Abstract
Atherosclerosis is associated with activation of the immune system. Intravenously applied normal polyclonal immunoglobulins (IVIg) have broad therapeutic applications in the treatment of autoimmune and systemic inflammatory diseases. Recently, IVIg have been shown to inhibit atherogenesis in experimental animal models. To investigate the role of the complement system in this process, we used third complement component-deficient (C3−/−) and control atherosclerosis-prone apolipoprotein E (ApoE) and low-density lipoprotein receptor (LDLR) double knock-out mice fed a normal diet. IVIg treatment reduced lesion fraction area in the aortic root of complement-sufficient mice whereas the lesion fraction area of C3−/− mice was not affected. Thus, complement activation plays a role in the anti-atherosclerotic effects of IVIg, possibly by C3-derived fragments generated through Fc-dependent complement activation.
Keywords: atherosclerosis, complement, C3, intravenous immunoglobulins, mice
Introduction
Atherosclerosis is a multi-factorial process in which subendothelial lipids accumulate in large arteries. The pathogenesis involves disturbed lipoprotein metabolism and immune activation. Macrophages and T lymphocytes infiltrate the artery wall, and autoantibodies to oxidized low-density lipoprotein (LDL) [1] and activated complement components [2–5] are also found in atherosclerotic lesions.
Intravenously applied normal polyclonal immunoglobulins (IVIg) have broad therapeutic applications in the treatment of infectious, autoimmune and systemic inflammatory diseases [6–8]. Immunomodulation by IVIg administration has been shown to effectively inhibit the progression of atherosclerosis both during fatty streak and plaque phases, possibly by the modulation of T cell activation and/or antibody production [9]. This work has been extended further by recent findings of Yuan and coworkers that the atheroprotective effect of IVIg is mediated by the Fc-portion of Ig [10].
To investigate the role of the complement system in this process, we used third complement component-deficient (C3−/−) and complement-sufficient control atherosclerosis-prone apolipoprotein E (ApoE) and low-density lipoprotein receptor (LDLR) double knock-out mice fed a normal diet. The IVIg treatment led to a reduction of lesion fraction area in the aortic root of the complement-sufficient mice, whereas it was not affected in C3−/− mice. Thus the complement system plays a role in the anti-atherogenic effects of IVIg.
Materials and methods
Mice
C3−/− [11] mice were crossed with ApoE−/− LDLR−/− [12] mice. Male C3−/−ApoE−/−LDLR−/− and C3+/+ApoE−/−LDLR−/− siblings from the mating of C3+/−ApoE−/−LDLR−/− parents were used for paired comparison to minimize the effect of other genes on the mixed genetic background (C57BL/6 J/129Ola). The mice were fed standard mouse chow. The study was approved by the ethics committee at Göteborg University.
Biological immunomodulator IVIg
When reconstituted for therapeutic use, Sandoglobulin® (Swiss Red Cross, Bern, Switzerland) contains 50 mg/ml of IgG, 25–35 mg/ml sucrose, 6–10 mg/ml glucose and 40–100 m M NaCl (osmolality 515 mosmol). For this study, a stock solution of 100 mg/ml (0·6 mM) of IVIg was prepared as described elsewhere [9].
IVIg treatment
Male C3+/+ (n = 5) and C3−/− (n = 5) ApoE−/−LDLR−/− mice received intraperitoneal injections of 10 mg IVIg daily over a 5-day period at the ages of 6 and 11 weeks and were killed at 16 weeks of age. This experimental paradigm was shown previously to reduce the atherosclerotic lesion size in ApoE−/− mice on a cholesterol-rich diet [9]. Age-matched, uninjected C3−/−ApoE−/−LDLR−/− and complement-sufficient ApoE−/−LDLR−/− male mice were used as control groups, as it has been shown that mice injected with human serum albumin (HSA) instead of IVIg have lesions fully comparable to uninjected mice [9].
Processing and analysis of the aorta
The vasculature was perfused with sterile phosphate-buffered saline (PBS). The heart and 2 mm of the ascending aorta were snap-frozen in optimal cutting temperature (OCT) embedding medium and cryosections cut from the aortic root. Four 10-µm sections were collected at 100-µm intervals starting 100 µm from the origin of the aortic valve cusps. Formaldehyde-fixed sections were stained with Oil Red O (Sigma-Aldrich, St Louis, MO, USA) and haematoxylin, and lesion size was analysed as described [9].
Histology and immunohistochemistry
Acetone-fixed aortic root sections were incubated with 1% (w/v) bovine serum albumin in PBS containing 0·05% Tween-20, and stained with rat anti-mouse monocyte and macrophage marker MOMA-2 (Serotec, Oxford, UK) or rat anti-mouse Fcγ receptor (CD32, FcγR) (BD Pharmingen, San Diego, CA, USA) antibodies. The antibodies were detected using biotin-conjugated rabbit anti-serum against rat immunoglobulins (Dako A/S, Glostrup, Denmark) followed by ABC Vectastain Elite kit (Vector Laboratories, Burlingame, CA, USA) and visualized by 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). For each lesion, cells positive for FcγR were recorded per total number of cells. Staining with MOMA-2 was registered as stained surface area (MOMA-2-stained surface/total lesion surface) rather than number of positive cells, since borders between individual cells could not be identified.
Statistical analysis
Data were analysed by using SPSS 10·0 and Statview 5·0 software. A paired Wilcoxon signed-rank test was used to assess the effect of genotype. An unpaired Mann–Whitney U-test was used to assess the effect of IVIg treatment. The results are presented as individual values and median. Differences were considered statistically significant at P < 0·05.
Results
IVIg treatment leads to reduced atherosclerosis in complement-sufficient but not in C3−/− mice
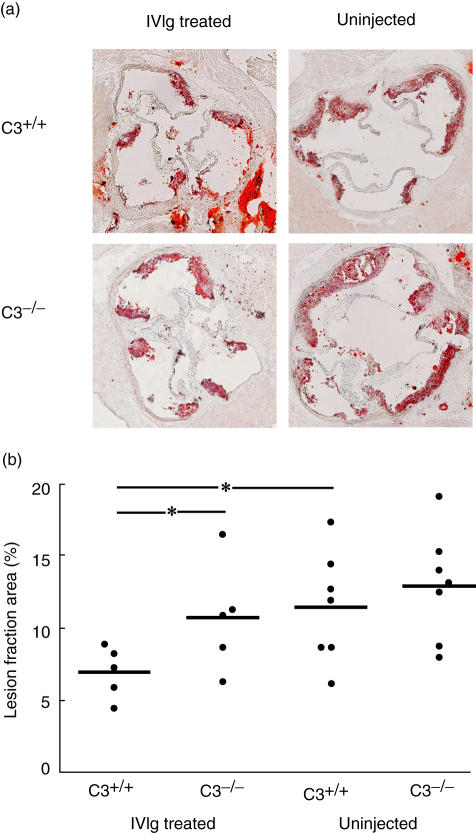
In serial sections of the aortic root, the Oil Red O-stained lesions of untreated C3−/−, C3+/+ as well as IVIg-treated C3−/− mice covered 11–13% of the aortic root. C3+/+ mice injected with IVIg showed a nearly 50% reduction of lesion formation (P < 0·05) with 7% of the cross-section area covered by lesions (Fig. 1).
Fig. 1.
Normal polyclonal immunoglobulins (IVIg) treatment reduces atherosclerotic lesion size in the aortic root. Third component-deficient (C3−/−)apolipoprotein E (ApoE)−/−low density lipoprotein receptor (LDLR)−/− and C3+/+ApoE−/−LDLR−/− mice were injected with IVIg. Uninjected age-matched mice were used as control. (a) Oil Red O staining of cross-sections of the aortic root. (b) Oil Red O-stained lesion fraction area was measured on cryosections of the aortic root. Horizontal bars represent sample median. *P < 0·05.
Increased inflammatory cell infiltration in all IVIg-treated mice
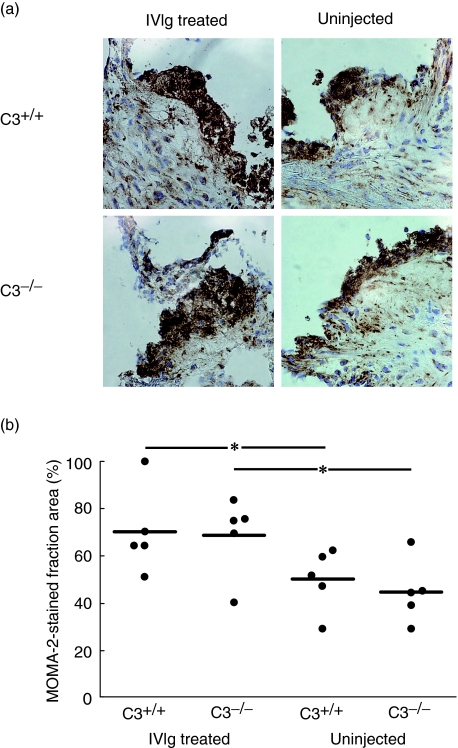
The proportion of lesion area stained by the monocyte and macrophage marker MOMA-2 was increased by 40% in IVIg-treated control mice compared to the uninjected control mice (P < 0·05). The same effect of the IVIg treatment was observed in the C3−/− mice (P < 0·05). The comparison of the injected C3−/−versus injected control mice showed no difference between the two groups (P = 0·8927), thus giving further support to the conclusion that the IVIg modulation of macrophage/monocyte infiltration of the lesion is not mediated by complement activation (Fig. 2).
Fig. 2.
Normal polyclonal immunoglobulins (IVIg) treatment increases macrophage/monocyte accumulation in the atherosclerotic lesions in the aortic root. (a) MOMA-2 staining of cross-sections of the aortic root from IVIg-treated and untreated third component-deficient (C3−/−) apolipoprotein E (ApoE)−/−low density lipoprotein receptor (LDLR)−/− and C3+/+ApoE−/−LDLR−/− mice. (b) MOMA-2 positive fraction area was measured on lesions on cryosections of the aortic root. Horizontal bars represent sample median. *P < 0·05.
Fcγ receptor (CD32) expression is not affected by C3 deficiency or IVIg treatment
The Fc receptors for IgG (Fcγ R) play a critical role in immunity by linking the IgG antibody-mediated responses with cellular effector and regulatory functions of the immune system [13]. Fcγ R (CD32) was expressed in lesions of both C3−/− and control mice. The distribution pattern did not differ between lesions of C3−/− and control mice, and was not affected by IVIg treatment (data not shown).
Discussion
It has been shown previously that administration of IVIg inhibits atherosclerosis in ApoE-deficient mice [9,10]. The immunomodulatory functions of the IVIg preparations seem to be responsible for this effect, as the IVIg injections were associated with anergization of T cells and reduction of IgM against oxidized LDL, whereas serum lipid levels were not affected [9,10]. We have reported previously that C3 deficiency leads to the formation of larger atherosclerotic lesions as assessed in en face preparations of the aorta, whereas the lesion size in cross-sections of the aortic root was not different from control ApoE−/−LDLR−/− mice [14]. Similarly, Buono and coworkers reported a larger lipid positive area in en face preparations of descending aorta but not in cross-sections of proximal aorta of C3−/−LDLR−/− mice [15]. Serial cryosections of the aortic root were used for evaluation of the affect of IVIg treatment on the formation of atherosclerotic lesions, because lesion size in cross-sections of the aortic root appears to be an atherogenesis parameter that is not affected primarily by C3 deficiency. By selecting such an evaluation paradigm, we were able to compare not only the injected versus uninjected groups, but also the injected C3−/−versus the injected control mice.
The principal finding of this study is that IVIg treatment inhibits progression of atherosclerotic lesions by a complement-dependent mechanism. Our study confirms that IVIg treatment has anti-atherosclerotic effects; however, these were seen only in complement-sufficient but not in C3-deficient ApoE−/−LDLR−/− mice. Interference with complement-mediated tissue damage, possibly by scavenging C3b and C4b, has been posited earlier in the literature as one of the possible mechanisms for the immunomodulatory effects of IVIg in autoimmune diseases associated with excessive complement activation [16–21]. This explanation for the anti-atherogenic effects of IVIg seems unlikely in the light of findings from our as well as other laboratories, that the lack of C3 does not lead to the reduction of lesion size as assessed in cross-sections of the aortic root [14,15]. Instead, it is likely that systemic activation of the complement cascade by IVIg leads to a systemic rather than local modulation of the host's immune response. This reasoning is also consistent with the fact that IVIg treatment has been shown to ameliorate efficiently a number of autoimmune and systemic inflammatory diseases [6–8].
Intravenous Ig activates complement in vitro as well as in vivo [19,22,23]. The Fc portion of IgG, which is the main constituent of IVIg preparation, is a potent activator of the classical pathway of complement activation. Upon complement activation, C3b becomes bound covalently to both Fab and Fc portion of IgG [24,25]. Through binding to complement receptors with affinity for C3b or its degradation products, the IgG-bound C3b or fragments thereof may regulate a number of immune functions such as phagocytosis, activation and proliferation of B lymphocytes, cell adhesion and cytotoxicity. Indeed, modulation of several of these functions, such as the recruitment of mononuclear leucocytes mediated by vascular leucocyte-adhesion molecules [26–28], chemokine production [29], antibody production [30–32] and differentiation of monocytes to macrophages [33] has been shown to ameliorate atherosclerosis in animal models. In support of this, a recent study demonstrated that intact IVIg, but not F(ab′)2 inhibit atherosclerosis in ApoE−/− mice [10]. The anti-atherogenic effect was associated with reduced titre of IgM antibodies to oxidized LDL [9], suppressed macrophage accumulation [9,10] and reduced T-cell activation [9,10]. Our data show that the anti-atherosclerotic effects of IVIg are mediated, at least partly, by complement. Taken together, it is conceivable that C3-derived fragments generated through Fc-dependent complement activation and complement receptor signalling are involved in this process. Interestingly, we found that the expression of CD32 (Fcγ R) in the lesions was not affected by lack of C3 or IVIg treatment. This would mean that IVIg inhibits atherogenesis by an Fc-portion-dependent mechanism different from the anti-inflammatory activity of IVIg in autoantibody-triggered inflammatory diseases in which the protective effect appears to be mediated through the induction of inhibitory Fc receptor expression on effector cells [34].
Our results show that the IVIg treatment is associated with an increased infiltration of the lesions with macrophages/monocytes, but this effect of IVIg does not seem to be mediated by complement activation. The finding of increased infiltration with macrophages/monocytes in mice that received IVIg is in contrast with reports showing reduction in macrophage accumulation and no effect on inflammatory infiltrate, respectively [9,10]. This discrepancy is probably caused by differences in experimental models (ApoE−/− mice on high fat diet versus ApoE−/−LDLR−/− mice on normal chow) and the evaluation paradigms such as antibodies against different markers (anti-Mac3, MOMA-2 and anti-I-Ab, respectively) labelling distinct subsets of inflammatory cells (tissue macrophages, monocytes/macrophages and MHC class II positive cells, respectively), and counting of positive cells versus positively stained lesion areas used in these three studies.
In conclusion, the complement system plays a role in anti-atherosclerotic effects of IVIg. IVIg treatment is associated with an increased accumulation of macrophages/monocytes in the lesions, although this does not appear to be mediated by complement activation products.
Acknowledgments
This work was supported by grants from the Swedish Heart-Lung foundation, the Swedish Medical Research Council (Projects 13470 and 6816), King Gustaf V's 80 years Foundation and Sigurd and Elsa Golje's Foundation.
References
- 1.Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang ASD, Katz A, Minta JO. C3 deposition in cholesterol-induced atherosclerosis in rabbits: a possible etiologic role for complement in atherogenesis. J Immunol. 1979;123:1117–22. [PubMed] [Google Scholar]
- 3.Hansson GK, Holm J, Kral IG. Accumulation of IgG and complement factor C3 in human aterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand Sect A. 1984;92:429–35. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- 4.Vlaicu R, Niculescu F, Rus HG, Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985;57:163–77. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- 5.Seifert PS, Messner M, Roth I, Bhakdi S. Analysis of complement C3 activation products in human atherosclerotic lesions. Atherosclerosis. 1991;91:155–62. doi: 10.1016/0021-9150(91)90197-b. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med. 1992;326:107–16. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- 7.Kaveri SV, Dietrich G, Hurez V, Kazatchkine MD. Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin Exp Immunol. 1991;86:192–8. doi: 10.1111/j.1365-2249.1991.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 9.Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson G. Immunoglobulin treatment reduces atherosclerosis in apoE knockout mice. J Clin Invest. 1998;102:910–8. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Z, Kishimoto C, Sano H, Shioji K, Xu Y, Yokode M. Immunoglobulin treatment suppresses atherosclerosis in apolipoprotein E-deficient mice via the Fc portion. Am J Physiol Heart Circ Physiol. 2003;285:H899–906. doi: 10.1152/ajpheart.00926.2002. [DOI] [PubMed] [Google Scholar]
- 11.Pekna M, Hietala MA, Rosklint T, Betsholtz C, Pekny M. Targeted disruption of the murine gene coding for the third complement component (C3) Scand J Immunol. 1998;47:25–9. doi: 10.1046/j.1365-3083.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 12.Witting PK, Pettersson K, Ostlund-Lindqvist AM, Westerlund C, Eriksson AW, Stocker R. Inhibition by a coantioxidant of aortic lipoprotein lipid peroxidation and atherosclerosis in apolipoprotein E and low density lipoprotein receptor gene double knockout mice. FASEB J. 1999;13:667–75. doi: 10.1096/fasebj.13.6.667. [DOI] [PubMed] [Google Scholar]
- 13.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 14.Persson L, Boren J, Robertson AK, Wallenius V, Hansson GK, Pekna M. Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in apolipoprotein E−/− low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2004;24:1062–7. doi: 10.1161/01.ATV.0000127302.24266.40. [DOI] [PubMed] [Google Scholar]
- 15.Buono C, Come CE, Witztum JL, et al. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–31. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- 16.Lutz HU, Stammler P, Jelezarova E, Nater M, Spath PJ. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn-IgG complexes. Blood. 1996;88:184–93. [PubMed] [Google Scholar]
- 17.Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. 1994;94:1729–35. doi: 10.1172/JCI117520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basta M, Fries LF, Frank MM. High doses of intravenous immunoglobulin do not affect the recognition phase of the classical complement pathway. Blood. 1991;78:700–2. [PubMed] [Google Scholar]
- 19.Mollnes TE, Andreassen IH, Hogasen K, Hack CE, Harboe M. Effect of whole and fractionated intravenous immunoglobulin on complement in vitro. Mol Immunol. 1997;34:719–29. doi: 10.1016/s0161-5890(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 20.Mollnes TE, Hogasen K, Hoaas BF, Michaelsen TE, Garred P, Harboe M. Inhibition of complement-mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its C1 binding properties. Scand J Immunol. 1995;41:449–56. doi: 10.1111/j.1365-3083.1995.tb03591.x. [DOI] [PubMed] [Google Scholar]
- 21.Wada J, Shintani N, Kikutani K, Nakae T, Yamauchi T, Takechi K. Intravenous immunoglobulin prevents experimental autoimmune myositis in SJL mice by reducing anti-myosin antibody and by blocking complement deposition. Clin Exp Immunol. 2001;124:282–9. doi: 10.1046/j.1365-2249.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz HU, Stammler P, Bianchi V, et al. Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood. 2004;103:465–72. doi: 10.1182/blood-2003-05-1530. [DOI] [PubMed] [Google Scholar]
- 23.Mollnes TE, Hogasen K, De Carolis C, et al. High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand J Immunol. 1998;48:312–7. doi: 10.1046/j.1365-3083.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 24.Anton LC, Alcolea JM, Sanchez-Corral P, Marques G, Sanchez A, Vivanco F. C3 binds covalently to the C gamma 3 domain of IgG immune aggregates during complement activation by the alternative pathway. Biochem J. 1989;257:831–8. doi: 10.1042/bj2570831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivanco F, Munoz E, Vidarte L, Pastor C. The covalent interaction of C3 with IgG immune complexes. Mol Immunol. 1999;36:843–52. doi: 10.1016/s0161-5890(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 26.Shih PT, Brennan ML, Vora DK, et al. Blocking very late antigen-4 integrin decreases leukocyte entry and fatty streak formation in mice fed an atherogenic diet. Circ Res. 1999;84:345–51. doi: 10.1161/01.res.84.3.345. [DOI] [PubMed] [Google Scholar]
- 27.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, McGregor JL. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(−/−) /ICAM-1(−/−) fed a fat or a chow diet. Arterioscler Thromb Vasc Biol. 2000;12:2630–5. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 28.Dong ZM, Wagner DD. Leukocyte–endothelium adhesion molecules in atherosclerosis. J Lab Clin Med. 1998;132:369–75. doi: 10.1016/s0022-2143(98)90107-x. [DOI] [PubMed] [Google Scholar]
- 29.Terkeltaub R, Boisvert WA, Curtiss LK. Chemokines and atherosclerosis. Curr Opin Lipidol. 1998;5:397–405. doi: 10.1097/00041433-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Palinski W, Miller EJ, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–5. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson G. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–14. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 32.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:721–4. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]