Abstract
Activation of the complement system contributes to the pathogenesis of ischaemia/reperfusion (I/R) injury. We evaluated inhibition of the classical pathway of complement using C1-inhibitor (C1-inh) in a model of 70% partial liver I/R injury in male Wistar rats (n = 35). C1-inh was administered at 100, 200 or 400 IU/kg bodyweight, 5 min before 60 min ischaemia (pre-I) or 5 min before 24 h reperfusion (end-I). One hundred IU/kg bodyweight significantly reduced the increase of plasma levels of activated C4 as compared to albumin-treated control rats and attenuated the increase of alanine aminotransferase (ALT). These effects were not better with higher doses of C1-inh. Administration of C1-inh pre-I resulted in lower ALT levels and higher bile secretion after 24 h of reperfusion than administration at end-I. Immunohistochemical assessment indicated that activated C3, the membrane attack complex C5b9 and C-reactive protein (CRP) colocalized in hepatocytes within midzonal areas, suggesting CRP is a mediator of I/R-induced, classical complement activation in rats. Pre-ischaemic administration of C1-inh is an effective pharmacological intervention to protect against liver I/R injury.
Keywords: classical complement, inhibition, liver ischaemia, rat model, reperfusion injury
Introduction
Normothermic ischaemia of the liver leads to hepatocellular injury which, depending on the time of ischaemia, is aggravated by restoration of oxygenated blood flow (reperfusion). Pathogenic mechanisms involved in I/R injury have been extensively investigated. Studies in models of myocardial [1–3] intestinal [4], renal [5] and hepatic [4,6–8] I/R have provided evidence for the role of complement in the pathogenesis of I/R injury [9].
The effect of complement activation during liver I/R is diverse. Several complement components become localized in ischaemic liver [7,10–12], presumably facilitating opsonization of injured cells for phagocytosis [13]. Furthermore, plasma levels of activated complement components are increased after liver I/R in pigs [12] and humans [10]. Complement activation products as the anaphylatoxins and the terminal complement membrane attack complex (MAC) have deleterious effects on the liver and contribute to neutrophil activation, vasoconstriction, impaired microcirculation, increased vascular permeability and cell lysis. In rats, depletion of complement before ischaemia has shown to attenuate superoxide generation by Kupffer cells and accumulation of neutrophils in the liver during reperfusion, thereby suppressing liver I/R injury [14]. Inhibition of the complement pathways by administration of soluble complement receptor type 1 (sCR1) just after the onset of liver ischaemia, significantly reduces endothelial complement C3 deposition and the amount of liver necrosis [7]. Taken together, these studies demonstrate that reperfusion of the ischaemic liver may induce activation of complement. Hence, inhibition of the complement cascade presents a potential strategy to reduce the inflammatory damage in the postischaemic, reperfused liver [9].
Main activators of complement by the classical pathway are IgM or IgG antibody-antigen complexes, whereas bacteria trigger the alternative and lectin pathways [15,16]. Also, cytokines [17], the release of intracellular proteins [18,19] and reactive oxygen species [20,21] may be involved in triggering the activation of complement. The molecular mechanism of the observed activation of complement during liver I/R is not clear. A previous study from our department in humans suggests a contribution of the acute phase protein C-reactive protein (CRP) [10].
Although some studies show that the activation of complement in ischaemic liver occurs via the alternative pathway [7,14,22], the involvement of C4, and hence the classical pathway has been demonstrated in human liver [10]. C1-inhibitor (C1-Inh) is a member of the serine protease inhibitor (serpin) family and is a major inhibitor of the classical complement pathway [23]. Because of its anti-inflammatory properties, C1-inh has been evaluated in various animal models for diseases such as sepsis [24] and myocardial infarction [25–27]. These studies have yielded promising results, and initial studies with this compound in patients with these diseases have been started [28,29]. A recent, concise study reports that reperfusion-related microcirculatory disorders were minimized by C1-inh in rat liver [30]. In the present study, the role of the classical pathway in liver I/R and possible therapeutic use of C1-inh for reduction of liver I/R injury was investigated. Purified human C1-inh was administered at different dosages and at different time points in an in vivo rat model of partial liver ischaemia. A possible mechanistic role of CRP in the initiation of complement activation was investigated by measurement of specific CRP-complement complexes in plasma [31].
Materials and methods
Animals
This study was approved by the Animal Experiment Committee of the Academic Medical Centre, University of Amsterdam, the Netherlands. Male Wistar rats (n = 35; n = 5 per group; 325–375 g) were purchased from Broekman (Someren, the Netherlands). All rats were allowed to adapt to the laboratory environment for 7 days with free access to water and standard laboratory chow (Hope Farms, Woerden, the Netherlands). Rats were housed under standard environmental conditions with a 12-h light/dark cycle. Before use in experiments, rats were fasted overnight with free access to water.
Anaesthesia
All animals were anaesthetized via inhalation of a mixture of O2: N2O (1: 1 l/min) and isoflurane 2–3% (Florene®, Abbott Laboratories Ltd, Queensborough, Kent, UK). After endotracheal intubation, rats were ventilated (Zoovent ventilator, Instruvet, Amerongen, the Netherlands) and anaesthesia was maintained with the same mixture. Adequate ventilation was verified by continuous monitoring of end-tidal CO2, assuring physiological pH during the entire procedure [32]. A silicone catheter (diameter 0.9 mm) was introduced into the left carotid artery and tunnelled subcutaneously to the back of the rats for the assessment of haemodynamic parameters during operation as well as for withdrawal of blood samples and injection of C1-inh, albumin or saline. Arterial blood pressure was maintained at approximately pre-operative levels by adjustment of the isoflurane levels. The animals were kept in supine position on a heating pad and rectal temperature was controlled at 37 °C with the use of a heating lamp [33].
Operative techniques
A midline laparotomy was performed. After dissection of the falciform ligament, the afferent vessels to the median and left lateral lobes were exposed by turning the hepatic lobes upwards. An a-traumatic vascular clip was applied to these vessels to induce partial hepatic ischaemia (70%) for 60 min, after which the clip was removed and subsequent reperfusion initiated. After surgical closure of the abdomen, the rat was allowed to regain consciousness and was provided with water and food.
After 24 h of reperfusion the rat was anaesthetized again for re-laparotomy. A cannula (diameter 0.4 mm) was inserted into the distal bile duct and bile was collected during 15 min as a parameter of hepatocyte function. Afterwards the rat was sacrificed under anaesthesia by haemorrhage, liver biopsies were taken, frozen in liquid nitrogen and stored at −80 °C or fixed in 4% (W/V) formaldehyde for future analyses.
Intervention
Five minutes before ischaemia (pre-I) or 5 min before reperfusion (at the end of ischaemia, end-I), C1-inh (Sanquin, Amsterdam, the Netherlands) at 100 IU/kg, 200 IU/kg or 400 IU/kg was administered intravenously. As a control 200 IU/kg human albumin (Cealb®, Sanquin) was administered intravenously end-I.
Blood sampling
Blood samples of 500 µl were collected prior to induction of ischaemia, after 90 min, 6 h and 24 h of reperfusion in tubes containing lithium heparin or K2EDTA. Blood was centrifuged (10 min at 2000× g at 4 °C). Aliquots of plasma were stored at −80 °C for further analysis.
Assessment of hepatocellular injury
Alaline aminotransferase (ALT) activity in heparin plasma was determined by routine spectrophotometry using alpha-ketoglutaric acid and pyridoxal phosphate (General Clinical Chemistry Laboratory, AMC, Amsterdam. the Netherlands).
ELISA
Functional human C1-Inh was detected as described before [34]. Briefly, C1-Inh was bound to plates coated with mAb RII against human C1-Inh [35] and detected with biotinylated C1s [36]. It should be noted that this end-stage assay measures the number of functional C1-Inh molecules and not the kinetics of the interaction between C1s and C1-Inh. Results were compared to those of normal plasma pool (NMP).
Activated rat C4 was detected with a novel ELISA [37]. Briefly, an IgG fraction of sheep polyclonal Ab against human C4 (SHC4; Department of Immune Reagents; CLB, Amsterdam, the Netherlands), which cross-reacts with activated rat C4 [37], was incubated at 2 µg/ml, final volume 100 µl, in 0·1 M carbonate/bicarbonate, pH 9·6, overnight at room temperature in Maxisorb plates (Nunc). Plates were washed with PBS 0·02% (w/v) Tween 20 (PBS-Tween). Rat plasma samples were appropriately diluted in PBS-Tween containing 0·2% (w/v) gelatin and 10 mM EDTA. One hundred µl of each dilution was incubated for 1 h at 4 °C, the plates being gently shaken. The plates were washed 5 times in PBS-Tween and incubated with biotinylated IgG fraction of SHC4, diluted in PBS-Tween-0·2% gelatin (PTG), for 1 h at room temperature. Plates were then washed 5 times in PBS-Tween and incubated with streptavidin-polymerized-HRP (Business Unit Reagents, Sanquin) 1: 10 000 diluted in PBS containing 2% (v/v) cow milk) for 25 min at room temperature. Finally, the plates were developed with 3, 3′, 5, 5′-tetramethylbenzidine (0·1 mg/ml in 0·11 M NaAc, pH 5·5, 0·003% H2O2) and stopped by addition of H2SO4. Absorption was measured at 450 nm. Levels of activated C4 in the plasma samples tested were compared to those in aged normal rat serum (NRA), which was used as an in house standard. NRA was prepared by incubating normal rat serum for 7 days at 37 °C in the presence of sodium azide.
Histochemistry for C1-inh, C3, C5b9 and CRP
Immune peroxidase labelling of human C1-inh, rat complement fragments C3 and C5b9 and CRP was performed on acetone-fixed frozen sections (8 µm) using mAbs mouse anti-human C1-inh (1: 40, clone RII IgG1; Sanquin Research), mouse anti-rat C3 (ED11, Serotec), mouse anti-rat C5b-9 (1: 40, clone 2A1, a kind gift of Dr W. Couser), rabbit anti-rat CRP (1: 100; affinity purified and absorbed with rat plasma depleted for CRP) and mouse anti-rat IgG (1: 40, Caltag, Burlingame, CA) as a control antibody for aspecific binding. Primary antibodies were detected with peroxidase-labelled rabbit anti-mouse IgG (1: 200, DAKO, Glostrup, Denmark) or peroxidase-labelled goat anti-rabbit IgG (1: 100, DAKO). Peroxidase activity was visualized by incubating the sections for 12 min with a medium containing 0·5 mg/ml diaminobenzidine (DAB), 10 µM hydrogen peroxide and 50 mM Tris-HCL buffer (pH 7·6). All antibodies were diluted in PTG buffer (PBS, 0·2% gelatin 0·02% Tween 20).
To demonstrate colocalization, single sections of I/R liver were sequentially stained for CRP and C5b9. Immunostaining for CRP was performed as described above and followed by intensive rinsing with double distilled water and PBS, incubation with mouse anti-rat C5b-9 and peroxidase-labelled rabbit anti-mouse IgG as described above and visualization (12 min) with 4-chloro-1-naphthol (2·24 mM, Sigma) dissolved in 0·2% dimethyl formamide and 0·3% ethyl alcohol, 10 µM hydrogen peroxide and 50 mM Tris-HCl buffer (pH 7·6).
Statistical analysis
Results are expressed as mean ± SEM. A paired Student t-test for analysis of matched data and one-way anova followed by a Newman-Keuls post-test for analyses between groups were performed using GraphPad Prism version 3·02 for Windows (GraphPad Software, San Diego California USA). A P-value < 0·05 was considered significant.
Results
Bile secretion
Bile secretion after 24 h of reperfusion was higher in all rats treated with any dose of C1-inh before induction of ischaemia as compared to albumin-treated rats. Rats treated with 200 IU/kg C1-inh before ischaemia (pre-I) showed better bile secretion than rats treated with the same dose at the end of ischaemia (end-I). Only treatment with 400 IU/kg of C1-inh at the end of the ischaemic period resulted in a significantly improved bile secretion when compared to albumin-treated rats (Fig. 1).
Fig. 1.
Bile secretion (means ± SEM) measured during 15 min after 24 h of reperfusion (24 h R). Rats were treated with albumin (white bar), 100 IU/kg C1-inh pre-I (black bar), 100 IU/kg C1-inh end-I (black chequered bar), 200 IU/kg C1-inh pre-I (dark grey bar), 200 IU/kg C1-inh end-I (dark grey chequered bar), 400 IU/kg C1-inh pre-I (light grey bar) and 400 IU/kg C1-inh end-I (light grey chequered bar). All rats treated with C1-inh pre-I, and rats treated with 400 IU/kg of C1-inh end-I, showed higher bile secretion than albumin treated rats. *significant versus albumin treated rats, P < 0·01. Rats treated with 200 IU/kg C1-inh pre-I showed better bile secretion than rats treated with the same dose at the end of ischaemia. ♯significant versus 200 IU pre-ischaemia, P < 0·05. Previous studies showed normal baseline levels of bile production in nonischaemic rats of 0·018 ml/min [32].
Hepatocellular injury
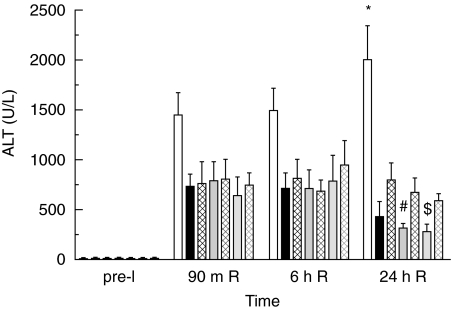
The increase of plasma ALT levels in rats treated with C1-inh was significantly less than that in the albumin-treated rats. Only after 24 h of reperfusion did this reduction in ALT levels reach statistical significance (P < 0·001) in all experimental groups. After 24 h of reperfusion, rats treated with either 200 or 400 IU/kg of C1-inh before ischaemia showed significantly lower ALT levels when compared to rats treated with 100, 200 or 400 IU/kg of C1-inh at the end of the ischaemic period (Fig. 2).
Fig. 2.
Plasma ALT (means ± SEM) measured pre-ischaemia (pre-I), after 90 m of reperfusion (90 m R), 6 h of reperfusion (6 h R) and 24 h of reperfusion (24 h R) in rats receiving albumin or C1-inh. After 24 h R, ALT levels in C1-inh treated rats were significantly lower than in albumin-treated rats (*P < 0·001) and ALT levels were lower in rats treated with C1-inh before ischaemia than in rats treated at the end of ischaemia (♯, $ significant versus end-ischaemia treated rats, P < 0·05). Bars are coded as described for Fig. 1.
Functional C1-inh
Administration of either 100, 200 or 400 IU/kg of human C1-inh resulted in an increase of plasma C1-inh levels by 1·9, 3·8 and 6·2 IU per ml, respectively (one IU is the amount present in pooled human plasma). Human C1-inh was not found in the rats treated with albumin. Plasma C1-inh levels between groups treated with either 100, 200 or 400 IU/kg of C1-inh remained significantly different during 24 h of reperfusion (Fig. 3). No differences in C1-inh levels existed between rats treated with equivalent amounts of C1-inh administered either before ischaemia or at the end of the ischaemic period. Mean plasma half-life of C1-inh was 4·4 h.
Fig. 3.
Percentage of functional C1-inh detected with ELISA compared to normal plasma pool (NMP) measured pre-ischaemia (pre-I), after 90 m of reperfusion (90 m R), 6 h of reperfusion (6 h R) and 24 h of reperfusion (24 h R) in rats receiving albumin or C1-inh. Plasma C1-inh levels between groups treated with either 100, 200 or 400 IU/kg of C1-inh remained significantly different during 24 h R (significance not shown). No differences were found between rats treated with equivalent amounts of C1-inh administered either pre-I or post-I. Bars represent means ± SEM. Bars are coded as described for Fig. 1.
Complement activation
All rats treated with C1-inh, irrespective of dosage and either administered before or at the end of ischaemia, had lower plasma levels of activated C4 after 90 min and 6 h of reperfusion as compared to the albumin-treated rats. After 24 h of reperfusion, no significant differences in activated C4 levels between C1-inh- and albumin-treated rats were observed (Fig. 4). Although activated C4 levels after 90 min of reperfusion were dissimilar in rats treated with equivalent amounts of C1-inh either before or at the end of ischaemia, statistical significance was not reached (C1-inh pre-I versus C1-inh end-I, 100 IU/kg P = 0·18, 200 IU/kg P = 0·09, 400 IU/kg P = 0·06). Complete inhibition of the classical pathway of complement could not be achieved with any dosage of C1-inh, since all rats still had a significant increase of activated C4 levels after 90 min of reperfusion when compared to pre-ischaemic levels. However, after 6 h of reperfusion, activated C4 levels in the C1-inh-treated experimental groups were not different when compared with pre-ischaemic levels. After 24 h of reperfusion activated C4 levels in several C1-inh-treated experimental groups were higher than pre-ischaemic levels. In albumin-treated rats activated C4 levels were consistently higher during reperfusion as compared to pre-ischaemic levels (Fig. 4).
Fig. 4.
Percentage of activated C4 detected with Elisa (means ± SEM) compared to aged normal rat plasma (NRA) measured pre-ischaemia (pre-I), after 90 m of reperfusion (90 m R), 6 h of reperfusion (6 h R) and 24 h of reperfusion (24 h R). Activated C4 levels in albumin-treated rats were higher during all time-points of reperfusion as compared to pre-ischaemic levels. After 90 min and 6 h of reperfusion, all rats treated with C1-inh had lower plasma levels of activated C4 as compared to the albumin-treated rats. Complete inhibition of complement activation could not be achieved since all rats after 90 min of reperfusion still had significantly increased activated C4 levels as compared to pre-ischaemic levels. *significantly different from C1-inh treated rats (P < 0·001); $significantly different from pre-I levels (P < 0·05). Bars are coded as described for Fig. 1.
Immune histochemistry for IgG, C1-inh, C3, C5b9 and CRP
In frozen sections of ischaemic-reperfused liver after control immune histochemistry without primary antibody, focal light brown staining was found on pericentral and midzonal hepatocytes, likely due to nonspecific adhesion of secondary antibodies and staining with the chromogen DAB. Upon incubation with rat IgG protein, as a control for nonspecific adhesion of plasma proteins to injured cells, a similar light brown staining in ischaemic foci was observed. In all ischaemic livers, intense dark brown staining was observed in granulocytes, showing high levels of endogenous peroxidase and nonspecific conversion of DAB colour product (Figs 5a,b).
Fig. 5.
Immune histochemistry for IgG (a,b), complement C3 (c,d) and C5b9 (e,f) and CRP (g,h) on a series of frozen sections of normal liver (a,c,e,g) and liver after 60 min of ischaemia and 24 h of reperfusion (b,d,f,h). In pericentral and midzonal areas, hepatocytes show clear staining for C5b9 and CRP (arrowheads). After ischaemia/reperfusion, complement C3 was mainly increased at the plasma membrane of hepatocytes (insert in d, arrowheads). No cytoplasmic staining of native C3 is observed in normal (c) or ischaemic-reperfused livers (d). Arrows point at granulocytes showing nonspecific, endogenous peroxidase staining. (cv, central vein; pv, portal vein; original magnification with 10× objective and for inserts 40× objective).
Human C1-inh was never demonstrated in livers at 24 after administration of the compound, neither on sinusoidal endothelium nor on hepatocytes or any other constituent of the liver. Rat complement C3 was found on the hepatocyte plasma membrane both in normal and ischaemic-reperfused livers. Occasionally, C3 staining was more intense in the cytoplasm of hepatocytes in pericentral and midzonal areas of I/R liver (Figs 5c,d).
Complement protein C5b9 as well as CRP were never observed in nonischaemic livers and appeared very intense in midzonal and pericentral hepatocytes in I/R livers. Incubation of serial sections and double staining of single sections showed that these proteins colocalized (Figs 5,e–h and 6).
Fig. 6.
In a single, frozen section of ischaemic-reperfused liver, sequential staining for CRP (brown reaction product) and complement C5b9 (blue reaction product) was performed. Co-localization of CRP and C5b9 is clearly shown in hepatocytes (arrows). (Original magnification with 100× objective).
Discussion
Inappropriate complement activation is an important mediator of I/R injury after major surgery [38,39]. Activation of the classical complement pathway in this type of tissue damage can occur via Ab-dependent as well as Ab-independent mechanisms, which in the latter case may involve the direct binding of C1q to damaged cells and in situ deposited acute phase proteins [39]. To prevent undesired effects of complement activation, the therapeutic application of complement inhibitor C1-inh, a physiological inhibitor of the serine proteases C1r and C1s of the classical pathway has been preliminary tested in man [23,28,40]. Herein, C1-inh was tested in a rat model of liver I/R injury at different doses and different time points. In our study exogenous administration of C1-inh significantly attenuated liver I/R injury after 24 h of reperfusion, which is in agreement with previous studies [26,41]. Furthermore, we show that pre-ischaemic administration of C1-inh was more effective in attenuating liver I/R injury than administration of C1-inh at the end of the ischaemic period. With respect to the recommended dose of C1-inh, our study suggests that a dose of 100 IU/kg is sufficient, as no additional effects were found using higher dosages.
We observed increased bile secretion, reduced ALT leakage and lower levels of activated C4 when C1-inh was administered before the start of ischaemia. This suggests that either C1-inh should be distributed thoroughly within the liver microcirculation or perhaps should be bound to the sinusoidal endothelium to exert its effect [42]. The observations also suggest that at least part of the complement activation is initiated during the period of ischaemia and is inhibited by C1-inh. Support for this reperfusion-independent activation of complement is provided by experiments in dogs undergoing experimental myocardial infarction. In these animals it was observed that C1-inh produced a significant cardioprotective effect when coronary vessels were permanently occluded (W.T. Hermens, C.E. Hack et al. unpublished observation).
Inhibition of an early step in the classical pathway is of relevance in view of the pro-inflammatory effects of early products of the complement activation cascade, such as C4a [43]. Although in this study the classical pathway of complement (i.e. level of activated C4) was only partially blocked by C1-inh, hepatic I/R injury (i.e. ALT leakage from hepatocytes) was significantly attenuated. This suggests that, although activation of the classical complement pathway is causally involved in harmful complement activation during liver I/R, C1-inh exerts its effects not only via reduction of classical complement activation. C1-Inh is also a major inhibitor of the lectin pathway of complement activation, the contact activation system and the intrinsic pathway of coagulation [23]. It is therefore endowed with anti-inflammatory properties. These additional effects of C1-inhibitor probably explain the therapeutic benefit of C1-Inh independent of an effect on the classical complement activation. Therefore, further definition of the contribution of the various complement pathways in liver I/R is of major importance for the development of an effective, specific, and safe treatment, e.g. in transplantation medicine.
The differences in liver I/R injury between albumin and C1-inh treated rats were most apparent after 24 h of reperfusion. This late beneficial effect of complement inhibition can be explained by the modulating effects of C1-inh on the inflammatory response, resulting in a blunted late phase of I/R injury, which is mainly propagated by activated neutrophils [14]. Furthermore, bile secretion, as parameter of hepatocyte function, did show differences between groups similar to the differences in ALT levels.
C1-inh has been found to bind to sinusoidal endothelium or the sinusoidal pole of the liver trabeculae, linked to sinusoidal endothelium, after 8 h of cold storage in UW solution containing C1-inh and 2 h of reperfusion [12]. We could not demonstrate that human C1-inh was retained by the livers at 24 h after administration of the compound. We did find C3 deposition on plasma membranes of hepatocytes both in normal and postischaemic liver. The mouse anti-rat antibodies did not discriminate between native C3 and activation fragments of C3. In normal livers, the low background staining in the cytoplasm of hepatocytes as well as the more intense staining of the hepatocyte membranes results from the synthesis of native C3 by these cells. In I/R livers, more cytoplasmic C3 was found in pericentral and midzonal hepatocytes. Whether this is native or activated C3 can not be distinguished. Deposition of the anti-C3 antibodies on liver cells shows marked heterogeneity, which is likely related to the mainly midzonal expression of I/R injury, but perhaps also to variability in sensitivity to complement activation. We made no attempt to quantify the amount of C3 in hepatocytes after administration of C1-inh or albumin, considering the large variation that results from assessment of liver sections [44].
The MAC or C5b9 complex was found in the same areas (and in the same hepatocytes as suggested by serial sectioning) as C3. This colocalization indicates that the increased binding of anti-C3 antibodies to the cytoplasm of hepatocytes represents deposition of activated C3. The coexistence of activated C3 and C5b9 on hepatocytes in areas renowned for ischaemic/reperfusion injury suggests that these hepatocytes probably die through MAC-mediated cell lysis.
CRP colocalized with both activated C3 and C5b9 in hepatocytes in ischaemically injured midzonal areas whereas periportal areas (notably most resistant to ischaemic injury) were negative for CRP and complement. These results suggest that CRP directly participates in local inflammatory processes, possibly via complement activation, after binding of a suitable ligand. Lagrand et al. [45] first hypothesized that CRP fixed to injured plasma membranes in infarcted myocardium could promote local activation of the complement system via the classical route in humans. Although a previous attempt failed to demonstrate this phenomenon in rats [46], recent findings have proven that rat CRP activates the endogenous complement system in rats [47]. Hepatocytes and sinusoidal endothelial cells, which have a low expression of complement-regulatory proteins in normal liver, might therefore be at risk for complement-mediated injury [48]. This may explain in part the high susceptibility of the liver to complement-mediated injury as shown in experimental models [6].
We conclude that our results provide further support for the role of classical complement activation in mediating liver I/R injury. Our study also demonstrates that pre-ischaemic administration of C1-inh is more effective in reducing liver I/R injury than administration just prior to reperfusion, providing an effective pharmacological intervention to protect against liver I/R. Furthermore, our data support the hypothesis for CRP-mediated complement activation in liver I/R.
Acknowledgments
The authors are grateful to Ineke Bos and Wim Bleeker for fruitful discussion and to Michael Kortleve, Toon Winkelman, Robin J. Hartman, Goos Huijzer and Esther Heikens for their assistance with the experiments.
References
- 1.Vakeva A, Laurila P, Meri S. Regulation of complement membrane attack complex formation in myocardial infarction. Am J Pathol. 1993;143:65–75. [PMC free article] [PubMed] [Google Scholar]
- 2.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;49:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation. 1990;81:156. doi: 10.1161/01.cir.81.1.156. [DOI] [PubMed] [Google Scholar]
- 4.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FDJ. Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–8. [PubMed] [Google Scholar]
- 5.Hebert LA, Cosio FG, Birmingham DJ. The role of the complement system in renal injury. Seminars Nephrol. 1992;12:408–27. [PubMed] [Google Scholar]
- 6.Jaeschke H. Pathophysiology of hepatic ischemia-reperfusion injury: The role of complement activation. Gastroenterology. 1994;107:583–6. doi: 10.1016/0016-5085(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 7.Chavezcartaya RE, Pinodesola G, Wright L, Jamieson NV, White DJ. Regulation of the complement cascade by soluble complement receptor type 1. Transplantation. 1995;59:1047–52. doi: 10.1097/00007890-199504150-00023. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H, Farhood A, Smith CW. Contribution of complement-stimulated hepatic macrophages and neutrophils to endotoxin-induced liver injury in rats. Hepatology. 1994;19:973–9. [PubMed] [Google Scholar]
- 9.Chan RK, Ibrahim SI, Verna N, Carroll M, Moore FD, Jr, Hechtman HB. Ischaemia-reperfusion is an event triggered by immune complexes and complement. Br J Surg. 2003;90:1470–8. doi: 10.1002/bjs.4408. [DOI] [PubMed] [Google Scholar]
- 10.Straatsburg IH, Boermeester MA, Wolbink GJ, et al. Complement activation induced by ischemia-reperfusion in humans: a study in patients undergoing partial hepatectomy. J Hepatol. 2000;32:783–91. doi: 10.1016/s0168-8278(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 11.Meyer zu Vilsendorf A, Link C, Jorns A, Nagel E, Kohl J. Preconditioning with the prostacyclin analog epoprostenol and cobra venom factor prevents reperfusion injury and hyperacute rejection in discordant liver xenotransplantation. Xenotransplantation. 2001;8:41–7. doi: 10.1034/j.1399-3089.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergamaschini L, Gobbo G, Gatti S, et al. Endothelial targeting with C1-inhibitor reduces complement activation in vitro and during ex vivo reperfusion of pig liver. Clin Exp Immunol. 2001;126:412–20. doi: 10.1046/j.1365-2249.2001.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 15.Tokuka A, Tanaka A, Kitai T, et al. Interrelationship of oxygen supply by hepatic artery and portal vein: rapid analysis of ischemia-reflow-induced changes in hepatic oxygenation in experimental and clinical subjects by tissue near- infrared spectroscopy. Eur Surg Res. 1994;26:342–52. doi: 10.1159/000129355. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi H, Miyauchi A, Karasawa Y, Hamamoto I, Maeba T, Tanaka S. Effect of warm ischemia and reperfusion injury on inducing major histocompatibility complex antigens on hepatocytes and nonparenchymal cells in the rat liver. Transplant Proc. 1993;25:3205–7. [PubMed] [Google Scholar]
- 17.Thijs LG, Hack CE, Strack Van Schijndel RJ, et al. Activation of the complement system during immunotherapy with recombinant IL-2. Relation to the development of side effects. J Immunol. 1990;144:2419–24. [PubMed] [Google Scholar]
- 18.Rossen RD, Swain JL, Michael LH, Weakly S, Giannini E, Entman ML. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extramyocardial mechanism of ischemic injury. Circulation Res. 1985;57:119–30. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- 19.Rossen RD, Michael LH, Kagiyama A, et al. Mechanism of complement activation following coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circulation Res. 1988;62:572–84. doi: 10.1161/01.res.62.3.572. [DOI] [PubMed] [Google Scholar]
- 20.Vogt W, Hesse D. Activation of the fifth component of human complement by oxygen-derived free radicals, and by methionine oxidizing agents: a comparison. Immunobiology. 1992;184:384–91. doi: 10.1016/S0171-2985(11)80595-4. [DOI] [PubMed] [Google Scholar]
- 21.Vogt W, von Damerau BZI, Nolte R, Brunahl D. Non-enzymic activation of the fifth component of human complement, by oxygen radicals. Some properties of the activation product, C5b-like C5. Mol Immunol. 1989;26:1133–42. doi: 10.1016/0161-5890(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann TG, Koeppel TA, Munch S, et al. Impact of inhibition of complement by sCR1 on hepatic microcirculation after warm ischemia. Microvasc Res. 2001;62:284–92. doi: 10.1006/mvre.2001.2342. [DOI] [PubMed] [Google Scholar]
- 23.Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev. 2000;52:91–112. [PubMed] [Google Scholar]
- 24.Jansen PM, Eisele B, de Jong IW, et al. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J Immunol. 1998;160:475–84. [PubMed] [Google Scholar]
- 25.Buerke M, Murohara T, Lefer AM. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion [see comments] Circulation. 1995;91:393–402. doi: 10.1161/01.cir.91.2.393. [DOI] [PubMed] [Google Scholar]
- 26.Buerke M, Prufer D, Dahm M, Oelert H, Meyer JDarius. Blocking of classical complement pathway inhibits endothelial adhesion molecule expression and preserves ischemic myocardium from reperfusion injury. J Pharmacol Exp Ther. 1998;286:429–38. [PubMed] [Google Scholar]
- 27.Horstick G, Heimann A, Gotze O, et al. Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation. 1997;95:701–8. doi: 10.1161/01.cir.95.3.701. [DOI] [PubMed] [Google Scholar]
- 28.Hack CE, Ogilvie AC, Eisele B, Jansen PM, Wagstaff J, Thijs LG. Initial studies on the administration of C1-esterase inhibitor to patients with septic shock or with a vascular leak syndrome induced by interleukin-2 therapy. Prog Clin Biol Res. 1994;388:335–57. [PubMed] [Google Scholar]
- 29.Horstick G, Berg O, Heimann A, et al. Application of C1-esterase inhibitor during reperfusion of ischemic myocardium: dose-related beneficial versus detrimental effects. Circulation. 2001;104:3125–31. doi: 10.1161/hc5001.100835. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann TG, Heger M, Munch S, Kirschfink M, Klar E. In vivo microscopy reveals that complement inhibition by C1-esterase inhibitor reduces ischemia/reperfusion injury in the liver. Transpl Int. 2000;13:S547–S550. doi: 10.1007/s001470050399. [DOI] [PubMed] [Google Scholar]
- 31.Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, Van den Bosch H. A role for secretory phospholipase a(2) and c-reactive protein in the removal of injured cells. Immunol Today. 1997;18:111–5. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- 32.Heijnen BH, Elkhaloufi Y, Straatsburg IH, Van Gulik TM. Influence of acidosis and hypoxia on liver ischemia and reperfusion injury in an in vivo rat model. J Appl Physiol. 2002;93:319–23. doi: 10.1152/japplphysiol.01112.2001. [DOI] [PubMed] [Google Scholar]
- 33.Heijnen BH, van Veen SQ, Straatsburg IH, Van Gulik TM. Pronounced effect of minor changes in body temperature on ischemia and reperfusion injury in rat liver. J Appl Physiol. 2001;91:265–8. doi: 10.1152/jappl.2001.91.1.265. [DOI] [PubMed] [Google Scholar]
- 34.de Smet BJ, de Boer JP, Agterberg J, Rigter G, Bleeker WK, Hack CE. Clearance of human native, proteinase-complexed, and proteolytically inactivated C1-inhibitor in rats. Blood. 1993;81:56–61. [PubMed] [Google Scholar]
- 35.Nuijens JH, Huijbregts CC, Eerenberg-Belmer AJ, et al. Quantification of plasma factor XIIa-Cl(-)-inhibitor and kallikrein-Cl(-)-inhibitor complexes in sepsis. Blood. 1988;72:1841–8. [PubMed] [Google Scholar]
- 36.Nuijens JH, Huijbregts CC, Cohen M, et al. Detection of activation of the contact system of coagulation in vitro and in vivo: quantitation of activated Hageman factor-C-1-inhibitor and kallikrein-C-1-inhibitor complexes by specific radioimmunoassays. Thromb Haemost. 1987;58:778–85. [PubMed] [Google Scholar]
- 37.Bos IG, van Mierlo GJ, Bleeker WK, et al. The potentiation of human C1-inhibitor by dextran sulphate is transient in vivo: studies in a rat model. Int Immunopharmacol. 2001;1:1583–95. doi: 10.1016/s1567-5769(01)00073-x. [DOI] [PubMed] [Google Scholar]
- 38.Dong J, Pratt JR, Smith RA, Dodd I, Sacks SH. Strategies for targeting complement inhibitors in ischaemia/reperfusion injury. Mol Immunol. 1999;36:957–63. doi: 10.1016/s0161-5890(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 39.Griselli M, Herbert J, Hutchinson WL, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asghar SS, Pasch MC. Therapeutic inhibition of the complement system. Y2K Update. Front Biosci. 2000;5:E63–E81. doi: 10.2741/asghar. [DOI] [PubMed] [Google Scholar]
- 41.Doherty JC, McMillen MA. Ischemic liver injury. J Am College Surgeons. 1998;186:606–7. [PubMed] [Google Scholar]
- 42.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–7. [PubMed] [Google Scholar]
- 43.Cooper NR. Biology of the complement system. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincot; 1999. [Google Scholar]
- 44.Fleming KA. Evidence-based cellular pathology. The Lancet. 2002;359:1149–50. doi: 10.1016/S0140-6736(02)08165-5. [DOI] [PubMed] [Google Scholar]
- 45.Lagrand WK, Niessen HW, Wolbink GJ, et al. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]
- 46.de Beer FC, Baltz ML, Munn EA, et al. Isolation and characterization of C-reactive protein and serum amyloid P component in the rat. Immunology. 1982;45:55–70. [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz Padilla N, Bleeker WK, Lubbers YT, et al. Rat C-reactive protein activates endogenous complement system. Immunology. 2003;109:564–71. doi: 10.1046/j.1365-2567.2003.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scoazec JY, Delautier D, Moreau A, et al. Expression of complement-regulatory proteins in normal and UW-preserved human liver. Gastroenterology. 1994;107:505–16. doi: 10.1016/0016-5085(94)90178-3. [DOI] [PubMed] [Google Scholar]