Abstract
The functional repertoire of T cells in abdominal aortic aneurysm (AAA) and the exact nature of aortic wall adaptive cellular immune responses still remains a matter of debate. In this study, we sought to determine whether type 1 or type 2 responses occur predominantly in human aneurysmal aortic lesions. We first examined the phenotype and cytokine secretion profile of T lymphocytes freshly isolated from aneurysmal aortic wall for comparison with their circulating counterparts using flow cytometry. We found that both populations of infiltrating CD4+ and CD8+T cells displayed a unique activated memory phenotype. In addition, we identified the presence in human aneurysmal aortic lesion of CD4+T cells producing high levels of interferon (IFN)-γ but not interleukin (IL)-4, reflecting their type 1 nature. Quantitative analysis of cytokine gene expression confirmed increased IFN-γ transcript levels in infiltrating cells compared to controls. We next analysed aortic wall responses using LightCycler-based quantitative real-time reverse transcription-polymerase chain reaction. Compared to control non-diseased aortic samples, we demonstrated that whole AAA tissues exhibited high mRNA levels of IFN-γ but not IL-4. Overexpression of the transcription factor T-bet in the absence of significant GATA-3 expression further assessed the type 1 polarization of aortic wall immune responses. These findings indicate that type 1 CD4+T cells predominate in human AAA lesions. This study has important implications for the pathogenesis of aneurysm disease. Through the production of IFN-γ, T cells may indeed contribute to orchestrate extracellular matrix remodelling.
Keywords: aortic aneurysm, helper T cells, interferon-γ, interleukins, transcription factors
Introduction
Lymphocyte infiltration of the vessel wall is a prominent feature of the chronic inflammatory response that characterizes human abdominal aortic aneurysm (AAA) lesion. Immunohistochemical studies of human AAA tissues have demonstrated the presence of a large number of T lymphocytes, including both CD4+ and CD8+ subsets in the outer media and adventitia [1–6]. A recent study investigating the phenotype of aneurysm-infiltrating cells revealed that T lymphocytes consist mainly of activated memory cells expressing a specific profile of adhesion and co-stimulatory molecules [7], suggesting the existence of a cellular immune response in the aortic wall. Interestingly, indeed, T cells may have significant implications in the pathophysiology of aneurysmal degeneration, as cytokines produced by T cell subsets, such as interferon-gamma (IFN-γ) and interleukin-4 (IL-4), have been shown to regulate macrophage expression of matrix metalloproteinases [8–14] (MMPs), thereby modulating proteolytic degradation of the wall connective tissue, and to induce apoptosis of medial smooth muscle cells [5,15]. Through these two pathways, infiltrating T cells could contribute to orchestrate extracellular matrix remodelling and promote weakening of the aortic wall, leading ultimately to aneurysm expansion. Both Th1-type and Th2-type immune responses have been identified in human studies and experimental animal models of AAA [16–23]. In the most recent report, Chan et al. [18] have described the presence of IFN-γ-producing type 1 lymphoid cells including CD4+ T helper cells, natural killer (NK) cells and NK T cells in human AAA samples.
Against this complex background, we first examined the phenotype and cytokine secretion profile of infiltrating T lymphocytes freshly isolated from aneurysmal aortic wall for comparison with their circulating counterparts using flow cytometry. Cytokine transcripts were quantified in peripheral and infiltrating cells using real-time reverse transcription-polymerase chain reaction (RT-PCR). We then investigated the nature of aortic wall immune responses. Cytokine gene expression was determined in whole aneurysmal and control non-diseased aortic tissues using quantitative real-time RT-PCR. The molecular basis of type 1 or type 2 dominant responses was further specified by analysing mRNA levels of transcription factors involved specifically in type 1 or type 2 differentiation such as T-bet and GATA-3.
Materials and methods
Aneurysmal and normal aortic tissue
Human infrarenal abdominal aortic aneurysm segments were obtained prospectively from patients undergoing elective open repair (n = 22). Demographic and clinical characteristics of the study population are summarized in Table 1. Patients with inflammatory or immune disorders, infectious diseases, cancer, corticosteroid therapy and/or immunosuppressive drugs were excluded. Median age was 73·5 years (95% CI 66·99–74·65; range, 54–82). Median maximal transverse diameter of the aneurysm measured by computed tomography scan imaging was 60 mm (95% CI 57·18–68·55; range, 40–90). Control non-diseased aortic samples were obtained from healthy organ donors [n = 4; median age, 30·5 (95% CI 13·29–49·71; range, 19–46) years; 75% males, 25% women]. The study protocol was approved by the local Medical Ethics Committee. All patients gave written informed consent. Immediately after tissue sampling, aortic specimens were divided into two portions: one section was directly processed for cell isolation, whereas the other was snap-frozen in liquid nitrogen and stored at −80°C until further RNA extraction.
Table 1. Characteristics of study population.
| Patient characteristics | Study population (n) |
|---|---|
| Number of patients | 22 |
| Male/female | 22/0 |
| Median (95% CI; range) age in years | 73·5 (66·99–74·65; 54–82) |
| Median (95% CI; range) AAA size in mm | 60 (57·18–68·55; 40–90) |
| Risk factors | |
| Family history | 3 |
| Smoking | 21 |
| Hypertension | 17 |
| Hyperlipaemia | 16 |
| Diabetes | 2 |
| Clinical presentation | |
| Asymptomatic | 15 |
| Abdominal pain | 3 |
| Rapid growth | 2 |
| Abdominal pain and rapid growth | 2 |
Cell isolation
Isolation of mononuclear cells from aneurysmal aortic wall
Fresh aneurysmal aortic tissue was minced into small pieces and digested for 1 h at 37°C with collagenase type I-S (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The resulting cell suspension was filtered through a 70 µm filter (Cell Strainer, Becton Dickinson Labware, Franklin Lakes, NJ, USA), and mononuclear cells were isolated by density gradient centrifugation on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway) according to the manufacturer's instructions. After washing with Hanks's balanced salt solution (HBSS) (Bio Whittaker, Verviers, Belgium), infiltrating mononuclear cells were resuspended in culture medium composed of RPMI-1640 (Bio Whittaker) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Bio Whittaker).
Isolation of mononuclear cells from peripheral blood
Circulating leucocytes were obtained before surgery from freshly collected heparinized peripheral blood samples. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway) according to the manufacturer's instructions, and were used as controls.
Flow cytometry
T cell immunophenotyping
The phenotype of peripheral and infiltrating lymphocytes was determined by flow cytometry immediately after the isolation procedure. Analysis of cell surface marker expression was performed by four-colour immunofluorescence using fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridin chlorophyll-a protein (PerCP)- and allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs). Surface antigens of lymphocytes were stained with mAbs against CD45, CD3, CD19, CD16, CD56, CD4, CD8, CD45RO, CD45RA, CD69, HLA-DR, CCR4, CCR5 and the corresponding isotype-matched irrelevant mAbs (Becton Dickinson, San Jose, CA, USA). Data were collected on 10 000 viable cells using a FACScan Flow Cytometer and CellQuest Software (Becton Dickinson, San Jose, CA, USA). Fluorescence channels were set at logarithmic gain. Lymphocytes were gated according to their forward versus side light-scatter properties. The T cell subpopulation was identified by gating on CD3 positive events. Results are expressed as percentage of positive cells.
Detection of intracytoplasmic cytokine expression
Flow cytometry was also used for the detection of intracytoplasmic cytokine expression in freshly isolated peripheral and infiltrating lymphocyte subsets. To this end, mononuclear cells were incubated at 106/ml with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma Chemical Co., St Louis, MO, USA) and 0·1 µg/ml calcium ionophore A23187 (Calbiochem-Behring, San Diego, CA, USA) for 6 h in culture medium (37°C, 5% CO2). Surface antigens were stained with FITC-conjugated anti-CD3 and PerCP-conjugated anti-CD8 mAbs. After fixation and permeabilization of the cells, intracellular cytokines were stained with PE-conjugated mAbs against IFN-γ, IL-2, IL-4, IL-13 (Becton Dickinson, San Jose, CA, USA) and IL-5 (Pharmingen, San Diego, CA, USA). Negative controls for cytokine expression were provided by unstimulated cells treated with Brefeldine A at 10 µg/ml (Sigma Chemical Co.) and by intracellular staining of stimulated cells with isotype-matched irrelevant PE-conjugated mAbs. Data were collected on 10 000 viable cells using a FACScan Flow Cytometer and CellQuest Software (Becton Dickinson). Fluorescence channels were set at logarithmic gain. Lymphocytes were gated according to their forward versus side light-scatter properties. The T cell subpopulation was identified by gating on CD3 positive events. Results are expressed as percentage of positive cells.
mRNA quantification using real-time RT-PCR
Cytokine mRNA levels in cells purified from peripheral blood and aneurysmal aortic wall
Quantitative real-time RT-PCR was used to determine cytokine mRNA levels in mononuclear cells purified from peripheral blood and aneurysmal aortic wall. Briefly, freshly isolated mononuclear cells were submitted to lysis in 300 µl lysis buffer from the MagNa Pure LC mRNA Isolation kit I (Roche Diagnostics GmbH, Mannheim, Germany) and stored at −20°C until analysis. RNA extraction, reverse transcription and real-time PCR were performed using MagNa Pure and LightCycler Instruments (Roche Diagnostics), as described previously [24,25]. Results are expressed as mRNA copy numbers per 107 copies of CD3-∈ mRNA. For CD3-∈ mRNA, 1 µl per reaction of a commercialized TaqMan pre-developed assay reagent (Applied Biosystems, Foster City, CA, USA) containing primers and probe for human CD3-∈ chain was used.
Cytokine and transcription factor mRNA levels in whole aortic tissue
Quantitative real-time RT-PCR was used to determine cytokine and transcription factor mRNA levels in aneurysmal and non-diseased aortic samples. Briefly, a portion of aortic tissue was snap-frozen, crushed under liquid nitrogen and dissolved in 300 µl lysis buffer from the MagNa Pure LC mRNA isolation kit II (tissue) (Roche Diagnostics GmbH, Mannheim, Germany) for mRNA extraction on MagNA Pure Instrument (Roche Diagnostics). Reverse transcription and real-time PCR were performed as described for purified cells. For transcription factors, PCR conditions and oligonucleotide sequences are listed in Table 2. Results are expressed as mRNA copy numbers per 107 copies of the housekeeping gene β-actin mRNA.
Table 2. Oligonucleotides for (real-time) polymerase chain reaction*.
| mRNA target | Oligonucleotides (5′→3′)† | Product size (bp) | Final concentration (nM)‡ |
|---|---|---|---|
| Primers and probes for real-time polymerase chain reaction | |||
| T-bet | F965: AACAATGTGACCCAGATGATTG | 145 | F 900 |
| R1109: TGAACTGGGTTTCTTGGAAAGT | R 600 | ||
| P1030: 6Fam-TGAGGTGAACGACGGAGAGCCA-Tamra-p | |||
| GATA-3 | F1049: CTCATTAAGCCCAAGCGAAG | 96 | F 900 |
| R1144: ATTCCTCCTCCAGAGTGTGGT | R 900 | ||
| P1077: 6Fam-CAGCCAGGAGAGCAGGGACGTC-Tamra-p | |||
| Primers for standard preparation by ‘classical’ polymerase chain reaction § | |||
| T-bet | F706: GATGTTCCCATTCCTGTCATTT | 594 | |
| R1299: TACTGGTTGGGTAGGAGAGGAG | |||
| GATA-3 | F931: CAGGGAGTGTGTGAACTGTGG | 440 | |
| R1370: AGATGTGGCTCAGGGAGGAC | |||
For a full description, see [24].
F, R and P indicate forward, reverse primers, and probes, respectively; numbers indicate the sequence position from GenBank accession numbers NM_013351 for T-bet and X58072 for GATA-3.
Final concentrations of forward (F) and reverse (R) primers.
Standard curves were generated from serial dilutions of polymerase chain reaction (PCR) products prepared by ‘classical’ PCR, for which specific conditions were as follows: denaturation at 95°C for 20 s, annealing at 60°C (T-bet) or 62°C (GATA-3) for 20 s and elongation at 72°C for 45 s, for a total of 35 cycles. MgCl2 final concentration was 1·5 mM.
Statistical analysis
Descriptive statistics were applied for all variables collected. Continuous variables were described as median with the corresponding lower and upper 95% confidence intervals (CIs). Differences between the groups were tested for significance by means of the non-parametric Mann–Whitney U-test. These analyses were performed using GraphPad InStatTM statistical software (GraphPad Software, Inc., San Diego, CA, USA). A P-value < 0·05 was considered to indicate statistical significance.
Results
CD4+ and CD8+ T lymphocytes in abdominal aortic aneurysm display an activated memory phenotype
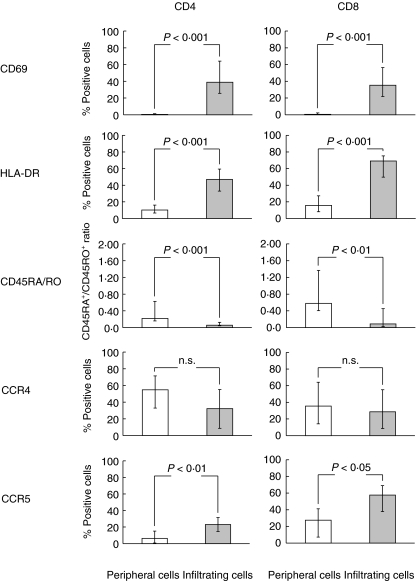
Flow cytometric immunophenotyping of mononuclear cells freshly isolated from aneurysmal aortic wall indicated that the vast majority of these cells are T lymphocytes. Indeed, CD3+ cells represented 79·74% (70·12–82·88) of infiltrating mononuclear cells (n = 13). Distribution of CD4+, CD8+ and CD56+ T cell subsets in aneurysmal lesion was comparable to that of control peripheral cells, with a predominance of CD4+ T cells (Table 3). We next examined the surface phenotype of T cells purified from aneurysmal aortic wall as compared to their circulating counterparts. As illustrated in Fig. 1, both infiltrating CD4+ and CD8+ T cells displayed an activated phenotype, as assessed by an increased expression of CD69 and HLA-DR activation antigens (n = 7), and predominantly expressed the CD45RO isoform characteristics of memory cells (n = 13). Median percentages of infiltrating and peripheral CD45RO+ cells represented 85·87% (84·28–90·51) versus 71·77% (59·19–74·94) of CD4+ T cells (P < 0·0001) and 81·55% (66·67–86·72) versus 50·82% (39·25–62·97) of CD8+ T cells (P < 0·001). Up-regulation of CCR5 expression was found on both infiltrating CD4+ and CD8+ T cells (n = 6). A tendency towards CCR4 down-regulation was noticed on infiltrating CD4+ T cells, but the data failed to reach statistical significance (n = 5).
Table 3. T cell subsets in abdominal aortic aneurysm.
| Peripheral cells (% positive cells) | Infiltrating cells (% positive cells) | |
|---|---|---|
| CD4+ cells | 66·55 | 58·28 |
| (56·86–72·61) | (51·07–62·45) | |
| CD8+ cells | 26·13 | 32·34 |
| (22·31–37·40) | (27·96–37·56) | |
| CD56+ NKT cells | 4·24 | 5·16 |
| (3·02–9·85) | (3·37–7·41) |
Mononuclear cells were purified from peripheral blood and aneurysmal aortic wall obtained from 13 patients undergoing open abdominal aortic aneurysm repair. Immediately after the isolation procedure, cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridin chlorophyll-a protein (PerCP)- and allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) against CD3, CD4, CD8, and CD56 antigens, and the corresponding isotype-matched irrelevant mAbs. Flow cytometric analysis of surface phenotype is shown after gating on CD3+ cells. Results are expressed as median percentage of positive cells; values within brackets represent the corresponding lower and upper 95% confidence intervals.
Fig. 1.
Phenotype of T cells in abdominal aortic aneurysm. Mononuclear cells were purified from peripheral blood and aneurysmal aortic wall obtained from 13 patients undergoing open abdominal aortic aneurysm repair. Immediately after the isolation procedure, cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridin chlorophyll-a protein (PerCP)- and allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) against CD45, CD3, CD4, CD8, CD69, HLA-DR, CD45RA, CD45RO, CCR4 and CCR5 antigens, and the corresponding isotype-matched irrelevant mAbs. Flow cytometric analysis of surface phenotype is shown after gating on CD3+CD4+ or CD3+CD8+ lymphocytes. Results are expressed as median (95% CIs) percentage of positive cells.
Increased production of IFN-γ by CD4+ T cells in abdominal aortic aneurysm
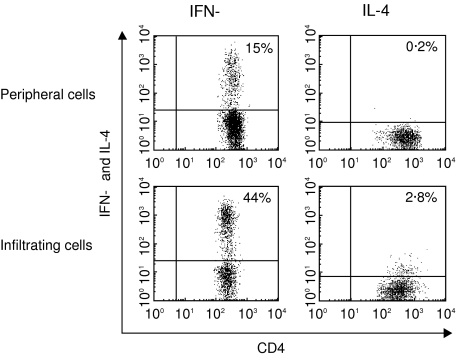
The cytokine secretion profile of freshly isolated infiltrating CD3+ T lymphocytes was first determined using flow cytometry after intracellular staining. In the absence of stimulation, no cytokines were detected. After 6 h of incubation with PMA and calcium ionophore, a clearly distinct cytokine profile of infiltrating CD3+ T lymphocytes was interestingly observed compared to control peripheral cells. Indeed, a significant proportion of infiltrating CD3+ T cells expressed IFN-γ, whereas IL-4, IL-5 and IL-13 were virtually absent (Table 4). This observation prompted us to examine the phenotype of IFN-γ-producing cells. As shown in Table 5 and Fig. 2, infiltrating CD4+ T cells exhibited a type 1 cytokine secretion profile, as indicated by their synthesis of high levels of IFN-γ and very low production of IL-4. Furthermore, a population of IFN-γ-producing CD8+ T cells was identified in aneurysmal aortic lesion, but data failed to reach statistical significance. The level of IFN-γ expression as assessed by median channel fluorescence intensity was similar in infiltrating CD4+ and CD8+ T cells (794·12 (335·77–1650·70) versus 718·80 (200·73–1120·10) (n.s.). Quantitative analysis of cytokine gene expression in cells purified from aneurysmal aortic wall and peripheral blood using LightCycler-based real-time RT-PCR confirmed high expression of IFN-γ in infiltrating cells compared to controls (342·842 (127·664–938·681) versus 30 184 (0·10–217 806) median mRNA copy number per 107 copies CD3-∈, P < 0·05).
Table 4. Increased production of interferon (IFN)-γ by infiltrating CD3+ T cells in abdominal aortic aneurysm.
| IFN-γ (% positive cells) | IL-2 (% positive cells) | IL-4 (% positive cells) | IL-5 (% positive cells) | IL-13 (% positive cells) | |
|---|---|---|---|---|---|
| Peripheral cells | 19·00 | 29·25 | 1·38 | 0·43 | 0·62 |
| (5·27–28·81) | (7·98–57·73) | (0·12–3·22) | (0·04–1·00) | (0·05–2·17) | |
| Infiltrating cells | 52·18* | 42·00 | 1·91 | 0·00 | 0·58 |
| (28·78–65·11) | (27·32–65·04) | (0·35–2·99) | (0·65–1·75) | (0·02–1·49) |
Mononuclear cells were purified from peripheral blood and aneurysmal aortic wall obtained from five patients undergoing open abdominal aortic aneurysm repair. After a 6-h culture with phorbol 12-myristate 13-acetate (PMA) and calcium ionophore, cell membranes were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and peridin chlorophyll-a protein (PerCP)-conjugated anti-CD8 monoclonal antibodies (mAbs). After fixation and permeabilization of the cells, intracytoplasmic cytokines were stained with phycoerythrin (PE)-conjugated mAbs against interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-5 and IL-13. Intracytoplasmic expression of cytokines was analysed by flow cytometry after gating on CD3+ lymphocytes. Results are expressed as median percentage of positive cells; values within brackets represent the corresponding lower and upper 95% confidence intervals.
P < 0·05.
Table 5. Cytokine profile of infiltrating T cell subsets in abdominal aortic aneurysm.
| CD4+ T lymphocytes | CD8+ T lymphocytes | |||
|---|---|---|---|---|
| IFN-γ (% positive cells) | IL-4 (% positive cells) | IFN-γ (% positive cells) | IL-4 (% positive cells) | |
| Peripheral cells | 15·42 | 1·38 | 25·93 | 2·26 |
| (4·02–23·01) | (0·12–2·84) | (5·05–59·29) | (0·81–3·18) | |
| Infiltrating cells | 43·85* | 1·56 | 67·78 | 2·44 |
| (26·05–58·21) | (0·15–2·99) | (32·89–90·98) | (0·29–3·51) | |
Mononuclear cells were purified from peripheral blood and aneurysmal aortic wall obtained from five patients undergoing open abdominal aortic aneurysm repair. After a 6-h culture with phorbol 12-myristate 13-acetate (PMA) and calcium ionophore, cell membranes were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and peridin chlorophyll-a protein (PerCP)-conjugated anti-CD8 monoclonal antibodies (mAbs). After fixation and permeabilization of the cells, intracytoplasmic cytokines were stained with phycoerythrin (PE)-conjugated mAbs against interferon (IFN)-γ and interleukin (IL)-4. Intracytoplasmic expression of cytokines was analysed by flow cytometry after gating on CD3+CD8− or CD3+CD8+ lymphocytes. Results are expressed as median percentage of positive cells; values within brackets represent the corresponding lower and upper 95% confidence intervals.
P < 0·01.
Fig. 2.
Detection of type 1 CD4+ T cells in abdominal aortic aneurysm. Representative dot plots showing interferon (IFN)-γ and interleukin (IL)-4 production by CD4+ T lymphocytes. Mononuclear cells were purified from peripheral blood and aneurysmal aortic wall obtained from five patients undergoing open abdominal aortic aneurysm repair. After a 6-h culture with phorbol 12-myristate 13-acetate (PMA) and calcium ionophore, cell membranes were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and peridin chlorophyll-a protein (PerCP)-conjugated anti-CD8 monoclonal antibodies (mAbs). After fixation and permeabilization of the cells, intracytoplasmic cytokines were stained with phycoerythrin (PE)-conjugated mAbs against interferon (IFN)-γ and interleukin (IL)-4. Intracytoplasmic expression of cytokines was analysed by flow cytometry after gating on CD3+CD8− lymphocytes. Data are from one of five experiments, which gave similar results.
Increased expression of IFN-γ and T-bet in aneurysmal aortic lesion
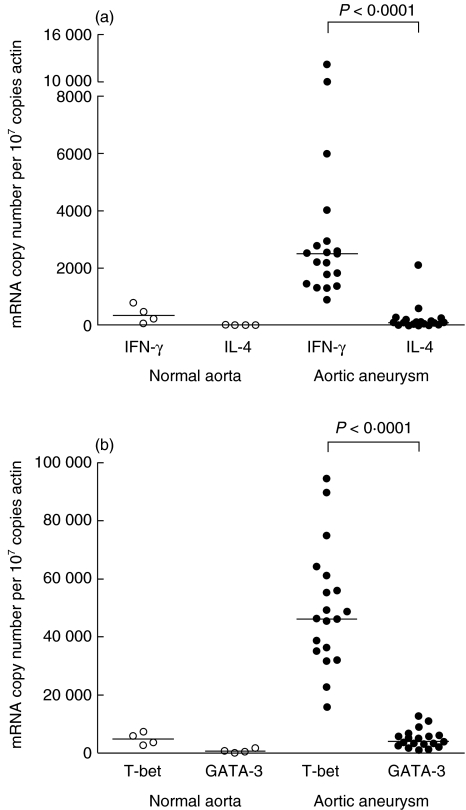
We next investigated the nature of aortic wall immune responses. To this end, IFN-γ and IL-4 mRNA levels were determined in whole aneurysmal and control non-diseased aortic samples using quantitative LightCycler-based real-time RT-PCR. Consistent with our observations on infiltrating T cell populations, high IFN-γ but not IL-4 transcripts were detected in aneurysmal aortic wall compared to controls (Fig. 3a). In a final series of experiments, we quantified in aortic tissues the expression of T-bet and GATA-3, transcription factors expressed preferentially in type 1 or type 2 cells, respectively. As shown in Fig. 3b, T-bet and GATA-3 were expressed at very low levels in control non-diseased aortic samples. By contrast, a clear overexpression of T-bet was measured in AAA tissues, whereas GATA-3 remained at background levels.
Fig. 3.
Interferon (IFN)-γ and T-bet predominate in aneurysmal aortic lesion. Quantitative analysis of cytokine (a) and transcription factor (b) transcripts was performed in aneurysmal (n = 19) and non-diseased (n = 4) aortic samples using LightCycler-based real-time reverse transcription-polymerase chain reaction (RT-PCR). Results are expressed as median mRNA copy number per 107 copies of the housekeeping gene β-actin mRNA. mRNA extracted from a Th2 clone of a patient with the hypereosinophilic syndrome was used as positive control for GATA-3 expression (433 491 GATA-3 mRNA copies per 107 copies of β-actin).
Discussion
Human AAA lesion is characterized typically by the presence in the media and adventitia of a great number of T cells including both CD4+ and CD8+ subsets [1–7]. Conflicting data are, however, available regarding the cytokine profile of infiltrating T cells, the exact nature of aortic wall adaptive cellular immune responses and the aetiological role of T lymphocytes and their cytokines in the initiation and progression of aneurysmal degeneration. On one hand, increased production of type 1-specific cytokine IFN-γ has been observed in culture supernatants of human AAA explants [16]. Elevated levels of IFN-γ have also been documented in the blood of patients with AAAs, IFN-γ concentrations showing positive correlation with aneurysm expansion [17]. Immunostaining and Western blot analysis of AAA tissues has further disclosed IFN-γ expression [19,20]. Based on these observations, a preference toward a dominant type 1 cytokine expression pattern in human aneurysmal aortic wall could be hypothesized. In a recent report, Chan et al. [18] have described the presence of IFN-γ-producing type 1 lymphoid cells including CD4+ T helper cells, NK cells, and NK T cells in AAA samples, and have further established that these infiltrating Th1 and NK T1 cells may regulate vascular smooth muscle cell proliferation and apoptosis, respectively. Moreover, recent demonstration that CD4+ T cells and IFN-γ play a pivotal role in the development of experimental AAA in a calcium chloride-induced mouse model [21] has further lent strong support to the notion that T cells and type 1-mediated responses are critically involved in the pathogenesis of aneurysm disease.
In contrast, there are several lines of evidence suggesting that type 2-mediated immune responses are key determinants of aneurysmal degeneration. Indeed, type 2-specific cytokines, particularly IL-4 and IL-10, have been detected in aneurysmal aortic tissues [19,20]. Schönbeck et al. have notably found that human AAA lesion predominantly displayed type 2-associated cytokines such as IL-4, IL-5 and IL-10, and correspondingly lack mediators associated with type 1 immune responses such as IL-2, IL-12, IL-18 and IL-15 compared to non-diseased and atherosclerotic occlusive tissues [20]. These findings led the authors to assume that dominance of type 2 responses within the aortic wall might contribute to direct atherosclerotic lesions towards aneurysm development rather than formation of occlusive atheroma. Using an aortic transplantation mice model, the same research group has subsequently provided the first clear evidence that polarization of T cell responses toward a type 2 phenotype can specifically mediate an inflammatory process leading to aneurysmal degeneration [22]. In this study, Shimizu et al. have indeed observed that interruption of IFN-γ signalling pathway with consecutive IL-4-driven inflammation in aortic transplants induced AAA formation associated with increased expression of MMP-9 and MMP-12, and that suppression of experimental AAA can be obtained by selective blockade of IL-4 using either anti-IL-4 blocking antibodies or concomitant genetic deficiency in IL-4. Besides, experiments from King et al. have indicated that IFN-γ deficiency enhanced the incidence and the severity of angiotensin II-induced AAA formation in apoE−/− mice [23].
In the current study we have demonstrated the predominance of type 1 CD4+ T cells in human AAA lesion. In an initial series of experiments, we found that populations of CD4+ and CD8+ T cells freshly purified from human aneurysmal aortic wall present a unique activated memory phenotype and exhibit a pattern of chemokine receptor expression compatible with a type 1-mediated response [26–28]. We next focused on the cytokine repertoire of infiltrating T cells using flow cytometry. Interestingly, we observed ex vivo that infiltrating CD3+ T cells display a type 1 cytokine profile [29,30], as indicated clearly by their dominant expression of IFN-γ. In addition, we identified the presence of IFN-γ-producing CD4+ T cells in aneurysmal aortic lesion. Along with the data published recently by Chan et al. [18], our findings closely corroborate the concept that Th1 cell infiltrates predominate in human end-stage AAA lesion. Their potential involvement in the pathogenesis of the disease is suggested by the recent convincing demonstration that absence of CD4+ T cells or targeted deletion of IFN-γ prevents the induction of experimental AAA in a calcium chloride-induced mouse model [21], AAA formation being largely reconstituted by administration of IFN-γ into CD4−/− mice or infusion of competent splenocytes from wild-type mice into IFN-γ−/− mice. In this study, Xiong et al. have also established both in vivo and in vitro that IFN-γ contributed to extracellular matrix metabolism and AAA formation in part through its ability to regulate macrophage production of MMP-2 and MMP-9. Besides, infiltrating CD4+ and CD8+ T cells are likely to trigger apoptosis of medial smooth muscle cells through the expression of death-promoting proteins and cytotoxic mediators such as perforin and Fas Ligand [5]. In a final series of experiments, we showed that whole AAA tissue contained high transcript levels of IFN-γ but not IL-4, further supporting the type 1 nature of aneurysmal aortic wall immune responses, and consistently overexpressed T-bet, a transcription factor specifically involved in type 1 T cell development and regulation [31–37], revealing a molecular basis of type 1 aortic wall responses. Such selective expression of T-bet in human AAA lesion strongly suggests a potential role for this transcription factor in promoting type 1-driven aortic wall inflammation. Because analyses of mRNA transcripts were performed on whole aortic wall samples, it is worth noting that several other cell types than conventional T cells might express T-bet, including NK and NK T cells which have been shown recently to contribute to IFN-γ production in AAA [18].
In summary, we found that most infiltrating T cells in human aneurysmal aortic lesion consist of CD4+ and CD8+ subsets expressing a unique activated memory phenotype. In addition, we identified the presence in the human aneurysmal aortic wall of IFN-γ-producing CD4+ T cells and demonstrated consistently increased expression of IFN-γ and T-bet. Taken together, these cellular and molecular findings indicate that type 1 CD4+ T cells predominate in human end-stage AAA lesion. Even though future efforts can be envisioned to elucidate the role of type 1 CD4+ T cells in the initiation and progression of aneurysmal degeneration, our observations are relevant for helping to clarify the pathobiology of human abdominal aortic aneurysm lesion and defining prospects for the prevention of aneurysm expansion. Indeed, uncovering the cytokine profile of infiltrating T cells and understanding the exact nature of aortic wall immune responses not only provide new insights into the pathogenesis of the disease, but could also serve as a basis for the development of novel management strategies directed at preventing aneurysm expansion, including therapeutic approaches based on the modulation of aortic wall immune responses, and designed to selectively target T cell activation and cytokine production.
Acknowledgments
This study was supported in part by grants from the Fondation Erasme (Brussels, Belgium) and the Fonds National de la Recherche Scientifique (Brussels, Belgium). Cécile Galle is a research fellow of the Fondation Erasme (Brussels, Belgium).
References
- 1.Koch AE, Haines GK, Rizzo RJ, et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–213. [PMC free article] [PubMed] [Google Scholar]
- 2.Stella A, Gargiulo M, Pasquinelli G, et al. The cellular component in the parietal infiltrate of inflammatory abdominal aortic aneurysms. Eur J Vasc Surg. 1991;5:65–70. doi: 10.1016/s0950-821x(05)80929-6. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman J, Scheib JS, Googe PB, Ichiki AT, Goldman MH. Inflammatory abdominal aortic aneurysm and the associated T cell reaction: a case study. J Vasc Surg. 1992;15:569–72. doi: 10.1067/mva.1992.32415. [DOI] [PubMed] [Google Scholar]
- 4.Satta J, Laurila A, Pääkkö P, et al. Chronic inflammation and elastin degradation in abdominal aortic aneurysm disease: an immunohistochemical and electron microscopic study. Eur J Vasc Endovasc Surg. 1998;15:313–19. doi: 10.1016/s1078-5884(98)80034-8. [DOI] [PubMed] [Google Scholar]
- 5.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 6.Bobryshev YV, Lord RSA. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154:15–21. doi: 10.1016/s0021-9150(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 7.Ocana E, Bohórquez JC, Pérez-Requena J, Brieva JA, Rodríguez C. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 8.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–57. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Zheng T, Zhu Z, et al. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin KY, Moon HS, Park HY, et al. Effects of tumor necrosis factor-α and interferon-γ on expressions of matrix metalloproteinase-2 and -9 in human bladder cancer cells. Cancer Lett. 2000;159:127–34. doi: 10.1016/s0304-3835(00)00522-x. [DOI] [PubMed] [Google Scholar]
- 11.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–30. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro SD, Campbell EJ, Kobayashi DK, Welgus HG. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990;86:1204–10. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chizzolini C, Rezzonico R, De Luca C, Burger D, Dayer JM. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte-macrophage colony-stimulating factor-differentiated human monocytes. J Immunol. 2000;164:5952–60. doi: 10.4049/jimmunol.164.11.5952. [DOI] [PubMed] [Google Scholar]
- 14.Sasaguri T, Arima N, Tanimoto A, Shimajiri S, Hamada T, Sasaguri Y. A role for interleukin-4 in production of matrix metalloproteinase 1 by human aortic smooth muscle cells. Atherosclerosis. 1998;138:247–53. doi: 10.1016/s0021-9150(97)00296-7. [DOI] [PubMed] [Google Scholar]
- 15.Geng YJ, Wu Q, Muszynski M, Hansson G, Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, tumor necrosis factor-α, and interleukin-1-β. Arterioscler Thromb Vasc Biol. 1996;16:19–27. doi: 10.1161/01.atv.16.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-γ but not IL-2 and IL-4: the possible role for IL-6 and interferon-γ in vascular inflammation. Agents Actions. 1994;42:159–62. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 17.Juvonen J, Surcel HM, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thomb Vasc Biol. 1997;17:2843–7. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 18.Chan WL, Pejnovic N, Hamilton H, et al. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and Th cells. Circ Res. 2005;96:675–83. doi: 10.1161/01.RES.0000160543.84254.f1. [DOI] [PubMed] [Google Scholar]
- 19.Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–6. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 20.Schönbeck U, Sukhova GK, Gerdes N, Libby P. Th2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-γ in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–12. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-γ signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–8. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King VL, Cassis LS, Daugherty A. Interferon-γ has opposing effects on angiotensin II-induced atherosclerosis and abdominal aortic aneurysm formation in apolipoprotein-E-deficient mice. Arterioscler Thomb Vasc Biol. 2003;23:a–78. [Abstract P448]. [Google Scholar]
- 24.Stordeur P, Poulin LF, Craciun L, et al. Cytokine mRNA quantification by real-time PCR. J Immunol Methods. 2002;259:55–64. doi: 10.1016/s0022-1759(01)00489-6. [Erratum in J Immunol Methods 2002; 262:229]. [DOI] [PubMed] [Google Scholar]
- 25.Stordeur P, Zhou L, Byl B, et al. Immune monitoring in whole blood using real time PCR. J Immunol Methods. 2003;276:69–77. doi: 10.1016/s0022-1759(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loetscher P, Uguccioni M, Bordoli L, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 31.Glimcher LH, Murphy KM. Lineage commitment in the immune system. The T helper lymphocyte grows up. Genes Dev. 2000;14:1261–70. [PubMed] [Google Scholar]
- 32.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 33.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–83. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 34.Murphy K, Reiner S. The lineage decisions of helper T cells. Nature Rev Immunol. 2002;2:33–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 35.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–41. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 36.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–9. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 37.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]