Abstract
Dendritic cell (DC)-based therapy has proved to be effective in patients with a variety of malignancies. However, an optimal immunization protocol using DCs and the best means for delivering antigens has not yet been described. In this study, 20 patients with malignant melanoma in stages III or IV were vaccinated with autologous DCs pulsed with a melanoma cell lysate, alone (n = 13) or in combination with low doses of subcutaneous (s.c.) interleukin (IL)-2 injections (n = 7), to assess toxicity, immunological and clinical responses. Monocyte-derived DCs were morphological, phenotypic and functionally characterized in vitro. Peripheral blood mononuclear cells (PBMC), harvested from patients either prior to and after the treatment, were analysed using enzyme-linked immunosorbent spot (ELISPOT). After vaccination, 50% of the patients tested (seven of 13) from the first group and (three of seven) from the second, showed an increase in interferon (IFN)-γ production in response to allogeneic melanoma cell lines but not to controls. Four of five tested human leucocyte antigen (HLA)-A2+ patients with anti-melanoma activity also showed specific T cell responses against peptides derived from melanoma-associated antigens. Delayed type IV hypersensitivity reaction (DTH) against melanoma cell lysate was observed in six of 13 patients from the group treated with DC vaccines only and four of seven from the group treated with the combination of DCs and IL-2. Significant correlations were found between DTH-positive responses against tumour lysate and both disease stability and post-vaccination survival on the stage IV patients. There were no toxicities associated with the vaccines or evidence of autoimmunity including vitiligo. Furthermore, no significant enhancement was observed as a result of combining DC vaccination with IL-2. Our data suggest that autologous DCs pulsed with tumour lysate may provide a standardized and widely applicable source of melanoma specific antigens for clinical use. It is safe and causes no significant side effects and has been demonstrated to be partially efficient at triggering effective anti-melanoma immunity.
Keywords: antigen presentation, cancer vaccines, dendritic cells, melanoma
Introduction
Immunological approaches for the treatment of cancer have been explored for more than a century. Immunotherapy against tumours have included the use of monoclonal antibodies conjugated with toxins, radionuclides or cytotoxic drugs [1], proinflammatory recombinant cytokines such as interleukin (IL)-2 and interferon (IFN)-α [2,3], adoptive therapies of lymphokine-activated killer cells (LAK) [4,5], and finally the use of antigen-presenting cells loaded with tumour-associated antigens [6–10]. An original methodological approach has been implemented during recent years, consisting of the use of autologous dendritic cells (DCs) loaded with tumour-associated antigens as a natural adjuvant in order to actively prime an effective immune response against tumour cells [11–15].
DCs originate in bone marrow and migrate from circulating blood to peripheral tissues, where they acquire the phenotype of mature antigen-presenting cells after interaction with pathogens or other inflammatory stimuli [16]. Mature DCs are characterized by the expression of high levels of major histocompatibility complex (MHC) Classes I and II molecules on cell surfaces as well as an increased expression of co-stimulatory molecules such as CD80 (B7·1) and CD86 (B7·2), adhesion molecules such as CD11c, CD54 (ICAM-1), CD58 (LFA-3) and CD102 (ICAM-3) and stimulatory molecules such as CD40 [17]. Physiologically, DCs can be isolated directly from peripheral blood, but the number of cells that can be recovered is limited by their low frequency in circulation, even after patients are pretreated with a DC-stimulating factor such as Flt3-L [18,19]. As an alternative, DCs can be generated on a much greater scale from CD34+ bone marrow precursors [20]. However, the isolation of CD34+ cells requires patients to be pretreated with granulocyte-colony stimulating factor (G-CSF) to increase mobilization of these cells from the bone marrow to the blood and a prolonged in vitro culture with a complex panel of cytokines [21,22]. Nevertheless, most of the clinical studies have been performed using dendritic cells produced from CD14+ monocytes derived from peripheral blood mononuclear cells (PBMC) cultured with granulocyte macrophage-colony stimulating factor (GM-CSF) and IL-4 (or alternatively IL-13) [11,23,24]. Monocytes treated in this way produce cells that are morphologically and functionally similar to immature DCs directly isolated from PBMC and require additional in vitro maturation with a stimulus such as tumour necrosis factor (TNF)-α [11].
The usefulness of DC-based vaccinations may depend not only on the vaccination schedules in terms of means of administration, dose and frequency, but also on the availability of these cells and the use of reproducible methods to generate them in large numbers. Additionally, the optimal tumour antigen delivery strategy has not yet been established. Autologous tumour cell lysate, tumour cells, antigenic peptides and mRNA have been used with DCs as immunization strategies, each generating promising but not yet satisfactory results [25,26]. Several clinical trials have used immature DCs, although most recent studies have also evaluated mature DCs, which have been proved to provide a stronger stimulus in activating T cells. Moreover, the use of a tumour lysate obtained from allogeneic melanoma cell lines, and which provides a standardized and widely applicable source of melanoma specific antigens for clinical use, has not been assessed extensively associated to DCs. In this report we describe an experimental approach used in our laboratory to generate large quantities of DCs to be used in a Phase I clinical trial consisting of the injection of 3–20 × 106 DCs pulsed with an allogeneic cell lysate derived from three melanoma cell lines in the presence of keyhole limpet haemocyanin (KLH) as adjuvant. The use of autologous DCs ensures that the antigen will be presented in a correct human leucocyte antigen (HLA) context. On the other hand, the allogeneic tumour cell lysate provides additional danger signals, thus contributing to an optimal immune response.
DC stimulation of anti-tumour T cells may be enhanced by additional cytokines that promote T cells expansion in vivo. Recombinant IL-2 (Proleukin®), an FDA-approved drug for the treatment of advanced melanoma and renal carcinoma, is a potent stimulator of lymphocyte proliferation and augments the activity of cytotoxic T lymphocytes (CTL) [27] either alone or in combination with other cytokines [2]. The systemic administration of low doses of IL-2 has a broad range of immunological effects, including the induction of specific T helper cells, natural killer and lymphokine-activated killer (LAK) cells, and ex vivo can also enhance the anti-tumour activity of adoptively transferred LAK cells [4] or specifically immune T lymphocytes [5,28,29]. Importantly, IL-2 alone can mediate regression of selected, established human tumours [3,30].
Based on these studies, a group of patients were vaccinated with autologous tumour lysate-pulsed DCs every 10 days on four different occasions. A second group of seven patients also received periodical subcutaneous (s.c.) injections of low doses of IL-2 between the vaccines to investigate whether the combination of DC therapy and cytokine injections improves the immune response of vaccinated patients.
During this study, in addition to the assessment of vaccine toxicity, immune monitoring of patients was also performed. Patient-derived PBMCs were checked pre- and post-vaccination for reactivity to melanoma cell lines and peptides derived from melanoma-associated antigens. Finally, the in vivo delayed type hypersensitivity (DTH) reaction against melanoma cell lysate was also studied in vaccinated patients.
Materials and methods
Trial eligibility
The Phase I clinical trial was designed to address the safety and immunological efficacy of immunizations with tumour lysate-loaded DCs. Twenty patients with stages III–IV metastasic melanoma, as defined by the modified 2001 American Joint Commission on Cancer (AJCC) stating system, were enrolled in this study, performed from January 2001 to August 2004. Patients were required to undergo head scans via magnetic resonance imaging (MRI) or computed tomography (CT) to ensure the absence of metastases, as well as CT scans of chest, abdomen and pelvis within 4 weeks before the therapy was started to evaluate the magnitude of the disease. Patients were also required to undergo radionuclide scintigraphy (EI) to evaluate the progression of melanoma bone metastasis. The eligibility criteria for vaccination included (i) histologically verified melanoma, (ii) an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, (iii) normal white blood cell (WBC) and platelet counts and (iv) HIV, hepatitis C antibody and hepatitis B surface antigen were required to be negative. Approval for the study was obtained from the Bioethical Committee for Human Research of University of Chile, Faculty of Medicine. All patients were required to understand the study and sign an informed consent.
Leukapheresis
Leukaphereses of stage IV melanoma patients were performed at the University of Chile Clinical Hospital Blood Bank Service using conventional techniques. Briefly, in order to obtain mononuclear cells from peripheral blood, patients were submitted to a single procedure using a mononuclear cell harvesting programme and a kit for white sanguineous cells in a cellular separator of continuous flow Cobe Spectra (Lakewood, CO, USA). ACD-A (sodium citrate 74·8 mM, right-twist monohydrate 123·6 mM and citric acid monohydrate 38·1 mM) was used as an anti-coagulant mixture in a blood/anti-coagulation proportion of 1 : 12. In each leukapheresis process, a volume equivalent to twice the patient's volaemia was taken.
Melanoma cell lines and cell lysate preparation
FMS mel, DF mel, BL mel, FM55 mel, DL mel, 0505 mel and BE mel are melanoma cell lines established at the Microbiology and Tumour Biology Centre, Karolinska Institute, Stockholm, Sweden. All lines derived from metastasic lesions and are HLA-A2+.
A melanoma cell lysate derived from a mixture of three allogeneic melanoma cell lines, FMS, DF and BL, was prepared as follows. The cell lines were obtained from metastasic lymph nodes. They were stained positive by the melanoma-specific markers; S-100, HBM45, A103, 9·2.27 and MC1R [31]. The cell lines were tested by polymerase chian reaction (PCR) for the absence of potential infecting virus or mycoplasma. Also the presence of contaminating bacteria was discarded after testing in agar. Equal amounts (7 × 106) of tumour cells from each cell line were mixed and washed three times in phosphate buffered saline (PBS). The PBS was discarded; the cell pellets were frozen rapidly in liquid nitrogen and then thawed at 37°C. This procedure was performed three times. The cell lysates were then sonicated with three pulses for 30 s and irradiated with a dose of 60 Gy. The protein concentration of the lysate was estimated after dilution in AIM-V medium by the Bradford's method using a Biophotometer (Eppendorf, Hamburg, Germany).
Generation of dendritic cells and administration to patients
PBMCs were obtained from buffy coats (patients CT001–CT006) or by leukapheresis (patients CT007–CT020). Leucocytes were isolated by density gradient separation with Ficoll-Hypaque (Axis-Shield, Oslo, Norway). Cells (3 × 107/well) were incubated in serum-free AIM-V therapeutic medium (Gibco BLR, Paisley, UK) at 37°C, 5% CO2 for 2 h in a six-well plate (Falcon cat. no. 3846 Becton Dickinson, Hershey, PA, USA). Non-adherent cells were removed, and the remaining cells were incubated for 7 days in the presence of 500 U/ml recombinant human IL-4 (rhIL-4) (US Biological, Swampscott, MA, USA) and 800 U/ml of GM-CSF (Shering Plough, Brinny Co., Ireland). The cultures were maintained for 7 days, replacing the medium every 2 days. At day 6, DCs were loaded overnight with a tumour cell lysate in the presence of 20 IU/ml of TNF-α (US Biological, Swampscott, MA, USA). On day 7, DCs were recovered and kept as described. Briefly, non-adherent cells were harvested and put on a 50 ml tube (Falcon cat. no. 2068, Becton Dickinson, Hershey, PA, USA), while adherent cells were incubated for 5 min with PBS and then removed carefully using a cell scraper (NUNC, Rochester, NY, USA), added to the tube, centrifuged and washed with PBS before freezing them down, using an automatic freezing system Cobe Spectra (Lakewood, CO, USA) in AIM-V medium containing 20% of autologous serum and 10% dimethylsulphoxide (DMSO). Viability of DCs was more than 97% after thawing, inclusive, if the sample cryogenic tubes were kept on ice for up to 8 h before counting. Patients CT001–CT006 were vaccinated intradermally (i.d.) in the leg or arm closest to intact lymph nodes with 3–5 × 106 DCs derived from autologous buffy-coat-derived PBMC, and patients CT007–CT020 were vaccinated i.d. with 1–2 × 107 DCs derived from autologous leukapheresis obtained via PBMC. Ten µg/ml KLH was co-administered with the vaccines (Calbiochem, San Diego, CA, USA) as adjuvant.
DC vaccine combined with low doses of rhIL-2
Seven patients (CT014-CT020) were vaccinated as described previously and treated additionally with 2·4 × 106 IU/m2 rhIL-2 (Proleukin®) (Chiron Emeryville, CA, USA), injected s.c. days 2, 3 and 4 after a second, third and fourth vaccination.
Morphological and phenotypical characterization of DC
The morphology of the DCs was evaluated using an inverse phase microscope (Leica DMIL, Leica Mikroskopie und Systeme Wetzlar, Germany). DCs were characterized phenotypically by flow cytometry using a panel of monoclonal antibodies (mAb) such as anti-CD14, HLA-ABC, HLA-DRDQDP, CD36, CD40, CD86 and CD83 conjugated with fluorescein isothiocyanate (FITC) and anti-CD11c anti CD1a and anti-CCR7 conjugated with phycoerythrin (PE) (BD PharMingen, San Diego, CA, USA). Briefly, 8 × 105 cells were recovered from the culture plate after a 10–15-min incubation at 37°C in PBS-ethylenediaminetetraacetic acid (EDTA) 1 × (KH2PO4 0·4 mm, Na2HPO4 0·6 mm, EDTA 0·2 g/l, pH 7·0). The cells were then centrifuged at 250 g for 5 min at room temperature, washed with PBS, fixed with 0·5% (v/v) formaldehyde in PBS, and incubated with conjugated mAbs for 30 min. Finally, the cells were washed twice with PBS and analysed in a flow cytometer FACSort (Beckton-Dickinson, San Diego, CA, USA). Data analysis was performed using the CellQuest program (Beckton-Dickinson). Small and dead cells were excluded from the analysis based on their light-scatter properties.
Measurement of propidium iodide-labelled apoptotic melanoma cells and FITC-dextran uptake by DCs
Dextran uptake activity was assessed by incubating 0·5 × 106 immature or mature dendritic cells with 0·5 × 106 propidium iodide (PI)-labelled apoptotic melanoma cells or FITC-conjugated dextran (0·2 mg/ml) for 2 h at 37°C in the darkness. Cells were washed carefully with PBS, and PI-labelled apoptotic melanoma cells and FITC-conjugated dextran uptake were quantified by flow cytometry. Spontaneous apoptotic cell or dextran incorporation was assessed by incubating DCs on ice. In some experiments, the dark pigmented DFB mel line were incubated after irradiation with immature DCs and observed at the microscope after 30, 60, 120, 180 min and finally after overnight incubation.
Skin testing
We assessed the patients for in vivo DTH to KLH or allogeneic tumour cell lysate. Skin tests were performed using 200 µg of KLH in aqueous solution injected i.d. in a volume of 100 µl using a tuberculin syringe and a 27-gauge needle. To analyse the tumour cell lysate specific reactivity, patients were evaluated using 400 µg/ml of tumour cell lysate in 200 µl aqueous solution, injected intradermally at a separated site, in a volume of 100 µl. Saline solution was used as a negative control. At least 5 mm of induration or erythema, read 48 h after intradermal injection, were required to score a skin test as positive. This evaluation was made 1 month after the end of therapy.
Culturing and expansion of TIL and of anti-melanoma CTL derived from melanoma patients
A single cell suspension from a metastasic lymph node from a melanoma patient (0505 TIL) was cultured in serum-free medium (AIM-V, Gibco), supplemented with 100 IU human rIL-2/ml (Chiron Emeryville, CA, USA). The culture was maintained in vitro for 4 weeks in the presence of the correspondent autologous melanoma cells (0505 mel), frozen and used after thawing. A CTL line specific for HLA-A2+ melanomas (DF CTL) was obtained after four rounds of weekly stimulation of peripheral blood leucocytes (PBL) with autologous tumour (DF mel). Briefly, 10 × 106 of PBL from patient DF were incubated periodically with irradiated autologous melanoma cells (DF mel) in the presence of 10 IU/ml of rIL-2. The IL-2 was always added 12 h after tumour stimulation. The melanoma cell lines used for stimulation were pretreated 24 h with 100 µg/ml IFN-γ (Genetech, San Francisco, USA), irradiated (6000 rad) and then added to the CTLs, after washing with PBS, in a effector : stimulator ratio of 20 : 1. Cytotoxic activity against the K562 and HLA-A2 negative melanomas was used to assess specificity. 51Cr-release assays against autologous tumour or against peptide loaded targets were performed after 3 or 4 weeks of in vitro culture for the TIL and 4–5 weeks for the PBMC-derived CTL.
Cytotoxic assays
To analyse peptide recognition, T2 cells were incubated for 2 h at 26°C together with 2 µg/ml peptide, washed and used as target cells in 51Cr-release assays. Cytotoxic tests were performed by incubating 51Cr-labelled target cells (melanoma cells or peptide-pulsed T2 cells) (1 × 106) with effector cells at various effector : target (E : T) ratios at 37°C for 5 h. Supernatants were harvested and radioactivity was determined using a gamma counter. The percentage of 51Cr release was calculated according to the following formula: % lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Enzyme-linked immunosorbent spot (ELISPOT) assays
One day prior to the assay, a 96-well, flat-bottomed plate (MAIPN1450; Millipore, Bedford, MA) was coated with 75 µl/well of anti-human IFN-γ monoclonal capture antibody (Mabtech, Nacka, Sweden) adjusted at 2 µg/ml in sterile 0·1 M bicarbonate buffer (pH 9·6) and incubated overnight at 4°C. The day of assay the plate was washed with cold PBS, and 200 µl/well PBS 1% bovine serum albumin (BSA) was added for 2 h at 37°C to block non-specific antibody binding. PBMCs were prepared as described above and adjusted to 1 × 106/ml. One hundred µl of cell suspension were incubated with tumour cells pretreated overnight at 37°C with 500 U/ml of IFNγ (E : T cell ratio of 10 : 1) in a total volume of 200 µl/well or with same amount of K562 cells as controls for natural killer (NK)-mediated activity. Negative controls for the assays were non-stimulated PBMC. Positive controls were stimulated with phytohaemagglutinin. For melanoma-specific CTL assays, DCs loaded with melanoma cell lysate or K562 cells (1 × 105 cells/well) were co-cultured with 5 × 104 or 2·5 × 104 effectors. For peptide-specific assays, T2 cells (1 × 104 cells/well) were co-cultured with 1 × 105 effectors in the presence of 10 µg/ml of HLA-A2 restricted antigenic peptides; MART1/Melan A27–35 (AAGIGILTV), gp100280–288 (YLEPGPVTA) and tyrosinase368–376 (YMDGTMSQV) or peptide HIV-1 p17 Gag77–85 (SLYNTVATL) as negative control. Cultures were incubated at 37°C, 5% CO2 for 4 h. After incubation, the plate was washed with cold PBS Tween 0·05% and then incubated with 0·5 µg/ml of a secondary biotinylated mAb specific for bound IFN-γ (Mabtech) for 20 h at 4°C The plate was washed again and then incubated with 0·5 µg/ml streptavidin–alkaline phosphatase (Mabtech) for 1 h at room temperature in dark. After alkaline phosphatase colour development (Bio-Rad, Richmond, CA, USA), spots representing IFN-γ secreted by a single cell were counted.
Enzyme-linked immunosorbent assay (ELISA)
Immature and mature DCs (0·3 × 106 cells) were incubated in 2 ml of RMPI 10% fetal bovine serum (FBS) during 24 h in the presence or absence of 1 × 106 IL-2-stimulated allogeneic irradiated PBL. The supernatants were collected and analysed in triplicate by ELISA. Capture and biotinylated detection antibodies and standards for human IL-10, TNF-α and IL-12 p70 (BD PharMingen, San Diego, CA, USA), were used according to the manufacturer's recommendations.
Clinical criteria
Patients are referred to as immunological responders when they show activity against tumour cell lysate in DTH assays or/and IFN-γ production in an ELISPOT assay. Stable disease (SD) was defined as less than a 25% change in tumour size with no new lesions developing for 6 weeks. Progressive disease (PD) was defined as a more than 25% increase in the perpendicular diameter of any measurable tumour, appearance of a new lesion or worsening of valuable disease. Time to progression (TTP) is the number of months that patients showed SD after the first vaccination. Post-vaccination survival is the number of months that patients survived after the first vaccination.
Statistical analysis
The differences in TTP and patient survival between the DTH responder and non-responder group were compared by Student's test using an Origin™ computer program, and were considered statistically significant at P < 0·05.
Results
Generation of monocyte-derived dendritic cells
The leukapheresis process, performed on 14 patients with stage IV malignant melanoma, allowed us to obtain an average of 4·8 × 109 of PBMC per patient, equivalent to the product of two volaemias. In our protocol, an average of 180 × 106 PBMCs were cultured in a six-well plate obtaining, at the end of the procedure, an average of 6·9 × 106 dendritic cells for each plate, which is equivalent to half a dose of DC vaccine (data not shown). A total of six doses could be obtained from one single leukapheresis procedure, but only four doses were used in patients.
Phenotypical and functional characterization of injected DCs
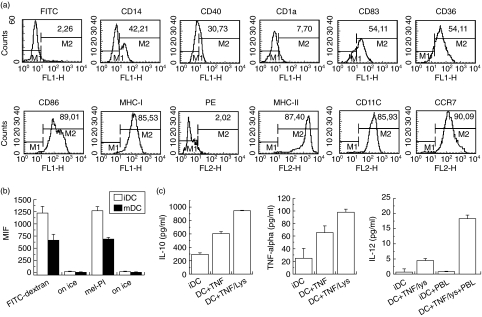
Prior to injection in patients, around 75–80% of the dendritic cells generated had a typical mature DC phenotype with the following average percentages (n = 6 patients) of cells expressing markers: CD14low (36·9%), CD40 (36·4%), CD1a (6·4%), CD83 (60·5%), CD36 (67·6%), CD11c (78%), CD86 (87·6%), HLA ABC (79·5%) and MHC class II (88·7%), which are similar to those obtained previously by other groups [23–26] (Fig. 1a represents marker expression of DCs obtained from patient CT003). To determine the potential capacity of our DCs to migrate, immature and mature cells were stained with a mAb against the molecule CCR7 before and after tumour lysate/TNF-α incubation. Mature DCs showed a marked expression in CCR7 (90%) (Fig. 1a), which is consistent with an increased migratory capacity for mature DCs as described previously [32].
Fig. 1.
Phenotypic and functional properties of mature dendritic cells (DCs) at day 7. (a) Surface marker expression of mature DCs at day 7. Cells were treated as described in Materials and methods, marked with conjugated antibodies and analysed by flow cytometry. Only large cells corresponding to the forward scatter were selected for this evaluation. (b) Comparison of phagocytic capacity of immature and mature DCs. DCs from day 6 (immature) and day 7 [matured with tumour necrosis factor (TNF)-α] were mixed with fluorescein isothiocyanate (FITC)-conjugated dextran or with propidium iodide (PI)-labelled apoptotic melanoma cells as described in Materials and methods. After 2 h, cells were analysed by flow cytometry. Experiments were performed twice with similar results. (c) Cytokines released by matured DCs. Cells were incubated for 48 h in presence or absence of TNF-α, tumour cell lysate and allogeneic peripheral blood leucocytes (PBL) and the supernatants analysed by enzyme-linked immunosorbent assay (ELISA) as described in Materials and methods. The figure represents three experiments with DCs from different patients.
In order to study the functional properties of DCs produced in our protocol, we performed phagocytosis assays. It is known that immature DCs have a strong phagocytic capability, whereas the mature DCs are better prepared as antigen-presenting cells [24]. In the absence of stimulation by TNF-α, immature dendritic cells demonstrated a twofold greater phagocytic capacity than mature DCs when they were exposed to fluorescein-labelled dextran (Fig. 1b). A significant difference (40% less phagocytosis mediated by mature DCs than immature DCs) was also observed when DCs were co-incubated with propidium iodide marked melanoma cells (Fig. 1b). The phagocytic process of melanoma apoptotic cells was very significant after 2 h of incubation and became complete after 12 h of incubation, as observed using melanin-pigmented DF mel cells (data not shown). After maturation with TNF-α and tumour cell lysate, the DCs used in our clinical protocol were shown to produce cytokines such as IL-10 and TNF-α, further supporting their mature phenotype (Fig. 1c, right and middle panels). The expression of IL-12 by DCs after TNF-α and tumour cell lysate stimulation was also checked, although the presence of this cytokine was below the detection limit of our ELISA kit (Fig. 1c, left panel). However, when mature DCs were mixed with allogeneic IL-2 activated irradiated PBL, they began to express significant amounts of IL-12. This observation indicates that IL-12 secretion by mature DCs would require additional stimuli besides TNF-α and cell lysate, such as CD40–CD40L interactions provided by activated T lymphocytes (Fig. 1c, left panel). In contrast, our immature DCs did not secrete IL-12 either in the presence or absence of allogeneic PBMC.
Antigen presentation capability of DCs loaded with melanoma cell lysate
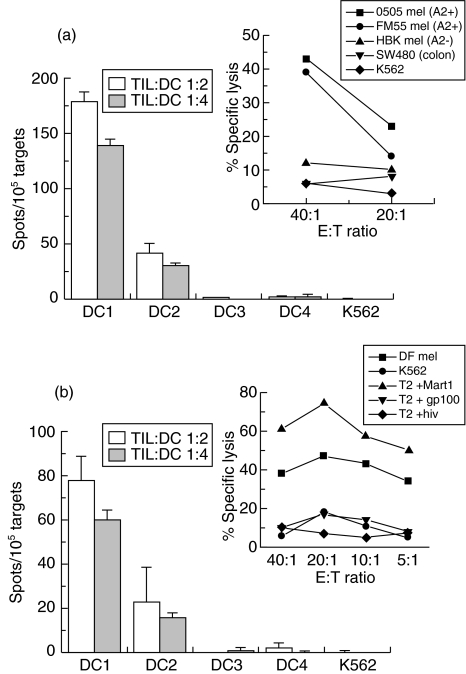
DCs obtained from four melanoma patients, two HLA-A2+ (DC1 and DC2) and two HLA-A2− (DC3 and DC4) were loaded with melanoma cell lysate and stimulated with TNF-α as described in Material and methods. DCs were then incubated together with two HLA-A2 restricted melanoma specific CTL lines (0505 TIL and DF CTL) and IFN-γ production by CTLs was measured by ELISPOT (Fig. 2). The 0505 TIL which recognize the autologous melanoma cell line (0505 mel) and an allogeneic HLA-A2+ melanoma cell line (FM55 mel), but not an HLA-A2− melanoma cell line (HBK mel) or a colon carcinoma cell line (SW480), can produce IFN-γ in response to two HLA-A2+ melanoma cell lysate-loaded DCs but not after stimulation with HLA-A2− DCs loaded with melanoma cell lysate (DC3) or with a prostate carcinoma cell lysate (DC4) or the NK sensitive prototype K562 (Fig. 2a). Similarly, the DF CTL which can also recognize the autologous melanoma cell line (DF mel) and T2 cells loaded with the MART1/Melan A27–35 (AAGIGILTV) epitope, but not K562 or T2 cells loaded with other epitopes, could also recognize DC1 and DC2 but not DC3, DC4 or K562 (Fig. 2b). These results indicate that our DCs are capable of presenting antigens derived from the melanoma cell lysate to melanoma-specific CTLs.
Fig. 2.
Mature dendritic cells (DCs) loaded with melanoma cell lysate can stimulate interferon (IFN)-γ release by human leucocyte antigen (HLA)-A2 restricted melanoma-specific cytotoxic T lymphocytes (CTL) lines. Melanoma-specific T lymphocytes derived from (a) 0505 TIL or (b) DF CTL lines were incubated with DCs derived from melanoma patients (DC1 and DC2 are HLA-A2+ cells loaded with melanoma cell lysate; DC3 are HLA-A2+ cells loaded with a prostate cancer lysate; and DC4 are HLA-A2− cells loaded with melanoma cell lysate) or K562 cells, as described in Material and methods and were then analysed by enzyme-linked immunosospot assay (ELISPOT), as described in Materials and methods. All ELISPOT experiments were performed in duplicate. Small graphs shows standard 51Cr release assays (a) 0505TIL and (b) DF CTL were incubated with 51Cr-labelled cells, as described in Material and methods.
Clinical trial criteria and safety
Twenty melanoma patients, stages III (n = 5) or IV (n = 15), were selected according to the inclusion criteria described in Materials and methods. The patients, 12 men and eight women, were 47 years old on average and all showed cutaneous or subcutaneous primary tumours (Table 1). For all patients, primary tumours or tumour-infiltrated lymph nodes were removed surgically prior to this study (Table 1). Six patients received 3–5 × 106 cells per dose and 14 received 15–20 × 106 cells per dose. Each patient was injected four times with the indicated amount of DCs and injections were separated by 10 days from each other (Table 2). The injected DCs were pretreated with TNF-α and with a tumour cell lysate derived from three different allogeneic melanoma cell lines and then injected (i.d.) to patients in the presence of KLH, as described in Materials and methods. To improve in vivo expansion of tumour-specific T cells after immunizations with DCs we tested IL-2 in a subgroup of patients. Patients CT014–CT020 also received nine s.c. injections of rhIL-2 (Proleukin®) as described in Material and methods. Vaccinated patients were monitored continuously for adverse reactions. Only two patients from the group that received DCs only experienced minor fever episodes associated potentially with the vaccination. On the other hand, all seven patients receiving DCs in combination with IL-2 showed weak fever, headache, flu-like symptoms associated with pain and reddening in the inoculation site. No additional adverse effects associated with patient treatment were detected during the study (Table 2). These results indicate that vaccination with autologous ex vivo produced DCs loaded with allogeneic cell lysate derived from melanoma cell lines is a safe procedure, even in combination with low doses of IL-2.
Table 1. Characteristics of enrolled patients.
| Patient | Age | Sex | Clinical stage | HLA | Primary tumour | Metastasis | Previous treatment (beside surgery) |
|---|---|---|---|---|---|---|---|
| CT001 | 34 | F | IV | A2+ | Cutaneous thorax | In transit mammary gland | – |
| CT002 | 31 | M | IV | A2− | Subcutaneous shoulder | Spinal column metastasis | – |
| CT003 | 34 | M | IV | A2+ | Cutaneous shoulder | Pulmonary metastasis | – |
| CT004 | 69 | M | IV | A2− | Cutaneous | Multiple metastasis | IL-2 |
| CT005 | 57 | M | IV | A2− | Cutaneous left leg | Pulmonary (2) metastasis | – |
| CT006 | 30 | M | IV | A2+ | Cutaneous shoulder | Positive left inguinal lymph node | – |
| CT007 | 53 | F | III | A2+ | Cutaneous EII | Positive iliac lymph node | – |
| CT008 | 37 | F | IV | A2+ | Subcutaneous left knee | Multiple pleuropulmonary metastasis | – |
| CT009 | 24 | F | III | A2- | Cutaneous shoulder | Positive axilar lymph nodes | – |
| CT010 | 39 | M | IV | A2+ | Malar right | Negative axilar lymph nodes | – |
| CT011 | 68 | F | IV | A2+ | Subcutaneous shoulder | Lymph nodes + liver metastasis | – |
| CT012 | 57 | F | IV | A2+ | Left plant | Positive axilar lymph nodes | – |
| CT013 | 62 | M | IV | A2+ | Cutaneous head | Positive cervical lymph nodes | – |
| CT014 | 64 | M | IV | A2+ | Cutaneous right leg | Abdominal metastasis | – |
| CT015 | 63 | M | IV | A2+ | Cutaneous head | Pulmonary metastasis | – |
| CT016 | 37 | F | III | A2+ | Malar left | Parotid gland | – |
| CT017 | 31 | M | IV | A2+ | Cutaneous shoulder | Positive left cervical lymph node | – |
| CT018 | 46 | M | IV | A2− | Cutaneous EII | Positive right axilar lymph nodes | IL-2 |
| CT019 | 57 | F | III | A2+ | Cutaneous left leg | Positive left inguinal lymph node | – |
| CT020 | 51 | M | IV | A2− | Malar left | Pulmonary metastasis | – |
IL: interleukin; HLA: human leucocyte antigen.
Table 2. Clinical, immunological and adverse effects on vaccinated patients.
| Patient | DC (106)/ dose | Adjuvant | Adverse reactions | ELISPOT response | DTH KLH (mm) | DTH lysate (mm) | Stable disease (months) | Post-vaccination survival (months) |
|---|---|---|---|---|---|---|---|---|
| CT001 | 3–5 | KLH | Negative | + | + (6) | + (10) | 23 | 26 |
| CT002 | 3–5 | KLH | Fever | nt | − | − | − | 1 |
| CT003 | 3–5 | KLH | Negative | + | + (7) | + (7) | 20 | 21 |
| CT004 | 3–5 | KLH | Negative | − | + (7) | − | 18 | 21 |
| CT005 | 3–5 | KLH | Weak fever, knee inflammation. Anti-nuclear antibodies negative, rheumatoid factor negative | − | + (6) | − | 0 | 18 |
| CT006 | 3–5 | KLH | Negative | + | + (6) | + (9) | 22 | 24 |
| CT007 | 15–20 | KLH | Negative | + | + (10) | + (18) | 20 | 22 |
| CT008 | 15–20 | KLH | Negative | − | + (7) | − | 1 | 5 |
| CT009 | 15–20 | KLH | Negative | − | + (7) | + (10) | 21 | 22 |
| CT010 | 15–20 | KLH | Negative | + | + (8) | + (10) | 0 | > 16 |
| CT011 | 15–20 | KLH | Negative | + | + (6) | − | 0 | 7 |
| CT012 | 15–20 | KLH | Negative | − | + (6) | − | 0 | 11 |
| CT013 | 15–20 | KLH | Negative | + | − | + (7) | 17 | > 18 |
| CT014 | 15–20 | KLH + IL-2 | Weak fever | + | + (8) | − | 0 | 1 |
| CT015 | 15–20 | KLH + IL-2 | Weak fever, pain and reddening in inoculation site | + | + (6) | + (12) | 14 | > 15 |
| CT016 | 15–20 | KLH + IL-2 | Weak fever, pain and reddening in inoculation site | − | + (10) | + (45) | 15 | > 16 |
| CT017 | 15–20 | KLH + IL-2 | Weak fever, headache, flu-like symptoms | − | + (8) | + (8) | 13 | > 14 |
| CT018 | 15–20 | KLH + IL-2 | Weak fever, headache GII, flu-like symptoms GII | + | + (6) | + (6) | 0 | 6 |
| CT019 | 15–20 | KLH + IL-2 | Weak fever, headache GII | − | + (7) | − | 13 | > 14 |
| CT020 | 15–20 | KLH + IL-2 | Weak fever | − | + (8) | − | 0 | > 5 |
DC: dendritic cells; ELISPOT: enzyme-linked immunospot assay; DTH: delayed type hypersensitivity; KLH: keyhole limpet haemocyanin; IL: interleukin.
Immunological responses of vaccinated melanoma patients
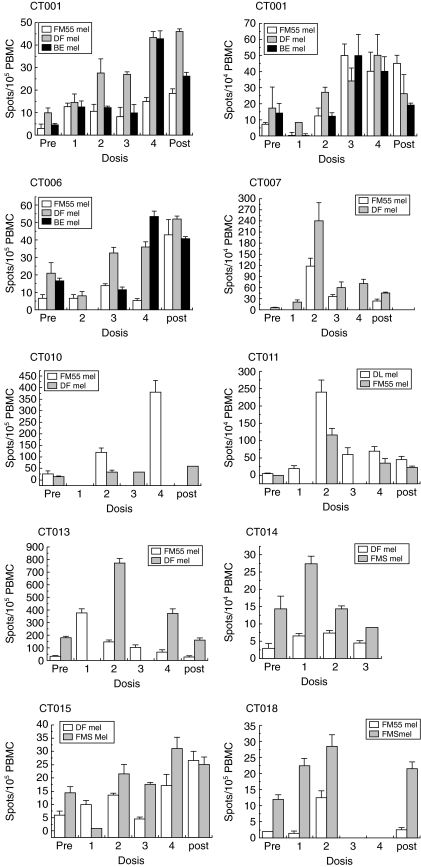
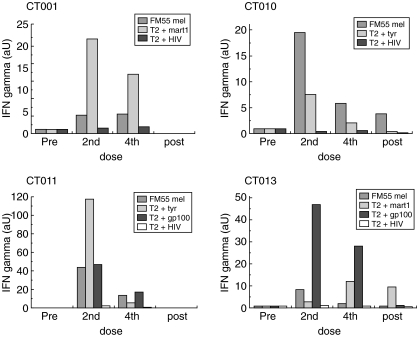
We used two detection methods to investigate the immunological effects of DC immunization in vaccinated patients. First, we determined the presence of melanoma-specific T cells in PBMC obtained from vaccinated patients using in vitro ELISPOT assays. Blood samples from patients were obtained before vaccination and after the first, second, third and fourth DC injections and also 1 month after the final vaccination. Our results show an increase of IFN-γ-secreting T cells against at least two different allogeneic melanoma cell lines in 10 of 19 patients tested (Fig. 3, Table 2). No detectable responses (less than five spots/105 PBMC) were observed against the NK sensitive cell line K562, thus discarding unspecific LAK cell activity (data not shown). T cell responses against tumours were defined as a positive response only when the number of spots at some point after vaccination was twice as high as that obtained on day 0 (Table 2). Six of 10 responders (CT001, CT003, CT006, CT010, CT015 and CT018) showed an increase in the number of IFN-γ spots after each vaccination, while four patients (CT007, CT011, CT013 and CT014) showed a marked response only after two DC injections. Thereafter, these patients showed a decrease in spot numbers after 3–4 weeks from the second DC injection (Fig. 3). To rule out the possibility that the reaction to melanoma cell lines reflected only an anti-allogeneic reaction, PBMCs from five HLA-A2+ patients were challenged against T2 cells loaded with HLA-A2 restricted synthetic peptides derived from known melanoma-associated antigens [33–35]. All five patients showed positive activity against a HLA-A2+ melanoma cell line (FM55) after the second and fourth doses, as measured by ELISPOT (Fig. 4). Patients CT001, CT010, CT011 and CT013 also showed a significant T cell reaction to T2 cells loaded with at least one of the synthetic peptides derived from the described melanoma associated antigens (MAA), such as MART1 [33], tyrosinase [34] or gp100 [35] (Fig. 4). Patient CT006 showed a strong reaction to the tumour line but a very weak reaction to the peptides mentioned, suggesting that the cells were either allospecific or recognized distinct, unidentified antigens (data not shown). In summary, these results indicate that DC vaccines loaded with melanoma cell lysate were able to elicit a T cell-mediated reaction to melanoma cells and that this response was directed at least partially against melanoma-associated antigens, although allospecificity cannot be discarded completely. In addition to the ELISPOT response, we performed in vivo measurement DTH reactions after injecting tumour cell lysate pre- and 1 month post-vaccination (Table 2). More than 50% of vaccinated patients (11 of 20) showed strong DTH reactions (more than 5 mm diameter of the erythema) against tumour cell lysate after the last dose (Table 2 and Fig. 5a). Besides two patients, who did not show a DTH response against KLH, the rest of the patients showed a significant DTH reaction against this xenogeneic protein, demonstrating that treated patients have a functional cellular immune system.
Fig. 3.
Interferon (IFN)-γ expression by melanoma specific T cells derived from peripheral blood mononuclear cells (PBMC) from responding patients. PBMCs from vaccinated patients CT001, CT003, CT006, CT007, CT010, CT011, CT013, CT014, CT015 and CT018 were obtained at different times and incubated with allogeneic melanoma cells, FM55mel, DFmel, FMSmel, DLmel or BEmel and with the natural killer (NK)-sensitive cell line K562 and were then analysed by enzyme-linked immunospot assay (ELISPOT), as described in Materials and methods. All ELISPOT experiments were performed in duplicate.
Fig. 4.
Specific response of peripheral blood mononuclear cells (PBMC) from melanoma-vaccinated patients to melanoma-associated antigen. PBMC from HLA-A2+ patients were tested against FM55 melanoma cell line; T2 cells loaded with melanoma associated-antigen peptides: MART1/Melan A27–35 (AAGIGILTV), gp100280–288 (YLEPGPVTA) and tyrosinase368–376 (YMDGTMSQV) or peptide HIV-1 p17 gag77–85 (SLYNTVATL) and the natural killer (NK)-sensitive cell line K562 as controls and were then analysed by enzyme-linked immunospot assay (ELISPOT), as described in Materials and methods. The arbitrary units (aU) correspond to the number of spots obtained after vaccinations normalized with the amount of spots obtained from prevaccination peripheral blood leucocytes (PBL). All ELISPOT experiments were performed in duplicate.
Fig. 5.
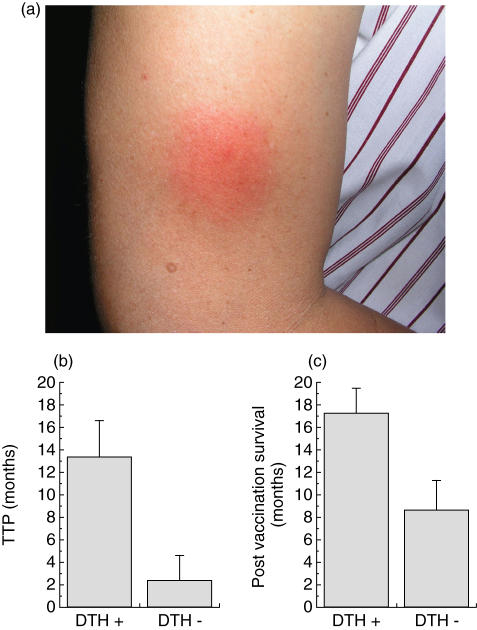
Immunological responses of vaccinated patients. (a) Typical DTH reaction to tumour cell lysate after two doses of dendritic cell (DC) vaccine. Similar reactions were detected in 11 out of 20 patients analysed. Correlation between delayed type hypersensitivity (DTH) positive response and (b) mean of time to progression (TTP) or (c) post- vaccination patient survival.
Clinical responses
From a clinical viewpoint, we observed stabilization of the disease (up to 23 months) in 11 patients according to criteria defined in Material and methods (Table 2). Two patients, CT001 and CT005, showed a partial regression of a breast subcutaneous and a cutaneous metastasis, respectively (data not shown). Significant correlations were found between DTH positive responder patients and a longer stability of the disease (P = 0·01362), and also longer post-vaccination patient survival (P = 0·0261) (Fig. 5b,c and Table 2). In effect, eight stage IV patients who showed a positive DTH reaction, as defined in Materials and methods, showed a median TTP of 13·37 months, while the group of eight stage IV patients who did not show DTH reactions to tumour cell lysate had a median TTP of 2·37 months (Fig. 5b). Similarly, the post-vaccination survival was also significantly longer in DTH responder patients (17·25 months) than in non-responders (8·625 months) (Fig. 5c). No significant correlations were found regarding ELISPOT reaction and TTP or survival. No signs of vitiligo were observed in any of the vaccinated patients (Table 2).
Discussion
In this study we demonstrate the feasibility of generating mature DCs from peripheral blood from stages III and IV melanoma patients for the treatment of malignant melanoma. The periodic injection of DCs loaded with an allogeneic melanoma cell lysate did not produce any major adverse effect on vaccinated patients, even when it was used in combination with low doses of IL-2. The preparations induced DTH reactions and in vitro IFN-γ production by tumour-specific and peptide-specific T lymphocytes.
In spite of previous difficulties in the isolation and characterization of human DC, due to its low frequency relatively high amounts of DC can now be obtained in vitro from peripheral blood monocytes stimulated with GM-CSF and IL-4 [24,36]. Using this methodology, we standardized a leukapheresis method for the isolation of PBMC in numbers adequate for clinic use. The protocol detailed in this study also allowed us to generate an adequate amount of DCs (Table 1) with phenotypic (Fig. 1a) and functional properties (Figs 1b,c and 2a,b) appropriate for its utilization in the immunization of patients with malignant melanoma. DCs used in our protocol expressed specific markers corresponding to the expected mature DC phenotype, including the marker CCR7, which may ensure that injected DCs can migrate to lymph nodes. DCs also showed functional ex vivo properties for the induction of a T lymphocyte reaction to melanoma after loading with melanoma-derived cell lysate (Figs 1 and 2). The DCs produced by our protocol showed, as expected, a marked increase in MHC Class I and Class II molecules, especially after maturation in the presence of a tumour lysate and TNF-α (Fig. 1a), and also a high expression level of the co-stimulatory molecule CD86, which improved the effectiveness of the T lymphocyte response (Fig. 1a). Numerous factors can induce and/or regulate the maturation of DCs, including lipopolysaccharide (LPS), bacterial DNA and a balance between pro- and anti-inflammatory cytokine signals [24,37]. In our protocol, we decided to use a combination of tumour lysate and TNF-α to induce maturation, antigen uptake and antigen presentation in a single step. As well as the expression of markers related to mature DCs, our DCs showed a high expression of cytokines such as TNF-α and IL-10 but not IL-12 (Fig. 1c), which is in line with observations made by others [38]. Although IL-12 expression is sometimes used as a maturation marker its expression seems to require additional stimulus such as the interaction of CD40 with CD40L expressed on activated T cells [39], in addition to TNF-α and cell lysate stimulus, as suggested by our experiment (Fig. 1c, left panel). This signal is probably provided in vivo after i.d. cell injection in patients. On the other hand, although the production of IL-10 by immature DCs, has been associated with tolerogenic effects and T lymphocytes anergy [40], it has also been described that IL-10 does not affect mature DCs antigen presentation or functional properties [40]. Moreover, despite the high levels of IL-10 and the barely detectable levels of IL-12 produced, monocyte-derived DCs were induced by bacterial infection or adenoviral vector to undergo phenotypic maturation and acquired antigen-presenting-cell functions, activating T helper 1 (Th1) and epitope-specific CD8+ T cells effector cells [41,42]. In our study, the IL-10 expressing DCs were also capable of stimulating IFN-γ expression by autologous T cells (Fig. 3) and also by melanoma specific CTL lines (Fig. 2), demonstrating their antigen presentation capability.
The cell population obtained after using our protocol was not completely homogeneous, but included a small population (20–40%) of semi-mature DCs or activated monocytes, which expressed low levels of CD14 (Fig. 1a). However, we preferred to not use any selection procedure, because those methods may affect DCs maturation, antigen presentation capability and cytokine release, as described recently [43]. Additionally, the observed low expression of CD1a, a non-traditional MHC molecule, showed that it can vary depending on the culture conditions used in the monocytes derived dendritic cells [44,45]. With respect to phagocytic capability, DCs produced in our laboratory showed the ability to ingest dextran molecules and apoptotic melanoma cells with higher efficiency in their immature than in their mature stage (Fig. 1b). In summary, these results indicate that cells produced in our laboratory from PBMCs from patients with malignant melanoma correspond to functional DCs that are able to acquire and present melanoma antigens and probably also induce an immunological in vivo response.
Our DCs were used in a Phase I clinical trial. The study demonstrated that vaccination with DCs loaded with tumour antigens is a safe procedure and can also induce a clinical and immunological response in patients with advanced cancer, as has also been shown using comparable protocols [11,46]. It has been shown that therapies based on immunization with DCs loaded with antigen-associated peptides [11,12,47] or autologous lysate of melanoma cells [11] induce specific cytotoxic T cell responses in various melanoma patients, and also showed objective tumour regressions [12,14]. However, the use of a mix of allogeneic melanoma cell lysate as an antigen source for DC vaccines has not yet been explored thoroughly. In fact, it has been demonstrated that although different melanoma cells share the majority of described MAA, their distribution varies considerably in cells obtained from different patients [31]. A mix of several melanoma cell lysates may cover a broad diversity of MAA, whose range will be presented in the context of autologous HLA isotype. The melanoma cell lines used as source for the cell lysate preparation showed significant expression of several melanoma markers, such as Mart-1/melanA, gp100, s100 protein, the melanoma-associated chondroitin sulphate proteoglycan (MCSP) and our recently described melanoma marker MC1R [31,48]. The cell lysate obtained not only delivered MAA to DCs, but also showed a strong capacity to induce DCs maturation, also in the absence of additional inflammatory signals (data not shown). Additionally, the tumour lysate provides a standardized and widely applicable source of melanoma specific antigens for clinical use.
In our Phase I study, 20 patients received all four vaccine injections and were examined for toxicity and/or immunological responses (Table 1). Seven patients (CT0014–CT020) also received low doses of rhIL-2. We have described previously that low doses of IL-2 given subcutaneously to melanoma patients increase the number of CD3+ T cells and memory T cells with only grades I–II WHO side effects, such as weak fever, transient local inflammation and induration of the injection site [49]. In our present study, there were no grades III or IV toxicities associated with the vaccines or major evidence of autoimmunity (Table 2). An increased IFN-γ production by PBMC in response to allogeneic melanoma cell lines but not to non-melanoma tumour controls was observed in 10 of 19 patients tested after the second vaccination (Table 2, Fig. 3). Four of five HLA-A2+ PBMC from patients with anti-melanoma activity also showed specific activity against peptides derived from melanoma-associated antigens (Fig. 4), indicating that the T cell response detected in the majority of patients PBMC was at least partially melanoma-specific and not only allogeneic-specific. Additionally, in vivo DTH reactions were observed in 11 of 20 patients tested tested with tumour cell lysate alone, indicating that the vaccine was able to induce cell-mediated immunological memory. Five patients (CT009, CT011, CT014, CT016 and CT017) showed divergent results between ELISPOT and DTH reactions, indicating that these methodologies may be capable of measuring independent immunological events (Table 2). From a clinical perspective, five stage IV melanoma patients remained stable for more than 20 months; nine patients showed a progression of the disease, and two died prior to final injection (Table 2). Stage IV patients who showed positive DTH reaction to tumour cell lysate (n = 8) had a significantly longer median TTP (13·37 months) than DTH non-reactive patients (n = 8) (2·37 months) (P = 0·01362) (Fig. 5b, Table 2). Similarly, stage IV DTH-responder patients showed a statistically significant (P = 0·0261) post-vaccination survival (17·25 months) compared to non-responders (8·62 months), as defined in Materials and methods (Fig. 5c, Table 2). In contrast, no significant differences were found between the groups with respect to ELISPOT reactivity and TTP or patient survival, indicating that the detected frequency of tumour-reactive T cells in blood is a less optimal response indicator than in vivo DTH reactions. However, the historically high variability of stage IV melanoma patient survival and the low number of patients studied here may not allow us to conclude that the observed correlations should have a major clinical relevance. Finally, partial regression of local breast subcutaneous metastasis was observed in patient CT001, and cutaneous partial regression was also observed in patient CT005 (data not shown). We did not observe significant differences in immunological or clinical responses between patients treated with IL-2 with respect to those treated with DC vaccines alone. No vitiligo or other autoimmune responses were observed in vaccinated patients. In summary, the administration of tumour lysate-pulsed DCs is non-toxic and capable of inducing specific immunological response to tumour antigens.
Acknowledgments
This work was supported by grants from the Fund for the Promotion of Scientific and Technological Development (FONDEF DO2I1088), the National Fund for Scientific and Technological Development, Chile (FONDECYT 1031005) and the Department of Research and Development of the University of Chile (DID). We thank Marisol Briones, Felix González and Manuel Salazar for technical help.
References
- 1.Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292–302. doi: 10.1016/S1470-2045(04)01467-6. [Review] [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Lotze MT, Yang JC, et al. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol. 1989;7:1863–74. doi: 10.1200/JCO.1989.7.12.1863. [DOI] [PubMed] [Google Scholar]
- 3.Lotze MT, Matory YL, Rayner AA, et al. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986;58:2764–72. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumour-infiltrating lymphocytes. Science. 1986;233:1318–22. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 6.Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255–60. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paglia C, Chiodoni M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumour antigen in vivo. J Exp Med. 1996;183:317–22. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W, Kong HL, Carpenter H, et al. Crystal dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumour immunity. J Exp Med. 1997;186:1247–56. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toes R, Ellen I, Van der Voort H, et al. Enhancement of tumour outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160:4449–56. [PubMed] [Google Scholar]
- 11.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumour lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 12.Baar J. Clinical applications of dendritic cell cancer vaccines. Oncologist. 1999;4:140–4. [PubMed] [Google Scholar]
- 13.Schuler-Thurner B, Dieckmann D, Keikavoussi P, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–6. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- 14.Lau R, Wang F, Jeffery G, et al. Phase I trial intravenous peptide-pulsed dendritic cell as in patients with metastatic melanoma. J Immunother. 2001;24:66–78. doi: 10.1097/00002371-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Thurner B, Haendle I, Roder C, et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koski GK, Kariko K, Xu S, Weissman D, Cohen PA, Czerniecki BJ. Innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J Immunol. 2004;172:3989–93. doi: 10.4049/jimmunol.172.7.3989. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S, Kagamu H, Yoshizawa H, et al. The duration of signaling through CD40 directs biological ability of dendritic cells to induce antitumour immunity. J Immunol. 2003;171:5828–36. doi: 10.4049/jimmunol.171.11.5828. [DOI] [PubMed] [Google Scholar]
- 18.de Vrics IJ, Eggert AA, Scharenborg NM, et al. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429–38. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svane IM, Soot ML, Buus S, Johnsen HE. APMIS. Vol. 111. 2003. Clinical application of dendritic cells in cancer vaccination therapy; pp. 818–34. [Review] [DOI] [PubMed] [Google Scholar]
- 21.Berger TG, Feuerstein B, Strasser E, et al. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Meth. 2002;268:131–40. doi: 10.1016/s0022-1759(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 22.Gatti E, Velleca MA, Biedermann BC, et al. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J Immunol. 2000;164:3600–7. doi: 10.4049/jimmunol.164.7.3600. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumour necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banchereau J, Steiman RM. Nature. Vol. 392. 1998. Dendritic cells and the control of immunity; pp. 245–52. [Review] [DOI] [PubMed] [Google Scholar]
- 25.Morisaki T, Matsumoto K, Onishi H, et al. Dendritic cell-based combined immunotherapy with autologous tumour-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell. 2003;16:175–82. doi: 10.1111/j.1749-0774.2003.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 26.Ridgway D. Cancer Invest. Vol. 21. 2003. The first 1000 dendritic cell vaccines; pp. 873–86. [Review] [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Grimm EA, McGrogan M, et al. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984;22:1412. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- 28.Oelke M, Moehrle U, Chen JL, et al. Generation and purification of CD8+ melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin Cancer Res. 2000;6:1997–2005. [PubMed] [Google Scholar]
- 29.Dunbar PR, Chen JL, Chao D, et al. Cutting edge. rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J Immunol. 1999;162:6959–62. [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907. [PubMed] [Google Scholar]
- 31.Salazar-Onfray F, López M, Lundqvist A, et al. Tissue distribution and differential expression of Melanocortin 1 Receptor, a malignant melanoma marker. Br J Cancer. 2002;87:414–22. doi: 10.1038/sj.bjc.6600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohl L, Mohaupt M, Czeloth N, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami Y, Eliyahu S, Sakaguchi K, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumour infiltrating lymphocytes. J Exp Med. 1994;180:347–52. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfel T, Van Pel A, Brichard V, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–64. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami Y, Eliyahu S, Jennings C, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumour-infiltrating T lymphocytes associated with in vivo tumour regression. J Immunol. 1995;154:3961–8. [PubMed] [Google Scholar]
- 36.Wong EC, Maher VE, Hines K, et al. Development of a clinical-scale method for generation of dendritic cells from PBMC for use in cancer immunotherapy. Cytotherapy. 2001;3:19–29. doi: 10.1080/146532401753156377. [DOI] [PubMed] [Google Scholar]
- 37.Spisek R, Bougras G, Ebstein F, et al. Transient exposure of dendritic cells to maturation stimuli is sufficient to induce complete phenotypic maturation while preserving their capacity to respond to subsequent restimulation. Cancer Immunol Immunother. 2003;52:445–54. doi: 10.1007/s00262-002-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagorsen D, Marincola FM, Panelli MC. Cytokine and chemokine expression profiles of maturing dendritic cells using multiprotein platform arrays. Cytokine. 2004;25:31–5. doi: 10.1016/j.cyto.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Kelsall BL, Stuber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40–CD40L interactions in Th1 T cell responses. Ann NY Acad Sci. 1996;795:116–26. doi: 10.1111/j.1749-6632.1996.tb52660.x. [DOI] [PubMed] [Google Scholar]
- 40.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–18. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 41.Fedele G, Stefanelli P, Spensieri F, Fazio C, Mastrantonio P, Ausiello CM. Bordetella pertussis-infected human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization and interleukin-23 expression. Infect Immun. 2005;73:1590–7. doi: 10.1128/IAI.73.3.1590-1597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehrotra S, Chhabra A, Chakraborty A, et al. Antigen presentation by MART-1 adenovirus-transduced interleukin-10-polarized human monocyte-derived dendritic cells. Immunology. 2004;113:472–81. doi: 10.1111/j.1365-2567.2004.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114:204–12. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley WG, Widen RH, Weiser AM, et al. The novel differentiation of human blood mononuclear cells into CD1a-negative dendritic cells is stimulated in the absence of exogenous cytokines by an extract prepared from pinecones. Int Immunopharmacol. 2003;3:209–23. doi: 10.1016/S1567-5769(02)00267-9. [DOI] [PubMed] [Google Scholar]
- 45.Xia CQ, Kao KJ. Monocyte-derived CD1a+ dendritic cells generated in two different culture systems: immunophenotypic and functional comparison. Scand J Immunol. 2003;57:324–32. doi: 10.1046/j.1365-3083.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- 46.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8. [PubMed] [Google Scholar]
- 47.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salazar-Onfray F, Nakazawa T, Chhajlani V, et al. Synthetic peptides derived from the melanocyte stimulating hormone receptor MC1R can stimulate HLA-A2 restricted CTL that recognize naturally processed peptides on human melanoma cells. Cancer Res. 1997;57:4348. [PubMed] [Google Scholar]
- 49.Masucci G, Svensson A, Hansson M, et al. Efficient harvest of in vivo IL-2 activated CD3+ lymphocytes for adoptive immunotherapy by selective leukopheresis (lymphocytapheresis) J Hematother. 1997;6:253–8. doi: 10.1089/scd.1.1997.6.253. [DOI] [PubMed] [Google Scholar]