Abstract
We have reported previously that Lactobacillus casei ssp. casei, together with specific substrate dextran, exhibited an adjuvant effect of stimulating humoral immune responses against bovine serum albumin (BSA) as a model antigen in BALB/c mice. In the present study, among the Lactobacillus species tested, L. casei ssp. casei with dextran significantly elevated the natural killer (NK) cell activites in spleen mononuclear cells from BALB/c mice in comparison to L. casei ssp. casei alone or other Lactobacillus species with or without dextran. Oral administration of L. casei ssp. casei together with dextran also resulted in a significant increase of NK cell activities in healthy human volunteers. Further, L. casei ssp. casei induced significant production of interleukin (IL)-12 in human peripheral blood mononuclear cells and IL-15 mRNA expression in the human intestinal epithelial cell line Caco-2. L. casei ssp. casei with dextran in food also significantly elevated the survival rate of BALB/c mice bearing Meth-A cells. Taken together, these results demonstrate that dietary synbiotic supplementation which is a combination of the L. casei ssp. casei used as a probiotic together with the dextran, a specific substrate as a prebiotic, efficiently elicits murine and human NK cell activities.
Keywords: cytokine, dextran, Lactobacillus casei ssp. casei, natural killer, synbiotic
Introduction
Potentiation of the intestinal mucosal barrier is thought to help protect from invasion by various pathogens. Lactic acid bacteria, Gram-positive and non-pathogenic organisms found in a wide variety of fermented food products [1], have been shown to provide health promoting and beneficial therapeutic effects toward the host [2,3], and are considered to function in a probiotic manner. Probiotic bacteria have been reported to prevent and treat inflammatory bowel disease in a number of previous studies [4]. They also protect infection by competing with pathogenic bacteria for attachment to gastrointestinal epithelium and enhance mucosal immune responses to pathogens [5].
Lactic acid bacteria have been found to have a variety of beneficial effects, including the prevention of carcinogenesis and tumour growth [6]. Lactobacillus casei and L. acidophilus were shown to inhibit the growth of transplantable tumour cells in experimental animals [7–9]. Biffi et al. also demonstrated that milk fermented with L. acidophilus and L. paracasei reduced the growth of a human breast cancer cell line MCF7 in vitro[10]. In addition, up-regulation of natural killer (NK) cell activity was observed in mice administered orally with L. casei in mice [11].
The immunoregulatory cytokine interleukin (IL)-12, a 70-kDa heterodimer formed by the covalent assembly of two chains, 40 kDa and 35 kDa, mediates interferon (IFN)-γ production from T cells and NK cells, and augments their cytotoxic activity against tumour cells [12]. Therefore, IL-12-activating capacity seems to be associated with anti-tumour activity. Interleukin (IL)-15 has a 4-helix bundle structure and exhibits IL-2-like functions, such as the induction of T cell and NK cell proliferation [13]. IL-15 is able to induce bone marrow-derived CD34-positive haematopoietic progenitor cells to differentiate into CD3-negative- and CD56-positive-NK cells [14] and up-regulates NK cell cytolytic functions [15]. Further, IL-15 mRNA is expressed by non-T cells, including those found in kidney, placenta and skeletal muscle tissue, as well as by macrophages and epithelial cells, unlike IL-2, which is induced only by T cells [16]. It was also reported that intestinal epithelial cells expressed IL-15 mRNA, which was up-regulated by interferon (IFN)-γ[17].
A synbiotic is a combination of live bacteria used as a probiotic and the specific substrate used as a prebiotic, which is defined as non-digestible food ingredients that affect the host beneficially by selective stimulation of some bacterial species in the colon [18]. It has been proposed that enhancement of probiotic bacteria in the intestines provides advantages to the host [19]. It was also demonstrated that synbiotic nutritional supplements consisting of probiotic L. paracasei and prebiotic fructooligosaccharides efficiently increased NK cell activity in elderly people [20]. Recently, we have shown that L. casei ssp. casei has a specific ability to metabolize macromolecular dextran, as oral administration of the bacteria in conjunction with dextran effectively enhanced humoral immune responses to BSA in BALB/c mice [21]. In the present study, we evaluated NK cell activities of L. casei ssp. casei administered together with dextran.
Materials and methods
Bacterial strains
L. casei ssp. casei JCM 1134T, L. paracasei ssp. paracasei JCM 1053 and L. acidophilus JCM 1132T were obtained from the Japan Collection of Microorganisms (Riken Biosource Center, Saitama, Japan).
Growth experiments
Growth experiments with the Lactobacillus species were carried out at 37°C by measuring optical density at 660 nm (OD660) with a colorimeter (Digital Bench Colorimeter Model 21150; Industrial & Chemical Measurement, Hillsboro, OR, USA). Fifty microlitres of each bacterial suspension (adjusted to 0·5 McFarland standard) was inoculated into 5 ml of deMan Rogosa Sharpe (MRS) broth (BD Biosciences, Mountain View, CA, USA) supplemented with or without 2% dextran, which has a molecular weight of 10 000 (Serva, Heidelberg, Germany), after which bacterial cell growth was monitored every 12 h. During the measurement of OD660, a non-inoculated culture medium was used as a blank.
Preparation of murine mononuclear cells
Eight-week-old male BALB/c mice (Charles River Japan, Inc., Yokohama, Japan), which received pellet chow (MF diet, Oriental Yeast Industry Co., Tokyo, Japan), were divided into two groups (each containing six mice). On days 0–9, the control group was given MF powdered chow (control group) and the dextran group received MF powdered chow with 75 mg/kg of dextran supplement. Mice in both groups received oral administrations with or without 1 × 107 CFU of various Lactobacillus species by gastric intubation with the aid of an intubation needle on days 0, 1 and 2. On day 9, murine mononuclear cells (MNC) as effector cells for a 51Cr-release assay were isolated from the spleen cells using Histopaque®1082 (Sigma, St Louis, MO, USA) separation.
Preparation of human peripheral blood mononuclear cells
This experiment was performed with eight healthy adult volunteers (six males, two females; average age 34·9 years). All subjects were informed regarding the study and each signed an informed consent form approved by the Ethics Committee of Asahi University (reference number 15007). On days 1–7, each received 1 × 1010 CFU of lyophilized L. casei ssp. casei and 1 g of dextran once per day. On days 0, 8 and 11, heparinized venous blood was sampled and subjected to fractionation using a Histopaque®1077 (Sigma) to obtain human peripheral blood mononuclear cells (PBMC) as effector cells for a 51Cr-release assay.
Cell lines
NK-sensitive YAC-1 (mouse T cell leukaemia) and K562 (human erythroleukaemia) cell lines were used as target cells for 51Cr-release assays, after being maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified incubator with 5% CO2. The culture medium was replaced every 3 days with fresh medium.
The human intestinal epithelial cell line Caco-2 was maintained in Eagle's minimum essential medium (EMEM; Sigma) supplemented with 10% FBS (Sigma) and 0·1 mM of non-essential amino acids (Gibco Laboratories, Grand Island, NY, USA). Meth A fibrosarcoma of BALB/c mouse origin was maintained by serial intraperitoneal passages in syngeneic BALB/c mice. The cells were prepared just prior to the experiment with mice peritoneal cavity specimens.
51Cr release assay
A standard 4-h 51Cr release assay was used to determine necrotic death of the target cells. Briefly, 1 × 106 target cells were labelled with 50 µCi of Na251CrO4 for 1 h at 37°C. Then, the cells were washed twice and resuspended in RPMI-1640 supplemented with 10% FBS, and YAC-1 (2 × 104 cells) or K562 (1 × 104 cells) cells were aliquoted in wells in volumes of 100 µl. Effector cells in an equal volume were also added six times to give the desired effector: target (E: T) cell ratios. The plates were incubated at 37°C for 4 h and then centrifuged at 370 g for 10 min. Next, the culture supernatants were decanted and samples were counted using a counter (Auto Well Gamma System ARC-380 CL; Aloka Co., Ltd, Tokyo, Japan). Medium alone or 1% Nonidet P-40 (Sigma) was added to labelled target cells to determine spontaneous (spons) and maximum (max) release, respectively. The percentage of killed cells (% kill) was calculated using the following equation: % kill = (experimental − spons)/(max − spons) × 100%. Before measurement of the cohort members, we examined different E: T ratios (1%, 3%, 11%, 33% and 100%) in a pilot study with a small amount of cytotoxic activity. We chose the ratio of 33%, which was the ratio at which differences in cytotoxic activity between individuals were most distinguishable.
IL-12 production
Human PBMC were isolated from heparinized venous blood sampled from a healthy adult donor as described above, then washed three times with phosphate buffered saline (PBS) (Sigma) and incubated with the indicated cells of Lactobacillus species in RPMI-1640 medium with 10% FBS at 37°C for 48 h. Prior to the experiment, we confirmed the ability of Lactobacillus species to survive in this culture condition. Culture supernatants were collected and analysed by enzyme-linked immunosorbent assay (ELISA) for secreted IL-12 (e Bioscience, San Diego, CA, USA). The results were determined using a standard curve prepared for each assay.
IL-15 mRNA expression
Caco-2 cells were co-cultured with 1 × 106 CFU/ml of Lactobacillus species or stimulated with 100 ng/ml of human IFN-γ (e Bioscience) in EMEM with 10% FBS at 37°C for 4 h. Total cellular RNA from the cells was extracted using an RNAqueous-4PCR (Ambion, Austin, TX, USA) according to the manufacturer's instructions. Extracted RNA (1 µg) was then reverse-transcribed into first-strand cDNA at 42°C for 40 min according to the manufacturer's instructions. PCR amplification was performed using the following oligonucleotide specific primers: for β-actin, sense 5′-GTG GGG CGC CCC AGG CAC CA-3′ and anti-sense 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′), and for human IL-15, sense 5′-GGA TTT ACC GTG GCT TTG AGT A-3′ and anti-sense 5′-TTC CTC CAG TTC CTC ACA TTC T-3′. Next, 5 µg of cDNA from the samples was amplified with 0·2 µM of the sense and anti-sense primers for the target gene in a 50-µl reaction mixture containing 75 U/ml of Ex Taq polymerase (Takara Biochemicals, Shiga, Japan). After initial denaturation at 94°C for 2 min, 35 cycles of denaturation (94°C for 30 s), annealing (60°C for 1 min) and extension (72°C for 1 min) for IL-15, and 27 cycles of denaturation (94°C for 30 s), annealing (58°C for 1 min) and extension (72°C for 1 min) for β-actin were performed using a PCR Express Thermal Cycler (Hybaid, Middlesex, UK). As a negative control, a non-RT sample was amplified by PCR. Following PCR, 10 µl of the total amplified product was electrophoresed on an ethidium bromide-stained 1% agarose gel and fluorescence was visualized under UV light.
NK cell fraction in human PBMC
Human PBMC were labelled for analysis with fluorescein isothiocianate (FITC)-conjugated anti-human CD3 (clone UCHT1 +) and R-phycoerythrin (RPE)-conjugated anti-human CD56 (clone MOC-1) antibodies (Dako Dual-Colour Reagent, Dako, Glostrup, Denmark). The cells were then washed with PBS and fixed with 1% paraformaldehyde. The percentage of CD56-positive/CD3-negative NK cells in the lymphocyte fraction was analysed using a FACS Calibur with Cell Quest software (BD Biosciences, San Jose, CA, USA) after gating on the forward- and side-scatter profile.
Anti-tumour activity against tumour-bearing mice
The BALB/c mice that received the MF diet were divided into four groups (each containing 12 mice). Starting from 7 days before inoculation with tumour cells, the mice were given MF powdered chow (control group), MF powdered chow with 75 mg/kg of dextran supplement (dextran group), 5 × 105 CFU/day of lyophilized L. casei ssp. casei in MF powdered chow (L. casei group) or 5 × 105 CFU/day of lyophilized L. casei ssp. casei in MF powdered chow with 75 mg/kg of dextran supplement (L. casei–dextran group), and then inoculated intraperitoneally with Meth A cells at a dose of 1 × 104 cells/mouse. Survival was monitored for up to 80 days.
Statistical analysis
The normality of NK cell activities was confirmed by χ2 for goodness of fit, and the statistical significance of NK cell activities in mice and humans was quantified using unpaired and paired t-tests, respectively. Survivals were calculated starting from the day of inoculation of Meth A cells. Survival curves were drawn according to the Kaplan–Meier method, with differences analysed by a log-rank test. The significance level was set at 5%.
Results
Cell growth of Lactobacillus species in the presence of dextran
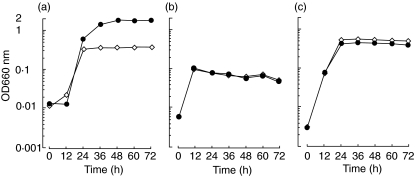
To evaluate the ability of dextran to be utilized with L. casei ssp. casei, L. paracasei ssp. paracasei and L. acidophilus, we monitored cell growth by measuring OD660. Increased cell growth with the addition of dextran was observed in the case of L. casei ssp. casei(Fig. 1). In contrast, L. paracasei ssp. paracasei and L. acidophilus showed almost identical cell growth patterns, regardless of the presence or absence of dextran.
Fig. 1.
Cell growth of Lactobacillus species in the presence or absence of dextran. Fifty microlitres of a bacterial suspension of Lactobacillus casei ssp. casei (a), L. paracasei ssp. paracasei (b) or L. acidophilus (c) (adjusted to 0·5 McFarland standard) was inoculated onto 5 ml of deMan Rogosa Sharpe (MRS) broth supplemented with (closed circle) or without (open diamond) 2% dextran, and then incubated aerobically at 37°C. Bacterial cell growth was monitored by measuring optical density at 660 nm (OD660) with a colorimeter every 12 h. During the measurement of OD660, a non-inoculated culture medium was used as a blank. Experiments were done at least three times and representative results are presented.
Effects of L. casei ssp. casei on NK cell activities in dextran-fed BALB/c mice
To examine the effects of Lactobacillus species with or without dextran toward host cells, we investigated NK cell activities in BALB/c mice administered orally with Lactobacillus species (Fig. 2). All the bacteria tested significantly increased the level of NK cell activity in non-dextran-fed mice. Among them, oral administration of L. casei ssp. casei, but not L. paracasei ssp. paracasei or L. acidophilus, resulted in a significant increase of NK cell activity in dextran-fed mice compared with non-dextran-fed mice. These results suggest that the specific utilizing ability of dextran by L. casei ssp. casei contributes to an up-regulation of NK cell activity.
Fig. 2.
Effects of Lactobacillus casei ssp. casei on natural killer (NK) cell activities in dextran-fed mice. BALB/c mice received MF powdered chow (non-dextran-fed) or MF powdered chow with 75 mg/kg of dextran supplement (dextran-fed), and then were administered orally with or without 107 colony-forming units (CFU) of various Lactobacillus species by gastric intubation on days 0, 1 and 2. On day 9, mononuclear cells (MNC) were isolated from spleen cells and NK cell activities were measured as described in Materials and methods. The mean values were significantly different from non-administration in non-dextran-fed mice (**P < 0·01, *P < 0·05) and administration of L. casei ssp. casei in non-dextran-fed mice (†P < 0·01).
IL-12 production by human PBMC co-cultured with L. casei ssp. casei
It has been reported previously that administration of Lactobacillus species killed by UV radiation induced IL-12 production by human PBMC [22]. In the present study, we examined the IL-12-producing activity in human PBMC co-cultured with Lactobacillus species. We found that L. casei ssp. casei induced greater IL-12 production in a cell number-dependent manner compared with L. paracasei ssp. paracasei and L. acidophilus(Fig. 3).
Fig. 3.
Interleukin (IL)-12 production by human peripheral blood mononuclear cells (PBMC) co-cultured with Lactobacillus casei ssp. casei. The cells were co-cultured with the indicated doses of live L. casei ssp. casei, L. paracasei ssp. paracasei or L. acidophilus for 48 h. IL-12 production was analysed by enzyme-linked immunosorbent assay (ELISA). Data are shown as the mean ± s.e.m. of three independent experiments.
Expression of IL-15 mRNA in human intestinal epithelial cells co-cultured with L. casei ssp. casei
Because intestinal epithelial cells have been reported to express IL-15 [17], we examined IL-15 mRNA expression in Caco-2 cells co-cultured with Lactobacillus species by reverse transcription-polymerase chain reaction (RT-PCR). Caco-2 cells constitutively expressed IL-15 mRNA, and all the tested bacteria as well as IFN-γ clearly induced the expression (Fig. 4). RT-PCR analysis of β-actin expression confirmed the quality of all RNA preparations and no band was detected for the non-RT sample by PCR (data not shown).
Fig. 4.
Interleukin (IL)-15 mRNA expression in Caco-2 cells co-cultured with Lactobacillus casei ssp. casei. The cells were co-cultured with 1 × 106 colony-forming units (CFU)/ml of Lactobacillus species for 4 h. Interferon (IFN)-γ (100 ng/ml) was used as a positive control stimulant. IL-15 mRNA expression was analysed by reverse transcription-polymerase chain reaction (RT-PCR), with β-actin assayed as a positive control. Lanes: M, 100 base pairs (bp) ladder marker; 1, Caco-2 cell alone; 2, L. casei ssp. casei; 3, L. paracasei ssp. paracasei; 4, L. acidophilus; and 5, IFN-γ.
Effects of feeding of L. casei ssp. casei with dextran on NK cell activities in humans
To investigate the effects of oral L. casei ssp. casei together with dextran, we examined NK cell activities in eight healthy human volunteers (Fig. 5). The average NK cell activity was 28·7 ± 9·8% (range 12·7–45·3%) on 1 day before supplementation, which increased to 43·9 ± 15·9% (range 19·9–64·1%) on day 8, 1 day after finishing supplementation for 7 days. A significant increase was also observed on day 11, 4 days after finishing the supplementation period (41·7 ± 13·2%; range 17·9–62·0%). Interestingly, the percentage of NK cells in the peripheral blood lymphocyte fractions showed a tendency to continue increasing after supplementation (data not shown). These results suggest that orally ingested L. casei ssp. casei together with dextran facilitates the number and ability of NK cells in human.
Fig. 5.
Effects of orally ingested Lactobacillus casei ssp. casei together with dextran on human natural killer (NK) cell activities. Eight healthy adult volunteers received 1 × 1010 colony-forming units (CFU) of lyophilized L. casei ssp. casei and 1 g of dextran once per day from days 1–7. On days 0, 8 and 11, peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood and NK cell activities were measured as described in Materials and methods. The mean values were significantly different from experimental day 0 (**P < 0·01, *P < 0·05).
Anti-tumour activities of L. casei ssp. casei with dextran
Figure 6 shows that the results for BALB/c mice that survived the trials using autologous Meth A cells. The survival rate of the control group was 8·3%, whereas that of the dextran and L. casei groups was 33·3%, respectively. Furthermore, the L. casei–dextran group showed a significant increase in survival rate (50%) compared to the others. These results indicated that feeding of L. casei ssp. casei together with dextran markedly elevated anti-tumour activities.
Fig. 6.
Effects of feeding Lactobacillus casei ssp. casei together with dextran on the survival rate of Meth A-bearing mice. BALB/c mice received MF powdered chow (control group), MF powdered chow with dextran (dextran group), lyophilized L. casei ssp. casei in MF powdered chow (L. casei group) or lyophilized L. casei ssp. casei in MF powdered chow with dextran (L. casei–dextran group), and then were inoculated intraperitoneally with Meth A cells at a dose of 1 × 104 cells/mouse. A P-value of 0·029 was determined using a Kaplan–Meier product limited-survival analysis between the L. casei–dextran and control groups.
Discussion
We have demonstrated previously that L. casei ssp. casei is specifically capable of utilizing dextran [21]. It has been also shown that oral administration of the bacteria in conjunction with dextran effectively augments humoral immune responses in mice. In the initial experiment of this study, we investigated the effects of dextran on bacterial cell growth of three Lactobacillus species using a colorimeter (OD660). Those results showed that L. casei ssp. casei could utilize dextran and additional growth was seen, compared with in the absence of dextran (Fig. 1). Therefore, we concluded that dextran supplementation plays an important role in the further growth of L. casei ssp. casei.
It has been demonstrated previously that an intravenous injection of L. casei LC 9018 augmented the NK cell activities of spleen cells in BALB/c mice [23]. Takagi et al. also showed that oral administration of L. casei Shirota enhanced NK cell activity and caused a delay of 3-methylcholanthrene-induced carcinogenesis in mice [24]. In the present experiments, we found that oral administration of L. casei ssp. casei JCM 1134T augmented NK cell activities in BALB/c mice, while dextran supplementation resulted in further increases of only the activities of L. casei ssp. casei (Fig. 2). These results indicate that the dextran-utilizing ability of L. casei ssp. casei contributes to further augmentation of NK cell activity (Figs 1 and 2).
Little is known about the mechanisms of increased NK cell activity by oral administration of Lactobacillus species. It was reported that L. plantarum, L. rhamnosus and L. paracasei ssp. paracasei activated human PBMC to induce IL-12 [22,25]. The major biological activities of IL-12 are directed toward T cells and NK cells, in which it increases cytokine production, proliferation and cytotoxicity [26]. Thus, IL-12 production is considered to be related closely to NK cell activity. Our data also showed that L. casei ssp. casei clearly induced IL-12 production by human PBMC (Fig. 3). In addition, we demonstrated that L. casei ssp. casei as well as the other Lactobacillus species used in this study induced IL-15 mRNA expression in the human intestinal epithelial cell line Caco-2 (Fig. 4). Reinecker et al. also reported that IL-15 mediated the proliferation of Caco-2 cells [17], while IL-15 induced by intestinal epithelial cells was shown to mediate the activation of intestinal intraepithelial NK cells [27]. Together, these results suggest that the cytokines induced by L. casei ssp. casei are related closely to the activation of NK cells.
Dietary probiotic supplementation, such as food fermented with lactic acid bacteria, has been demonstrated to have health-promoting effects through improvement of the intestinal microflora and host immune system [3]. It was demonstrated that daily intake of fermented milk containing L. casei DN114001 for 8 weeks enhanced the innate immune defence in healthy middle-aged humans [28]. Gill et al. also showed that dietary consumption of milk supplemented with L. rhamnosus and Bifidobacterium lactis for 3 weeks enhanced NK cell activity in elderly people [29]. Furthermore, oral supplementation of a synbiotic diet containing fructooligosaccharides, along with L. acidophilus and Bifidobacterium species, was reported to dramatically facilitate weight gain in acutely ill children receiving antibiotics [30]. Femia et al. also demonstrated that the prebiotic inulin in conjunction with probiotics L. rhamnosus and B. lactis efficiently exerted protective effects on azoxymethane-induced colon cancer in rats [31]. In the present study, oral administration of L. casei ssp. casei together with dextran resulted in a further increase of NK cell activities in humans and mice (Figs 2 and 5), as well as of the percentage of NK cells in the lymphocyte fraction in healthy human volunteers (data not shown). Furthermore, Meth A-bearing BALB/c mice, which received L. casei ssp. casei together with dextran, had a greatly increased survival rate compared with the control group (Fig. 6). Together, these results suggest that oral supplementation of synbiotic materials enhance host immune functions and augment anti-tumour activities.
In conclusion, our results indicate clearly that a new synbiotic supplement consisting of the probiotic L. casei ssp. casei and the prebiotic dextran would be highly efficacious when given as an oral immunoadjuvant. Moreover, dextran, which is utilized specifically by L. casei ssp. casei, appears to play a pivotal role as both a selective prebiotic material and adjuvant.
Acknowledgments
We thank Mr Mark Benton for his critical reading of the manuscript.
References
- 1.Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85:88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- 2.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–78. [PubMed] [Google Scholar]
- 3.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82:279–89. [PubMed] [Google Scholar]
- 4.Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm Bowel Dis. 2000;6:107–15. doi: 10.1097/00054725-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001;73:S1124–30. doi: 10.1093/ajcn/73.6.1124S. [DOI] [PubMed] [Google Scholar]
- 6.de Roos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr. 2000;71:405–11. doi: 10.1093/ajcn/71.2.405. [DOI] [PubMed] [Google Scholar]
- 7.Asano M, Karasawa E, Takayama T. Antitumor activity of Lactobacillus casei (LC 9018) against experimental mouse bladder tumor (MBT-2) J Urol. 1986;136:719–21. doi: 10.1016/s0022-5347(17)45035-x. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki T, Hashimoto S, Yokokura T. Effects on antitumor activity and cytokine production in the thoracic cavity by intrapleural administration of Lactobacillus casei in tumor-bearing mice. Med Microbiol Immunol (Berl) 1996;185:157–61. doi: 10.1007/s004300050026. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh GH, Royle PJ, Playne MJ. A probiotic strain of L. acidophilus reduces DMH-induced large intestinal tumors in male Sprague–Dawley rats. Nutr Cancer. 1999;35:153–9. doi: 10.1207/S15327914NC352_9. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, Coradini D, Larsen R, Riva L, Di Fronzo G. Antiproliferative effect of fermented milk on the growth of a human breast cancer cell line. Nutr Cancer. 1997;28:93–9. doi: 10.1080/01635589709514558. [DOI] [PubMed] [Google Scholar]
- 11.Hori T, Kiyoshima J, Yasui H. Effect of an oral administration of Lactobacillus casei strain Shirota on the natural killer activity of blood mononuclear cells in aged mice. Biosci Biotechnol Biochem. 2003;67:420–2. doi: 10.1271/bbb.67.420. [DOI] [PubMed] [Google Scholar]
- 12.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 13.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 15.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 17.Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–13. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 18.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 19.Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 20.Bunout D, Barrera G, Hirsch S, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parenter Enteral Nutr. 2004;28:348–54. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Asai Y, Yasuda K, Sakamoto H. Oral immunoadjuvant activity of a new synbiotic, Lactobacillus casei subsp. casei in conjunction with dextran in BALB/c mice. Nutr Res. 2005;25:295–304. [Google Scholar]
- 22.Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–82. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;27:209–17. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 24.Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599–605. doi: 10.1093/carcin/22.4.599. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–62. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–11. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita N, Hiroi T, Ohta N, Fukuyama S, Park EJ, Kiyono H. Autocrine IL-15 mediates intestinal epithelial cell death via the activation of neighboring intraepithelial NK cells. J Immunol. 2002;169:6187–92. doi: 10.4049/jimmunol.169.11.6187. [DOI] [PubMed] [Google Scholar]
- 28.Parra MD, Martinez de Morentin BE, Cobo JM, Mateos A, Martinez JA. Daily ingestion of fermented milk containing Lactobacillus casei DN114001 improves innate-defense capacity in healthy middle-aged people. J Physiol Biochem. 2004;60:85–91. doi: 10.1007/BF03168444. [DOI] [PubMed] [Google Scholar]
- 29.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly. an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264–71. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 30.Schrezenmeir J, Heller K, McCue M, et al. Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections. Clin Pediatr. 2004;43:239–49. doi: 10.1177/000992280404300305. [DOI] [PubMed] [Google Scholar]
- 31.Femia AP, Luceri C, Dolara P, et al. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–60. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]