gut, have a wide range of features in common which are associated with their function in antigen presentation. As sentinels of the immune system, DC travel from the blood to the peripheral tissue to capture foreign antigens. Thereafter, they migrate to the draining lymphoid organs to prime naive CD4+ T cells into Th1 or Th2 effector cells and induce a subgroup of central memory T cells. In the immune system, two main subsets of DC can be found: myeloid DC (mDC) and plasmacytoid DC (pDC) [2,3]. The longest-known member of the myeloid DC system are the classical Langerhans cells (LC), which are characterized by their primary marker, the tennis-racket-shaped Birbeck granules in combination to their surface expression of CD1a. LC reside in different peripheral tissues such as the basal and suprabasal layers of the epidermis, and the mucosal tissue and are present even in normal, uninflamed skin.
It has been shown by different research groups that HSV-1 infection of immature DC in vitro results in morphological changes and the down-regulation of the expression of co-stimulatory molecules such as CD80, CD86, CD40 the DC-marker CD1a, the adhesion molecule CD54 (ICAM-1) [4] and major histocompatibility class (MHC) I molecules (Fig. 2). This leads to the inhibition of MHC class I-dependent antigen presentation of infected DC and interferes with the recognition of DC by CD8+ T cells. The inhibition of MHC I is achieved by the formation of HSV gene product infected cell protein (ICP)47 with TAP to ICP47–TAP complex, which blocks the translocation of the MHC class I peptide complex to the cell surface in vivo [1,5–7]. Further, HSV infected DC secrete lower levels of interleukin (IL)-12 in consequence of stimulation with lipopolysaccharides (LPS) in vitro. IL-12 is required for the polarization of naive T cells into Th1 helper cells, which are characterized by a high interferon (IFN)-γ secretion. Further, IL-12 is produced during the maturation of DC. Together with a weaker stimulatory capacity toward T cells [8], the lower IL-12 production might mirror the lower maturation stage of HSV-infected DC and a lower capacity to induce sufficient amounts of IFN-producing Th1 T cells. In accordance with these observations, infection of mature DC with HSV reduces, similar to immature DC, the stimulatory capacity of these cells toward T cells and diminishes the expression of the maturation marker CD83 on the surface of these cells in vitro [9]. CD83 is a 45 kDa glycoprotein and member of the Ig-family, which is up-regulated during maturation of DC and involved in immune responses of DC [10]. Recently, a soluble form of CD83 has been described, which blocks DC-mediated T cell stimulation [11]. It is important to note that the HSV-dependent loss of CD83 surface expression does not result from a reduced mRNA synthesis but from a degradation of CD83 in the lysosomal compartments [10,12] and might underlie the lower T cell stimulation of HSV-infected DC.
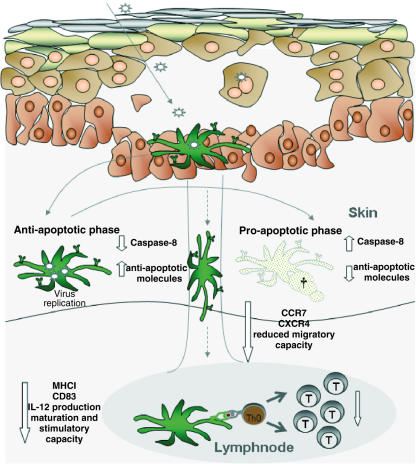
Fig. 2.
Interaction of herpes simplex virus (HSV) with dendritic cells (DC). Infection with HSV of the skin goes along with the ballooning degeneration of epithelial cells which lose intracellular adhesion, leading to intra-epidermal vesicles. Infection of skin DC with HSV induces a primary anti-apoptotic phase with rapid viral replication and a second pro-apoptotic phase of DC. Further, HSV infection down-regulates the surface expression of co-stimulatory molecules and MHC molecules on DC and slows down the maturation and migration of DC to the peripheral lymphnodes, which together results in a lower stimulation of T cells by DC.
It has been demonstrated repeatedly that defence strategies of the host against HSV consist of DC-mediated T helper cell responses and antibody production. To down-regulate CD4 T cell responses, HSV is able to interrupt MHC II antigen processing of several cells by the reduction of the expression of the invariant chain and the interaction of the viral envelope glycoprotein B (gB) with HLA-DR and HLA-DM polypeptides [13].
Furthermore, it has been shown that CD4+ helper T cells are required for the priming of cytotoxic T lymphocytes (CTL). In this regard, studies have shown that ‘licensing’ of DC by CD4+ T cells is essential not only for the initial expansion of HSV-specific CTL, but also for the generation of memory CTL and immunity to HSV [14]. In terms of HSV infections, CD4+ T cell dependence of the primary phase of T cell expansion has been observed [15]. This means that initiation of CD8+ T cell-mediated responses to cutaneous HSV-1 infections is critically dependent on the presence of antigen-presenting DC which present virus-derived antigens [16].
As another effective defence strategy, HSV induces apoptosis of attacking DC. As a characteristic feature, the induction of apoptosis by HSV represents a biphasic mechanism with an early phase, in which rather anti-apoptotic mechanisms are induced by HSV, and a late phase in which HSV-mediated pro-apoptotic mechanisms predominate (Fig. 2). It has been shown that HSV glycoprotein D induces NF-κB activation and thereby protection against Fas-induced apoptosis by the reduction of caspase-8 activity and up-regulation of intracellular anti-apoptotic molecules in the early, anti-apoptotic phase [17]. The inhibition of apoptosis in the early phase of HSV infection is aimed at ensuring the survival of HSV-infected cells for a sufficient viral replication. In combination with a delayed activation of T cells by HSV-infected DC, these sequential mechanisms might enable HSV to replicate for a longer time before effective defence strategies of the host are induced [18]. In the second phase, HSV induces apoptosis of immature DC by the induction of the caspase-8 mechanism, an up-regulation of tumour necrosis factor (TNF)-α, TNF-related apoptosis ligand (TRAIL) and p53 in combination with a down-regulation of the cellular FLICE-inhibitory protein (c-FLIP). Together, this leads to the effective incapacitation and elimination of DC by HSV.
It is well known that DC take up antigens and migrate to the peripheral lymph nodes to present the processed antigens to T cells and induce sufficient T cell responses. As another escape mechanism of HSV, the down-regulation of the chemokine receptors CCR7 and CXCR4 on mature DC and a subsequently lower migration to the respective chemokine ligands CCL19/macrophage-inflammatory protein (MIP)-3β and CXCL12/stem cell derived factor (SDF-1)α has been observed. It is therefore supposed that HSV influences mature DC in their capability to migrate to the peripheral lymph nodes and impairs the DC-mediated induction of inhibitory, antiviral immune responses in this way [19] (Fig. 2).
In which way does HSV interact with and enter DC?
The viral envelope of HSV contains 11 different membrane glycoproteins, of which four (i.e. gD, gH, gL and gB) are essential for the viral entry of HSV [20–23]. As the first step the HSV virion envelope/glycoprotein C (gC) and/or gB binds to surface glycosaminoglycans such as heparin sulphate. The interaction of virion gD with one of its receptors triggers the penetration of the virus, which occurs through fusion of the viral envelope with the cell membrane and requires glycoproteins gB, gD, gH and gL [24] (Table 1).
Table 1. Summary of the most relevant interactions of herpes simplex virus (HSV) with dendritic cells (DC). The most important effects of HSV on DC are summarized.
| HSV and DC interaction | In vitro | In vivo | Reference |
|---|---|---|---|
| Down-regulation of co-stimulatoy molecules and MHC molecules | x | [4] | |
| Formation of ICP47-TAP complex which blocks the MHC I translocation | x | [1,5–7] | |
| Reduction of the expression of the invariant chain | x | [13] | |
| Reduced production of interleukin-12 after lipopolysaccharide stimulation | x | [8] | |
| Loss of CD83 surface expression | x | [10,12] | |
| Anti-apoptotic mechanisms | x | [17] | |
| Pro-apoptotic mechanisms | x | [18] | |
| Down-regulation of chemokine receptors and lower migration | x | [19] |
In general, three classes of different surface molecules can serve as gD receptors for HSV entry into cells: a member of the TNF receptor family, two members of the immunoglobulin (Ig) superfamily and specific sites in heparin sulphate generated by the action of certain isoforms of 3-sulphotransferase. The members of the Ig superfamily are nectin-1 (also known as Prr1 or HveC) and nectin-2 (also known as Prr2 or Hveb). Nectins contain three extracellular Ig-like domains, a transmembrane membrane domain and a cytoplasmic tail. Further, nectins form dimers and mediate cell adhesion through interaction with other nectins. Nectin-1 allows entry of HSV-1 and HSV-2, while for nectin-2 the entry is restricted to specific types of HSV such as HSV-2. Nectin-1 co-localizes with E-cadherin at adherent junctions of epithelial cells. This nectin-1/E-cadherin system increases viral entry and spreading of HSV from cell to cell [25]. Nectin-1 is expressed only weakly on DC, while nectin-2 and herpes virus entry mediator (HVEM) are expressed at higher levels on immature DC. Upon maturation, nectin-2 expression is further up-regulated while nectin-1 and HVEM expression remain unaffected.
HVEM belongs to the TNF family and activates NF-κB and AP-1 via TNF receptor-mediated mechanisms [26]. HVEM represents the cell receptor for gD and plays a role in mediating death receptor-associated apoptosis. Enhanced soluble levels of HVEM could be detected in the sera of patients with allergic diseases [18].
HSV-infection occurs in a sequentially ordered cascade in which α-proteins (immediate-early genes), to which ICP0, ICP4, ICP22 and ICP27 belong, induce the synthesis of β-proteins (early) and γ-proteins (late). The early genes give rise to viral proteins required for the replication of the viral genome, while late genes encode the structural components of the virion. After lytic infection HSV avails the host's synthesis machinery to sustain its own reproduction.
What role do pattern recognition receptors (PRRs) play on DC?
Recently, a new group of pattern recognition receptors, which belong to the innate immune system, have been described. Toll-like receptors (TLR) represent phylogenically conserved transmembrane proteins and over the last years, several TLR family members and their respective agonists have been identified [27]. TLR bind microbial proteins at the cellular surface or endosomes and activate cytoplasmic signal transduction pathways, which are regulated by adaptor proteins. TLR3 bind double-stranded RNA synthesized by viruses, TLR7 and TLR8 are required for the recognition of viral single stranded RNA and TLR9 can be activated by unmethylated cytosine guanine oligodeoxynucleotide motifs common to both bacterial and viral DNA, and are highly present in double-stranded genomes of HSV [28,29]. In addition, a strain of HSV is able to activate TLR2 and cause herpes encephalitis in mice [10]. So far it is known that stimulation of plasmacytoid dendritic cells (pDC) with inactivated HSV after HSV recognition via TLR9 requires the expression of the adapter molecule MyD88 and induces the production of IFN-α. As one of the most important mechanisms, IFN-α production of pDC is triggered by outside–in signal transduction pathways via TLR7 and TLR9 [30]. With regard to the downstream signalling events which follow TLR9 activation on pDC, it has been elucidated that IFN-α production is dependent on the enhanced expression of interferon regulatory factor-7 (IRF-7) and NF-κB activation. The interferon regulatory factors are direct regulators of IFN-α and IFN-β production, which under HSV infection undergo phosphorylation and nuclear translocation. IRF-7 alone is able to activate both IFN-α and IFN-β genes, while IRF-3 activates mainly the IFN-β gene. Further, LPS mediates up-regulation of TLR4 expression and increases the capacity of these cells to produce IFN-α via an enhanced IRF-7 level [31]. As well as the TLR9-dependent pathways to induce IFN-production by pDC, recent research papers describe additional, TLR9-independent ways of HSV-induced IFN production by pDC which need to be elucidated in more detail in the future [32]. The induction of type I IFN production by mDC plays a pivotal role for DC to bypass the HSV-mediated inhibition of antigen presentation. Together with IL-12 production, IFN-production induces indirect bystander activation and maturation of even DC in the uninfected cell fraction, implying that not one DC subtype but distinct DC-subset prime HSV-specific cytotoxic T-lymphocytes [33]. Further, the direct contact of the HSV envelope with DC induces direct maturation of DC via NF-κB and p38 mitogen-activated protein kinase (MAPK) dependent pathways [34].
It has been shown recently that HSV glycoprotein D is also able to bind to mannose receptor (CD206), which is expressed by macrophages but also by subtypes of DC, and induce the production of IFNα/β by DC. This second mechanism of IFN-induction seems to represent an alternative way of TLR-independent IFN-α production by DC and might link innate and adaptive immune responses to HSV.
Considering the production of type II IFN, it has been shown in a mouse model that HSV infection reduces the production of type II IFN (i.e. IFN-γ) by pDC and thereby lowers T cell proliferation in a paracrine way [35].
Conclusions
HSV represents a flexible and clever pathogen, which has developed a high number of immune evasion strategies to defend itself effectively against the attacks of the host cells. HSV takes advantage of the crucial position of DC in antigen presentation and T cell stimulation processes. The knowledge about the sophisticated interplay of HSV with DC enables us to introduce effective prevention strategies such as the development of effective HSV vaccines or new methods using the wide variety of HSV functions as promising targets for immunization strategies.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG NO454/1–4 and DFG FOR 423 NO454/2–3) and BONFOR.
References
- 1.Wutzler P, Doerr HW, Farber I, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations − relevance for the incidence of genital herpes. J Med Virol. 2000;61:201–7. doi: 10.1002/(sici)1096-9071(200006)61:2<201::aid-jmv5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Vandenabeele S, Wu L. Dendritic cell origins: puzzles and paradoxes. Immunol Cell Biol. 1999;77:411–19. doi: 10.1046/j.1440-1711.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 3.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 4.Mikloska Z, Bosnjak L, Cunningham AL. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virol. 2001;75:5958–64. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jugovic P, Hill AM, Tomazin R, Ploegh H, Johnson DC. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J Virol. 1998;72:5076–84. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomazin R, Hill AB, Jugovic P, et al. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–66. [PMC free article] [PubMed] [Google Scholar]
- 7.York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;20:525–35. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 8.Pollara G, Speidel K, Samady L, et al. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis. 2003;187:165–78. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- 9.Kruse M, Rosorius O, Kratzer F, et al. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T cell stimulatory capacity. J Virol. 2000;74:7127–36. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurt-Jones EA, Chan M, Zhou S, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345–51. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hock BD, Kato M, McKenzie JL, Hart DN. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int Immunol. 2001;13:959–67. doi: 10.1093/intimm/13.7.959. [DOI] [PubMed] [Google Scholar]
- 13.Neumann J, Eis-Hubinger AM, Koch N. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol. 2003;171:3075–83. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- 14.Smith CM, Belz GT, Wilson NS, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–40. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- 15.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–8. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 16.Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–6. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medici MA, Sciortino MT, Perri D, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J Biol Chem. 2003;19:36059–67. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, Fernandez M, Herc K, et al. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virol. 2003;77:11139–49. doi: 10.1128/JVI.77.20.11139-11149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prechtel AT, Turza NM, Kobelt DJ, et al. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J Gen Virol. 2005;86:1645–57. doi: 10.1099/vir.0.80852-0. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HM, Wang L, Pangburn MK, Lambris JD, Lubinski J. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J Immunol. 2000;165:4528–36. doi: 10.4049/jimmunol.165.8.4528. [DOI] [PubMed] [Google Scholar]
- 21.Judson KA, Lubinski JM, Jiang M, et al. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J Virol. 2003;77:12639–45. doi: 10.1128/JVI.77.23.12639-12645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubinski JM, Jiang M, Hook L, et al. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol. 2002;76:9232–41. doi: 10.1128/JVI.76.18.9232-9241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules. roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94:655–67. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–53. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Sakisaka T, Taniguchi T, Nakanishi H, et al. Requirement of interaction of nectin-1alpha/HveC with afadin for efficient cell–cell spread of herpes simplex virus type 1. J Virol. 2001;75:4734–43. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrop JA, McDonnell PC, Brigham-Burke M, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 29.Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlender J, Hornung V, Finke S, et al. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–15. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol. 2004;173:1535–48. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- 32.Hochrein H, Schlatter B, O'Keeffe M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–21. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 34.Pollara G, Jones M, Handley ME, et al. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J Immunol. 2004;173:4108–19. doi: 10.4049/jimmunol.173.6.4108. [DOI] [PubMed] [Google Scholar]
- 35.Bjorck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J Immunol. 2004;172:5396–404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]