Abstract
The c-Jun N-terminal kinase (JNK) participates in intracellular signalling cascades that mediate inflammatory responses. Therefore, the JNK signalling may be involved in gastric injury and inhibition of this pathway may form the basis of a new strategy for the treatment of gastric injury. The aim of this study was to determine whether JNK participates in the formation of gastric lesions in an experimental model. Acute gastric injury was induced in Sprague-Dawley rats by intragastric administration of 100% ethanol. The amount of phospho-JNK in the rat stomach was determined using immunohistochemistry and Western analysis. Animals received subcutaneous injections of a specific JNK inhibitor SP600125 or vehicle and the extent of mucosal damage in the stomach was determined. Western analysis revealed early phosphorylation of JNK and, to a lesser extent, p38 as well as late phosphorylation of the p42/44 extracellular signal-related kinases during the development of gastric lesions. JNK was phosphorylated in epithelial cells and in occasional mononuclear cells present at lesion sites. These cells were rarely found in samples from control specimens. Treatment with SP600125 significantly reduced the extent of gastric lesions. These findings indicate that experimental gastric injury is associated with activation of the JNK signalling pathway, and also suggest that JNK inhibitors may play a role in the treatment of gastric injury in humans.
Keywords: c-Jun N-terminal kinase (JNK), experimentally induced gastric lesions, rats
Introduction
Mitogen-activated protein kinases (MAPKs) are part of intracellular signalling cascades that transduce signals from diverse extracellular stimuli to initiate inflammatory cellular responses [1,2]. Several subgroups have been identified within the MAPK family, including the p42/44 extracellular signal-related kinases (ERKs), p38 MAPKs, and c-Jun N-terminal kinases (JNKs). Of these, JNK has been implicated as an important regulator of the coordinated release of cytokines by immunocompetent cells and of the response of neutrophils to inflammatory stimuli [3,4]. Many different stiumuli can activate JNK, including lipopolysaccharide and other bacterial products, cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1, growth factors, and stresses such as heat shock, hypoxia, and ischemia/reperfusion. In addition, JNK activates the expression of a variety of genes involved in inflammation, such as those encoding TNF-α, IL-1, and IL-6 [5–10].
Although the functions of many of the MAPKs have been determined using specific inhibitors, no specific JNK inhibitor has been available until very recently. Recently, SP600125 has been identified as a specific inhibitor of JNK [11]. The in vivo effects of SP600125 on the inhibition of cytokine synthesis and protection against tissue injury have been evaluated in several animal models of inflammation including adjuvant-induced arthritis [12] and pulmonary inflammation [13]. Accordingly, pharmacological inhibition of JNK has been proposed as a potential therapeutic strategy for the treatment of gastric injury.
Ethanol penetrates deeply into the gastric mucosa because of its high lipid solubility and causes microvascular damage, resulting in ulcerative lesions [14,15]. This injury is characterized by a group of highly varied and complex cellular and biochemical events in which cytokines and growth factors play an important role in modulating inflammation [16–20]. A study by Pai et al. [21] demonstrated that ERK, a member of the MAPK family, promotes the repair of ethanol-induced gastric lesions in rats. However, to the best of our knowledge, nothing is known about the role of JNK in the setting of gastric injury.
The aims of the present study were to determine the level of expression of activated JNK in ethanol-induced gastric lesions, and to use SP600125 to examine whether JNK blockade can suppress this injury.
Materials and methods
JNK inhibitor
SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) is a reversible ATP-competitive inhibitor of JNK synthesized by the Department of Chemistry at the Signal Research Division of Celgene (San Diego, CA, USA). Its chemical and biochemical properties have been reported elsewhere [11,12].
Induction of gastric injury
Seven-week-old female Sprague-Dawley rats weighting 180–200 g (SLC Co., Ltd, Shizuoka, Japan) were used for this study. Gastric injury was induced by intragastric administration of 2 ml of 100% (v/v) ethanol [14,20]. The studies were approved by the Animal Research Committee of Kurume University.
Temporal assessment of experimental gastric injury
Rats were killed before or 1, 6, 12 or 24 h or 4 or 7 days after, the induction of gastric injury. The stomach and proximal duodenum were removed, opened along the greater curvature, rinsed with ice-cold saline, and photographed in a standardized fashion as described below. Regions of the stomach with macroscopic evidence of damage were excised and processed for Western blotting and immunohistochemistry as outlined below.
Evaluation of gastric lesions
A semiquantitative scale (0, normal mucosa; 1, 1–4 small petechia; 2, 5 or more petechia or haemorrhagic streaks up to 4 mm in length; 3, erosions longer than 5 mm or confluent haemorrhages) was used to assess the extent of gastric mucosal lesions [22].
Western analysis
Stomach tissue was obtained from rats was frozen immediately in liquid nitrogen and stored at −80 °C. Thereafter, the tissue was homogenized in 2–4 ml of lysis buffer containing 50 mM Tris-HCl (pH 8·0), 0·5% NP-40, 1 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mM sodium vanadate, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM phenylmethylsulphonyl fluoride and a protease inhibitor cocktail (Sigma Chemical Co, St. Louis, MO, USA). Extracts were cleared by pelleting cellular debris at 20 630 g at 4 °C for 15 min, and then diluted with lysis buffer to an approximate protein concentration of 2 mg/ml. Total tissue extracts were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were detected by Western blotting using antiphospho-JNK (Santa Cruz Biotechnology, Santa Cruz, CA, USA), antiphospho-p38 (Cell Signalling Technology, Beverly, MA, USA), antiphospho-ERK (Santa Cruz Biotechnology), anti-JNK (Cell Signalling Technology), antip38 (Cell Signalling Technology), and anti-ERK (Cell Signalling Technology) antibodies.
Immunohistochemistry
Immunohistochemistry was performed using paraffin-embedded rat stomach sections. A rabbit polyclonal antibody against phospho-JNK (Santa Cruz Biotechnology) was used as the primary antibody. Horseradish peroxidase staining was achieved using an avidin-biotin complex kit (Vectastain; Vector Laboratories, Burlingame, CA, USA). Each slide was stained with diaminobenzidine tetrahydrochloride substrate and counterstained with haematoxylin.
Effect of in vivo administration of SP600125
SP600125 (30 mg/kg) or vehicle (40% polyethylene glycol, PEG 400, in PBS) was injected subcutaneously. The dosing regimen used was based on previous in vivo studies [12]. The treatment was administered 2 h before ethanol exposure and repeated 12 h later. Rats were killed 24 h after the induction of injury. Thereafter, gastric mucosal damage was assessed by stomach weight and the lesion scale described above.
Statistical analysis
Values are expressed as the mean ± SEM. Student's t-test was used to compare the differences between the means of two groups. A value of P < 0·05 was considered statistically significant.
Results
Time course of gastric damage
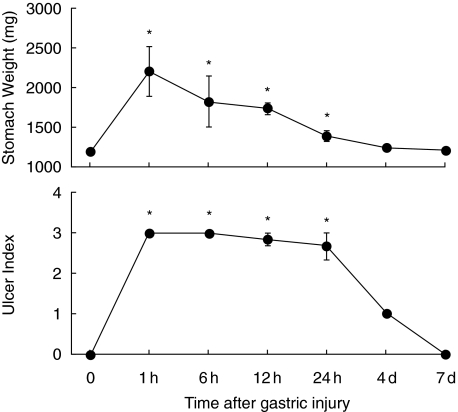
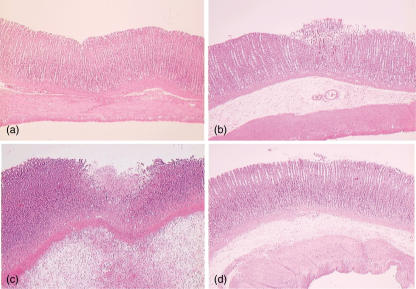
The topical application of 100% ethanol resulted in severe tissue damage as assessed by stomach weight and lesion index (Fig. 1). The stomach weight peaked at 1 h and gradually declined thereafter. The lesion index increased at 1 h and remained elevated 24 h after ethanol exposure. Figure 2 shows the temporal changes in the microscopic appearance of rat gastric mucosa exposed to ethanol. Disruption or exfoliation of epithelial cells with inflammatory infiltrates and submucosal oedema was observed at 1 h and 24 h but the damage had completely recovered at 4 days.
Fig. 1.
Time course for changes in gastric tissue damage. Gastric injury was induced by intragastric administration of 100% ethanol and tissue damage was assessed by stomach weight and lesion morphology (range from 0, normal to 3, maximal activity) at the indicated time-points. Each point represents the mean ± SEM for 4–8 animals. P < 0·05 versus control.
Fig. 2.
0. Histological manifestations of gastric lesions (a) before and (b) 1 h, (c) 24 h and (d) 4 days after exposure to 100% ethanol (haematoxylin and eosin).
Time course of MAPK expression
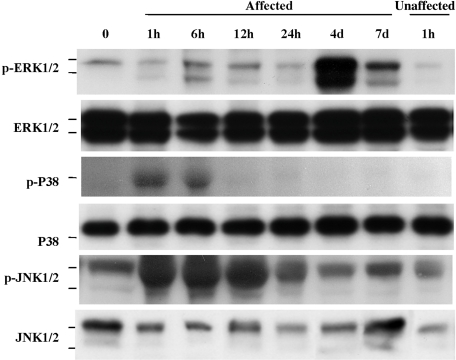
During the course of ethanol-induced gastric injury, the expression of MAPKs at the lesion sites was monitored. As shown in Fig. 3, all 3 MAPKs were activated but the profiles of activation were quite different. The phospho-JNK expression increased rapidly and peaked at 1–12 h, then gradually returning to control levels of phosphorylation. This profile paralleled the extent of mucosal damage as assessed by stomach weight and lesion index. Similarly, p38 was phosphorylated but the level of phosphorylation was modest and of short duration. In contrast to the JNK and p38 MAPK profile, ERK was phosphorylated with a peak at 4 days, which correspond to the time when mucosal damage began to recover. It is therefore likely that, of the 3 MAPKs, JNK plays a central role in the development of this injury.
Fig. 3.
Time course-changes in the extent of MAPKs phosphorylation during ethanol-induced gastric injury in rats. Gastric injury was induced by intragastric administration of 100% ethanol. Expression of phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, and JNK in tissue homogenates was determined by Western blotting. A representative Western blot of three separate experiments is shown.
JNK localization
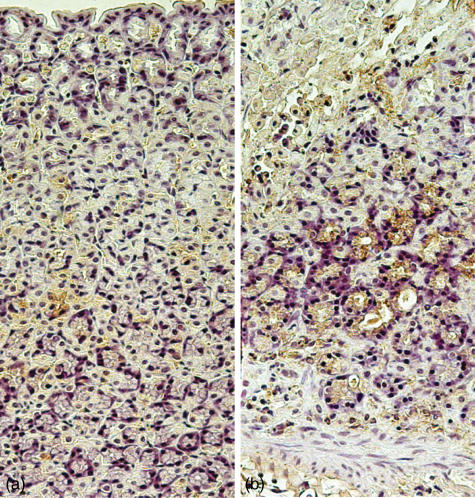
To identify the cells expressing phospho-JNK in the rat gastric mucosa, immunohistochemical staining was performed. As shown in Fig. 4, epithelial cells at the lesion sites stained positive for phospho-JNK in an ethanol-exposed animal killed 24 h after the induction of injury. Some phospho-JNK immunoreactivity was also detected in mononuclear cells. Phospho-JNK immunoreactivity was very rare in control animals.
Fig. 4.
Localization of phospho-JNK immunoreactivity in stomach sections of rats (a) before and (b) 24 h after intragastric administration of 100% ethanol. Staining of epithelial cells and occasional mononuclear cells for phospho-JNK is demonstrated. Control slides incubated with control IgG or saline showed no immunoreactivity, and staining was greatly reduced after preincubation of the primary antibody with recombinant phospho-JNK (not shown).
Treatment with SP600125
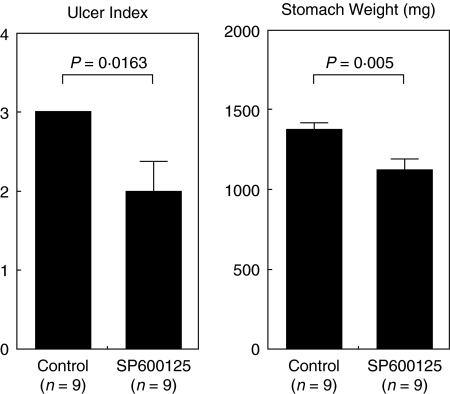
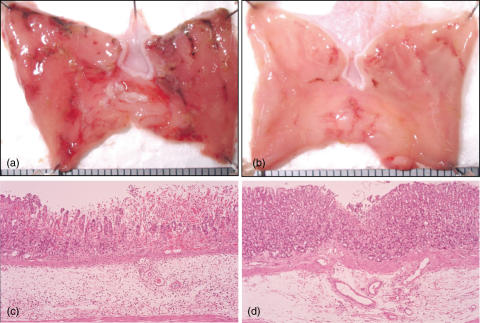
Because an association between the extent of these lesions and JNK activation was observed, we examined whether JNK signalling is involved in the development of gastric injury by using a specific JNK inhibitor. Treatment with SP600125 initiated 2 h before the induction of injury significantly inhibited the appearance of gastic lesions when compared to the vehicle-treated group (Fig. 5). Representative photographs of ethanol-induced gastric lesions after SP600125 treatment are shown in Fig. 6. Gastric damage was mild in the SP600125-treated stomach (Fig. 6b) compared with the vehicle-treated stomach (Fig. 6a). Severe mucosal and submucosal inflammation with evidence of erosive injury was observed in the vehicle-treated stomach (Fig. 6c), whereas the extent of injury was markedly attenuated by SP600125 (Fig. 6d).
Fig. 5.
Effects of SP600125 on tissue damage in ethanol-induced gastric injury in rats. Rats received SP600125 (30 mg/kg) or vehicle by subcutaneous injection 2 h before and 12 h after exposure to ethanol, and the severity of injury was evaluated by stomach weight and lesion morphology (range from 0, normal to 3, maximal activity) 24 h following the induction of injury. Bars represent mean values ± SEM for 9 rats per group.
Fig. 6.
Photographs showing the macroscopic (a,b) and microscopic (c,d) appearance of ethanol-induced gastric injury after treatment with vehicle (a,c) or SP600125 (b,d). Rats received SP600125 (30 mg/kg) or vehicle by subcutaneous injection 2 h before and 12 h after induction of gastric injury. The stomach was opened along the greater curvature 24 h after the induction of injury. Note that severe mucosal and submucosal inflammation with erosive injury was less severe in the SP600125-treated rat (haematoxylin and eosin). Original magnification ×100.
Discussion
Based on the importance of cytokines in gastric injury, JNK is likely to act as a key regulator of tissue damage via effects on AP-1 [1,2]. However, previous studies of JNK function in vitro and in vivo have been limited by the lack of availability of a specific inhibitor. A recently developed specific JNK inhibitor, SP600125 [11,12], provided us with a more precise way to investigate the role of JNK in the development of gastric injury.
We first examined the local expression of phospho-JNK during the evolution of ethanol-induced gastric injury. We found that ethanol activates all 3 MAPK pathways and that the profile of activation is characterized by a rapid and high level of activation of JNK, a rapid but modest activation of p38, and a late activation of ERK. Therefore, of the three MAPKs, JNK appears to be a key regulator in the development of gastric lesion formation.
Although transient and not as dramatic, p38 MAPKs activation also increased with lesion progression. However, the in vivo effect of p38 inhibitors against gastric damage is controversial. Takahashi et al. [23] reported a beneficial effect of a p38 kinase-selective inhibitor FR167653 in Helicobactor pylori-induced gastritis in Mongolian gerbils. In contrast, Kobayashi et al. [24] have shown an impairment of ulcer healing and angiogenesis by FR167653 in acetic acid-induced gastric ulceration in rats. The exact role of p38 MAPKs in gastric injury needs further investigation.
In contrast to these two MAPKs that are activated with lesion progression, the activation of ERK occurred during the healing process. These data are consistent with extensive work performed by Pai et al. [21] who examined the ERK activation profile in the same model. They also demonstrated an impairment of mucosal healing in animals treated with the ERK inhibitor Tyrphostin A46. Taken together, it seems likely that ERK signalling induces mucosal repair rather than mucosal destruction in this gastric lesion model.
With respect to the cellular source of JNK in our model, phospho-JNK was detected mainly in epithelial cells at lesion sites. Indeed, gastric epithelial cells have been shown to produce and release cytokines and chemokines in response to various stimuli [25–28]. Thus, cytokines secreted by epithelial cells may influence immunocompetent cells in a manner that contributes to development of gastric injury. Mononuclear leucocytes are unlikely to contribute significantly to phospho-JNK expression because of the relatively small number of mononuclear cells that stained positive.
Given the high degree of activation of JNK signalling following the induction of experimental injury, investigating the role of JNK in the initiation of injury was the next step. In vivo inhibition studies using a specific JNK inhibitor demonstrated a decrease in gastric damage in animals treated with SP600125, suggesting a key role for JNK signalling in the lesion progression. However, the finding that SP600125 treatment led to a reduction in the gastric damage, but did not completely abolish it, can be explained by the presence of other signal transduction pathways involved in inflammatory and immune reactions, such as NF-κB [29] and Janus kinases (JAK)/signal transducer and activator of transcription (STAT) [30], as well as p38 MAPKs. These pathways warrant further investigation. To date, there have been several reports on the effectiveness of SP600125 in the treatment of inflammatory disorders [12,13]. However, to the best of our knowledge, our study is the first to demonstrate the up-regulation of JNK in the setting of gastric mucosal injury, and to demonstrate that SP600125 ameliorates mucosal damage.
In conclusion, our study demonstrated that experimental gastric injury is associated with activation of the JNK signalling pathway, and also suggest that JNK inhibitors may play a role in the treatment of gastric injury in humans.
References
- 1.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–5. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 2.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 3.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 4.Kallunki T, Su B, Tsigelny I, Sluss H, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 5.Jain J, Valge-Archer VE, Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992;148:1240–50. [PubMed] [Google Scholar]
- 6.Foletta VC, Segal DH, Cohen DR. Transcriptional regulation in the immune system: all roads lead to AP-1. J Leukocyte Biol. 1998;63:139–52. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 7.Finkenzeller G, Technau A, Marme D. Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun. 1995;208:432–9. doi: 10.1006/bbrc.1995.1356. [DOI] [PubMed] [Google Scholar]
- 8.Schanke JT, Marcuzzi A, Podzorski RP, Van Ness B. An AP1 binding site upstream of the kappa immunoglobulin intron enhancer binds inducible factors and contributes to expression. Nucl Acids Res. 1994;22:5425–32. doi: 10.1093/nar/22.24.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Knethen A, Callsen D, Brune B. NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol Biol Cell. 1999;10:361–72. doi: 10.1091/mbc.10.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40:222–33. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 11.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eynott PR, Nath P, Leung SY, Adcock IM, Bennett BL, Chung KF. Allergen-induced inflammation and airway epithelial and smooth muscle cell proliferation: role of Jun N-terminal kinase. Br J Pharmacol. 2003;140:1373–80. doi: 10.1038/sj.bjp.0705569. Epub: 17 November 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarnawski A, Hollander D, Stachura J, Krause WJ, Gergely H. Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology. 1985;88:334–52. doi: 10.1016/s0016-5085(85)80191-8. [DOI] [PubMed] [Google Scholar]
- 15.Szabo S. Mechanisms of mucosal injury in the stomach and duodenum. time-sequence analysis of morphologic, functional, biochemical and histochemical studies. Scand J Gastroenterol. 1987;127:21–8. doi: 10.3109/00365528709090946. [DOI] [PubMed] [Google Scholar]
- 16.Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany BL. Gastric mucosal apoptosis induced by ethanol: effect of antiulcer agents. Biochem Mol Biol Int. 1997;42:247–54. doi: 10.1080/15216549700202631. [DOI] [PubMed] [Google Scholar]
- 17.Jones MK, Itani RM, Wang H, Tomikawa M, Sarfeh IJ, Szabo S, Tarnawski AS. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol. 1999;276:G1345–55. doi: 10.1152/ajpgi.1999.276.6.G1345. [DOI] [PubMed] [Google Scholar]
- 18.Liu ES, Cho CH. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion. 2000;62:232–9. doi: 10.1159/000007821. [DOI] [PubMed] [Google Scholar]
- 19.Szabo IL, Kawanaka H, Jones MK, Pai R, Soreghan BA, Baatar D, Husain SS, Tarnawski AS. Activation of hypoxia inducible factor-1alpha in gastric mucosa in response to ethanol injury: a trigger for angiogenesis? Life Sci. 2001;69:3035–44. doi: 10.1016/s0024-3205(01)01410-2. [DOI] [PubMed] [Google Scholar]
- 20.Matsui Y, Mitsuyama K, Tomiyasu N, Toyonaga A, Sata M. Efficacy of vascular endothelial growth factor in the treatment of experimental gastric injury. Digestion. 2002;66:99–105. doi: 10.1159/000065591. [DOI] [PubMed] [Google Scholar]
- 21.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology. 1998;114:706–13. doi: 10.1016/s0016-5085(98)70584-0. [DOI] [PubMed] [Google Scholar]
- 22.Szabo S, Trier JS, Brown A, Schnoor J, Homan HD, Bradford JC. A quantitative method for assessing the extent of experimental gastric erosions and ulcers. J Pharmacol Meth. 1985;13:59–66. doi: 10.1016/0160-5402(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S, Keto Y, Fujita T, Uchiyama T, Yamamoto A. FR167653, a p38 mitogen-activated protein kinase inhibitor, prevents Helicobacter pylori-induced gastritis in Mongolian gerbils. J Pharmacol Exp Ther. 2001;296:48–56. [PubMed] [Google Scholar]
- 24.Kobayashi N, Kataoka T, Ono A, Tsukimi Y, Okabe S. Role of p38 mitogen-activated protein kinase in the healing of gastric ulcers in rats. J Physiol Pharmacol. 2001;52:195–210. [PubMed] [Google Scholar]
- 25.Beales IL, Calam J. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine. 1997;9:514–20. doi: 10.1006/cyto.1996.0195. [DOI] [PubMed] [Google Scholar]
- 26.Kraft M, Riedel S, Maaser C, Kucharzik T, Steinbuechel A, Domschke W, Luegering N. IFN-gamma synergizes with TNF-alpha but not with viable H. pylori in up-regulating CXC chemokine secretion in gastric epithelial cells. Clin Exp Immunol. 2001;126:474–81. doi: 10.1046/j.1365-2249.2001.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiraoka S, Miyazaki Y, Kitamura S, Toyota M, Kiyohara T, Shinomura Y, Mukaida N, Matsuzawa Y. Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2001;281:G735–42. doi: 10.1152/ajpgi.2001.281.3.G735. [DOI] [PubMed] [Google Scholar]
- 28.Nakachi N, Klein TW, Friedman H, Yamamoto Y. Helicobacter pylori infection of human gastric epithelial cells induces IL-8 and TNFalpha, but not TGFbeta1 mRNA. FEMS Immunol Med Microbiol. 2000;29:23–6. doi: 10.1111/j.1574-695X.2000.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Fujita T, Yamamoto A. Role of nuclear factor-kappaB in gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1296–304. doi: 10.1152/ajpgi.2001.280.6.G1296. [DOI] [PubMed] [Google Scholar]
- 30.Tebbutt NC, Giraud AS, Inglese M, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–97. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]