Abstract
Humoral immunity in response to an octavalent O-polysaccharide-toxin A conjugate Pseudomonas aeruginosa vaccine is well studied, and a phase III clinical study in cystic fibrosis (CF) patients is currently ongoing. In contrast, little is known about cellular immunity induced by this vaccine. Fifteen healthy volunteers were immunized on days 1 and 60. Parameters of cellular immunity were studied before vaccination on day 1, and on day 74. Analyses included flow cytometry of whole blood and antigen-induced proliferation of and cytokine production by lymphocyte cultures. The effects of immunization on the composition of peripheral blood lymphocytes as determined by flow cytometry were minor. In contrast, after immunization a highly significant increase of proliferation in response to stimulation with detoxified toxin A was noted: the stimulation index rose from 1·4 on day 1 to 42·2 on day 74 (restimulation with 0·4 µg/ml; P = 0·003). Immunization led to significant production of interferon (IFN)-γ and tumour necrosis factor (TNF)-α by antigen-stimulated lymphocytes. In contrast, no significant induction of interleukin (IL)-4 or IL-10 was observed. In conclusion, immunization of healthy volunteers led to activation of cellular immunity including strong antigen-specific proliferation and cytokine production. In CF patients priming of the cellular immune system towards a Th1-like pattern would be of potential advantage. Therefore, confirmatory analyses in immunized CF patients with and without chronic infection with P. aeruginosa are foreseen.
Keywords: cellular immunity, conjugate vaccine, human, Pseudomonas aeruginosa
Introduction
Cystic fibrosis (CF) is the most common inherited disease affecting Caucasians [1]. There is a basic defect in ion transport, which results in the accumulation of sticky mucus on epithelial surfaces in many organs, particularly the lungs. This alteration of the respiratory system forms the environment for severe bacterial infections that are difficult to eradicate, most prominently with Staphylococcus aureus and Pseudomonas aeruginosa. As a result of the recurrent infections, the lungs are progressively destroyed, and exercise capacity becomes limited [2]. In order to prevent colonization and ultimately chronic lung infection with P. aeruginosa in CF patients, we have developed an octavalent P. aeruginosa O-polysaccharide-toxin A conjugate vaccine (‘PA vaccine’) against the eight most prevalent serotypes of P. aeruginosa[3–9].
Currently several clinical studies are running; among them a phase III, double-blind, randomized, placebo-controlled, multi-centre trial with over 450 patients in four European countries to determine the efficacy of the PA vaccine in preventing respiratory tract infection with P. aeruginosa. Whereas humoral immunity in response to the vaccine is well understood, its effect on cellular immunity has not been studied so far. However, animal and human studies have shown that in P. aeruginosa lung infection cellular immunity plays a central role by shaping the type of inflammation ensuing from infection [10–13]. Earlier studies in rats [10] indicated induction of a Th1-like inflammation pattern after immunization with PA vaccine which would be potentially beneficial for CF patients [13]. To confirm these findings in humans, this pilot trial in healthy volunteers was performed, prior to further studies in CF patients.
Materials and methods
Study design and immunization
The study was a phase I, open, non-randomized, uncontrolled, single-centre trial. It was performed at the Clinical Investigation Unit of the University Hospital of Berne (Inselspital), Switzerland with approval by the local Ethics Committee. Fifteen healthy males were recruited and, after having given informed consent, they were immunized intramuscularly with PA vaccine containing per dose 15 µg O-polysaccharide of each of the following eight lipopolysaccharide (LPS) serotypes according to the International Antigen Typing System (IATS): 1, 3, 4, 5 (Fisher Immunotype 7), 6, 10, 11 and 16 (Fisher Immunotype 3). A total of 120 µg polysaccharide and 182 µg carrier protein P. aeruginosa exotoxin A was administered. The development and initial evaluation in healthy volunteers and CF patients has been described extensively [3–9]. Based on previous experience, immunizations were performed on days 1 and 60. Blood for analysis of cellular immunity was taken on day 1 (prevaccination baseline) and 2 weeks after the 2nd immunization at day 74, which represents an ideal time-point for analysis of antiprotein and antipolysaccharide responses.
Measurement of specific serum IgG by enzyme-linked immunosorbent assay (ELISA)
Serum IgG antibodies against all vaccine components (LPS of eight different P. aeruginosa serotypes isolated using standard phenol extraction techniques and toxin A of P. aeruginosa) were measured by ELISA. P. aeruginosa LPS antigen (5 µg/ml) was bound to polystyrene microtitre plates (NUNC Maxisorp, Denmark) by methylated human serum albumin. Toxin A was directly coated at a concentration of 1 µg/ml. After washing, serially diluted sera and standard reference serum were incubated for 2 h at ambient temperature. Plates were washed, and incubated with alkaline phosphatase-labelled goat anti-human IgG antibody (Sigma, St Louis, MO, USA) for 1·5 h. After further washing, plates were developed with 4-nitrophenylphosphate (Merck, Darmstadt, Germany) and optical density (OD) at 405 nm was measured using a Spectromax ELISA-plate reader (Paul Bucher AG, Switzerland). OD values were transformed to µg/ml with SoftMax Pro software version 3·1.1 (Molecular Devices, Sunnyvale, CA, USA) using a standard curve of a human reference serum.
Flow cytometry
Anti-coagulated blood (100 µl/tube) was dispensed into individual 5 ml FACS tubes (BD Biosciences, Basel, Switzerland) and 2 ml of lysing solution (BD Biosciences) was added for 5 min. Thereafter tubes were centrifuged (500 g, 5 min), the supernatant was discarded, pellets were suspended in 3 ml CellWash (BD Biosciences), and the tubes again centrifuged as above. After discarding the supernatant, cells were incubated for 20 min at room temperature with 20 µl of the following fluorescently labelled antibodies (all ready-to-use from BD Biosciences): phycoerythrin (PE)-Cy5 CD19, PE CD27, fluorescein isothiocyanate (FITC) IgM, FITC IgG, PE-Cy5 CD3, antigen-presenting cells (APC) CD4, FITC CD8 and PE-Cy5 CD45RA. After incubation cells were washed, pellets resuspended in 400 µl CellFix (BD Biosciences) solution and measured immediately using a FACSCalibur and BD CellQuestTM Pro Software (version 4·0.2).
Antigen-induced proliferation
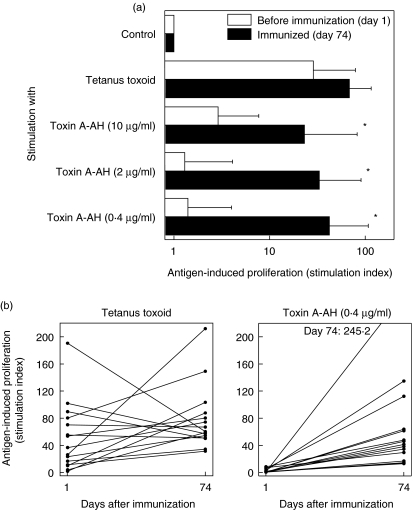
Antigen-induced T cell proliferation was assessed by measuring incorporation of [3H]-thymidine and the stimulation index (SI) was calculated assuming that proliferation in the absence of antigen (medium control) corresponds to an SI of 1 (Fig. 1). As an internal positive control, proliferation induced with tetanus toxoid was measured.
Fig. 1.
Antigen-induced proliferation. (a) Lymphocytes from peripheral blood were cultured in the presence of various antigens before (open bars) and after (filled bars) immunization with P. aeruginosa O-polysaccharide-toxin A conjugate (PA) vaccine. Incorporation of [3H]-thymidine during the last 18 h of a 7-day culture period was measured. Mean + s.d. of 15 subjects is shown, *P < 0·05 by two-tailed, paired t-test between days 1 and 74. (b) Stimulation index after in vitro restimulation with tetanus toxoid or toxin A-AH at day 1 (before) and day 74 (after immunization) of all 15 individual subjects.
Lymphocytes from 9 ml anticoagulated blood were isolated by Ficoll (Sigma; Fluka Chemie GmbH, Buchs, Switzerland) density centrifugation (400 g, 30 min). Cells from the interphase were collected, washed twice with phosphate-buffered saline (PBS) and suspended in RPMI-1640 (Sigma) containing 8% human serum (Sigma) and 50 µg/ml gentamycin (Sigma). Cells were counted and plated at 1·5 × 105 cells/well in 96-well round-bottomed plates (Costar; Corning, NY, USA). The following antigens were added: tetanus toxoid at 2 µg/ml and P. aeruginosa Toxin A-AH (toxin A modified with adipic acid dihydrazide as a linker for subsequent coupling to a polysaccharide antigen, concurrently destroying the toxicity of the carrier protein) at 10, 2 or 0·4 µg/ml. Cultures were grown in triplicate in a final volume of 200 µl/well at 37°C for 7 days. For the last 18 h of culture 1 µCi/well [3H]-thymidine (Amersham, Dübendorf, Switzerland) was added, and thereafter the cells were harvested with a Skatron Combi Cell Harvester. Beta-emission was measured on a Canberra Packard Tri-Carb Liquid Scintillation Analyser.
Antigen-induced cytokine production
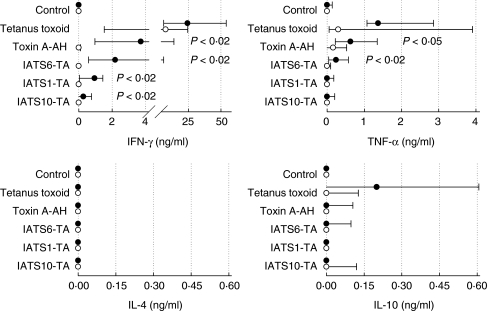
To assess cytokine production in response to P. aeruginosa antigens, lymphocytes were stimulated with toxin A-AH or with individual components of the vaccine, i.e. with conjugates of O-polysaccharides of three different serotypes (IATS-6, IATS-1, IATS-10) with toxin A, followed by ELISA to determine cytokine levels in the cell culture supernatant fluids. Interferon (IFN)-γ was measured as a prototypical Th1-cytokine, interleukin (IL)-4 and IL-10 as typical Th2 cytokines and tumour necrosis factor (TNF)-α as a marker of inflammation.
Lymphocytes were isolated as described above. Cells were counted and plated at 1·5 × 105 cells/well in 96-well round-bottomed plates (Costar). The following antigens were used for stimulation, all at 2 µg/ml: tetanus toxoid, toxin A-AH, individual polysaccharide-toxin A conjugates of IATS serotypes 6 (IATS6-TA), 1 (IATS1-TA) and 10 (IATS10-TA). Cultures were grown in 16 replicates in a final volume of 200 µl/well at 37°C for 3 days. At the end of culture, supernatant fluids were collected and stored at −70°C until the content of various cytokines was determined using commercial assay systems for IFN-γ (BD Biosciences 555142), TNF-α (BD Biosciences 555212), IL-4 (BD Biosciences 555194) and IL-10 (BD Biosciences 555157), according to the manufacturer's instructions.
Statistical analysis
For the comparison of SI (proliferation) before and after immunization a paired, two-tailed t-test was applied. Cytokine data were analysed by Friedman repeated-measures analysis of variance on ranks followed by pairwise comparisons of the incubation with antigens before and after immunization using the Student–Newman–Keuls method.
Results
Vaccine-specific antibody response
Serum IgG antibody levels against vaccine components were determined for all 15 study subjects before and after immunization. As shown in Table 1, varying anti-LPS and -toxin A IgG titres, ranging from 1·5 µg/ml (IATS-10) to as high as 42·2 µg/ml (IATS-5) were detected before immunization (day 1). Immunization with PA vaccine on days 1 and 60 led to a titre-increase against all vaccine antigens. The increase ranged from 1·6-fold (IATS-11) to 23·6-fold (IATS-10), and was smaller for serotypes where high levels of specific antibody were already present before immunization. The titre-increase was significant for all antigens, except for serotype IATS-11. These data document successful immunization of the study subjects.
Table 1.
Specific serum IgG after immunization.
| Specific serum IgG; µg/ml (range) | ||||
|---|---|---|---|---|
| P. aeruginosa serotype | Before immunization (day 1) (n = 15) | Immunized (day 74) (n = 15) | Mean fold increase | Pa |
| IATS-1 | 17·9 (5·0–59·8) | 32·3 (9·7–59·6) | 1·8 | 0·008 |
| IATS-3 | 3·7 (1·1–44·7) | 38·5 (5·0–152·9) | 10·4 | 0·002 |
| IATS-4 | 4·8 (1·3–15·5) | 18·6 (3·8–48·3) | 3·9 | 0·0001 |
| IATS-5 | 42·2 (11·2–134·4) | 82·2 (46·3–160·2) | 1·9 | 0·019 |
| IATS-6 | 3·5 (1·0–15·9) | 50·1 (9·9–258·7) | 14·4 | 0·001 |
| IATS-10 | 1·5 (0·7–4·2) | 35·4 (6·4–145·6) | 23·6 | 0·001 |
| IATS-11 | 9·7 (0·9–63·3) | 15·5 (2·2–131·6) | 1·6 | 0·188 |
| IATS-16 | 29·4 (6·6–113·1) | 66·1 (24·7–153·6) | 2·2 | 0·009 |
| toxin A | 1·2 (0·9–4·7) | 5·1 (0·9–90·9) | 4·2 | 0·028 |
Paired, two-tailed t-test between day 1 and day 74.
Flow cytometry
The cellular composition of peripheral blood was analysed by flow cytometry in all 15 subjects (Table 2) before (day 1) and after immunization (day 74). No significant differences in the total number of cells were detected before and after immunization (data not shown). Whereas the percentage of lymphocytes remained constant, a decrease in monocytes (P = 0·019) with a concomitant increase of granulocytes (P = 0·054) was observed between day 1 and 74. The fraction of B cells remained constant, and no change in B cells expressing the memory marker CD27 was noted. The percentage of IgM-expressing memory B cells also remained unchanged, but a trend (P = 0·074) to a higher fraction of IgG+ memory B cells after immunization was observed. The proportion of T cells and CD4 and CD8 subsets did not change either. Surprisingly, a significant increase (P = 0·008) was observed in naive CD4 T cells (defined as CD8–, CD45RA+T cells). This trend was less pronounced (P = 0·095) for CD8+T cells. In summary, immunization did not lead to significant shifts in leucocyte populations in peripheral blood apart from an increase in granulocytes, IgG+ memory B cells and naive (CD45RA+) CD4 T cells.
Table 2.
Percentages of leucocyte subsets before and after immunization.
| % Positive cells (range) | |||
|---|---|---|---|
| Parameter (marker) | Before immunization (day 1) (n = 15) | Immunized (day 74) (n = 15) | Pg |
| Lymphocytes (FSC/SSC)a | 14·5 (8·8–25·8) | 12·5 (5·1–25·2) | 0·155 |
| Monocytes (FSC/SSC)a | 7·5 (5·1–12·1) | 5·9 (3·1–9·2) | 0·019 |
| Granulocytes (FSC/SSC)a | 66·3 (50·7–77·1) | 70·7 (52·4–84·2) | 0·054 |
| B cells (CD19+)b | 6·6 (1·2–12·6) | 7·2 (3·4–11·0) | 0·716 |
| Memory B-cells (CD19+ CD27+)c | 26·6 (17·1–47·0) | 25·9 (14·8–45·0) | 0·763 |
| IgM+ memory B cells (IgM+CD27+)c | 10·1 (4·5–34·0) | 9·9 (3·0–29·1) | 0·931 |
| IgG+ memory B cells (IgG+CD27+)c | 3·1 (0·9–6·2) | 4·5 (2·5–9·6) | 0·074 |
| T cells (CD3+)b | 72·6 (64·2–82·0) | 72·0 (59·2–85·1) | 0·731 |
| CD4+T cells (CD3+CD4+)d | 61·8 (46·3–71·4) | 62·8 (49·8–72·7) | 0·545 |
| CD8+T cells (CD3+CD8+)d | 36·9 (28·6–53·7) | 36·5 (27·3–50·2) | 0·545 |
| Ratio CD4/CD8 | 1·7 (0·9–2·5) | 1·7 (1·0–2·7) | 0·828 |
| Naive CD4+T cells (CD8–CD45RA+)e | 31·6 (20·0–48·8) | 37·7 (24·5–65·4) | 0·008 |
| Naive CD8+T cells (CD8+CD45RA+)f | 45·9 (26·7–67·0) | 51·0 (28·8–78·1) | 0·095 |
Among all cells
among all lymphocytes (defined by FSC/SSC)
among all B cells (CD19+)
among all T cells (CD3+)
among all CD4 T cells (CD3+CD8–)
among all CD8 T cells (CD3+CD8+)
paired, two-tailed t-test between day 1 and day 74.
Antigen-induced T cell stimulation
Tetanus toxoid was chosen as a positive control for these estimations because the entire study population had been immunized against tetanus and so was expected to have specific T cells. Robust proliferation in response to tetanus toxoid was indeed observed with lymphocytes taken before and after immunization. Interestingly, a trend to increased reactivity to tetanus toxoid was observed in several but not all individuals after immunization with PA vaccine (rise of SI from 28·7 before to 68·4 after immunization, P = 0·172; Fig. 1a, individual data in Fig. 1b). To investigate vaccine-specific T cells, lymphocytes were stimulated with a detoxified variant of the carrier protein toxin A (toxin A-AH). Some stimulation (SI = 2·9) of lymphocytes before immunization was observed with the highest restimulation concentration of toxin A-AH (10 µg/ml). This proliferation might be due to natural exposure of the study subjects to Pseudomonas, similar to the pre-existing serum IgG antibody levels present on day 1 (Table 1). Immunization resulted in a marked and highly significant (P = 0·04–0·003) increase in SI, e.g. a rise from 1·4 to 42·2 after stimulation with 0·4 µg/ml toxin A-AH (Fig. 1a; individual data in Fig. 1b).
Antigen-induced cytokine production
As shown in Fig. 2, no or only minute amounts of IFN-γ were produced in unstimulated (control) lymphocyte cultures. In contrast, strong IFN-γ production was stimulated in the presence of tetanus toxoid in lymphocytes taken before and after immunization. Generally, large variations between individual subjects were observed, reflected in the large range (25–75 percentile). Similar results were obtained for TNF-α. Very little or no IFN-γ was synthesized when lymphocytes taken before immunization were cultured in the presence of P. aeruginosa antigens. Immunization provoked strong production of IFN-γ. Interestingly, the four pseudomonal antigens induced production of varying levels of IFN-γ, i.e. the order of IFN-γ induction was IATS6-TA > IATS1-TA > IATS10-TA. Similar results were observed for TNF-α. In contrast, very low production of the Th2 cytokines IL-4 and IL-10 was stimulated. The production occurred in only a few patients, which is reflected in the large standard deviations. For some donors (n = 4) cultures for IL-4 and IL-10 were performed with antigen-specific restimulation for 7 days and additional PMA/ionomycin stimulation for the last 18 h of culture [13]. However, no significant induction of IL4 or IL-10 was observed (data not shown) even under these optimized culture conditions.
Fig. 2.
Antigen-induced cytokine production. Lymphocytes from peripheral blood were cultured in the presence of various antigens before (open circles) and after (filled circles) immunization with P. aeruginosa O-polysaccharide-toxin A conjugate (PA) vaccine. Content of cytokines was measured by enzyme-linked immunosorbent assay (ELISA) in cell culture supernatant fluid collected after 3 days of culture. Median and 25–75 percentile is shown. Statistical analysis by Friedman repeated-measures analysis of variances on ranks followed by the Student–Newman–Keuls method.
Discussion
This pilot study was conducted to evaluate cellular immunity in healthy individuals after immunization with our octavalent P. aeruginosa O-polysaccharide-toxin A conjugate vaccine. Humoral immune responses against the vaccine have been studied extensively. Specific serum IgG responses were monitored in a cohort of 25 CF patients who received basic immunization followed by yearly boosters over a period of 10 years ([9] and Zuercher et al. submitted). In these studies we found that yearly vaccination reduced the proportion of patients with chronic P. aeruginosa lung infection, and that protection was associated with vaccine-specific high-affinity serum IgG antibodies. Humoral immunity is also being analysed in a phase III, double-blind, randomized, placebo-controlled, multi-centre trial currently being undertaken in patients with CF to determine the efficacy of the vaccine in preventing respiratory tract infections with P. aeruginosa. In contrast, so far no aspects of cellular immunity induced by the vaccine have been studied. It is known that the response of immune cells to a conjugate vaccine varies according to the carrier protein [14–16]. These differences are reflected mainly in cytokine patters induced by antigen-specific restimulation. It is of great importance to estimate this, as it is a major determinant of vaccine effectiveness. In animal studies [11,12] and in CF patients [13], cytokine patterns directly determined the severity and outcome of lung infection with P. aeruginosa.
Measurement of specific serum antibody levels (Table 1) before and after immunization shows that immunization was successful and led to induction of robust serum IgG levels against all vaccine components. Some pre-existing specific serum IgG antibodies were detected against all vaccine antigens, particularly against the O-polysaccharide components. These stem most probably from natural exposure to the ubiquitously present P. aeruginosa.
In our flow cytometry analyses we generally observed only small shifts in cell (sub)populations before and after immunization. The impact of a vaccine on the quantitative and qualitative cellular composition of the immune system is relatively minor, and this is not surprising given the plasticity of the cellular immune system. Shifts in entire cell populations have been observed only after infection with strongly immunogenic pathogens; e.g. Hoshino et al. have shown [17] that infection with Epstein–Barr virus resulted in a transient, quantitative expansion of the entire CD8+T cell subset. In contrast, the effects due to immunization might be too mild to be detected on a quantitative level and might rather be reflected in qualitative changes of the cells. In this study some effects on the composition of peripheral blood leucocytes were shown by flow cytometry. After immunization a slight increase of granulocytes occurred, due probably to a mild inflammatory response caused by the vaccine. The concomitant induction of TNF-α supports this notion. Additionally, a trend to an increase in the fraction of IgG+ memory B cells, but not other B cell subtypes, was observed after immunization. This is anticipated as a normal response to the vaccine. Conjugate vaccines induce production of specific IgG antibodies, and thus an increase of IgG+ memory B cells is to be expected. Surprisingly, a significant increase in naive CD4+ cells (CD45RA+) was also noted. Contrary to this observation it has been shown that immunization or infection usually leads to an increase in T cells expressing activation and/or memory markers such as CD45RO [18,19]. However, in general the effects of immunization with the PA vaccine on peripheral blood leucocytes revealed by flow cytometry were minor.
In contrast, we measured strong proliferation of lymphocytes from the immunized donors in response to the carrier protein toxin A. Interestingly, immunization also resulted in a slight increase in tetanus-specific proliferation in some individuals (Fig. 1b). Similarly, an increase in tetanus-induced cytokine production was observed (Fig. 2). This finding is due most probably to bystander activation of tetanus-specific CD4+T cells. It has been shown that memory CD4+T cells have a low activation threshold, and that an increase in their turnover rate can be induced via various cytokine pathways in the absence of antigen [20]. As shown in Fig. 2, the levels of cytokines varied after stimulation with different antigenic preparations: the order of stimulation was IATS6-TA > IATS1-TA > IATS10-TA. This might be due to the fact that the concentration of 2 µg/ml that was used for in vitro restimulation was based on the polysaccharide content of the individual conjugates. In contrast, the content of toxin A differed slightly (4 µg/ml for IATS6-TA, 3·2 µg/ml for IATS1-TA and 3·1 µg/ml for IATS10-TA). These data indicate that the magnitude of cytokine production depended directly on the concentration of the protein component contained in the conjugate (toxin A), and that apparently the polysaccharide component had no or only a minor influence on cytokine induction. The same outcome would be expected for biofilm components that are mainly exopolysaccharides (alginate). Similar findings were obtained in a study of pneumococcal conjugate vaccines with different carriers [16]. Consequently, the quality of the immune response against a protein–polysaccharide conjugate might be determined exclusively by the choice of the carrier protein (see also below).
Analysis of cytokine production by peripheral lymphocytes after immunization revealed a distinct profile, with strong IFN-γ and TNF-α and few IL-4 and IL-10 responses. In a study using a similar conjugate vaccine, it has been shown previously that immunized rats developed a Th1-like inflammation upon lung challenge, whereas non-immunized rats developed a Th2-like inflammation [10]. Thus, our present findings are in line with this earlier study. Similar analyses after immunization with other conjugate vaccines showed that the cytokine pattern depended mainly on the carrier protein. Studies with S. pneumoniae capsular polysaccharide-CRM197 conjugate vaccine [14] or H. influenzae Type b-CRM197 conjugate vaccine [15] showed a mixed Th1/Th2 cytokine pattern, dependent on the activation of CRM197-specific CD4+T cells. Our results using toxin A from P. aeruginosa as the carrier protein suggest induction of a Th1-biased CD4+T cell response. In the context of P. aeruginosa infection in CF patients, pre-existing Th1-primed, antigen-specific cells might be an advantage. In a mouse model of lung challenge with alginate-embedded P. aeruginosa Moser et al. [11,12] have shown that mortality, lung pathology and bacterial loads were significantly less severe in C3H/HeN mice that show a Th1-like response upon challenge, compared with BALB/c mice that mount a Th2-like response. Similarly, in CF patients with chronic P. aeruginosa lung infection the ability of peripheral blood lymphocytes to produce IFN-γ was correlated positively with better lung function [13]. Together these studies suggest that steering immunity against P. aeruginosa Towards a Th1-like response with a vaccine might benefit the CF patient. This potentially advantageous effect of immunization with PA vaccine has to be confirmed in patients.
Acknowledgments
We would like to thank Dr Michael P. Horn, Silvana Manolio, Thomas Schwaar, Rolf Ryser and Chantal Zufferey for their excellent work.
References
- 1.Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–9. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 2.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryz SJ, Jr, Fuerer E, Cross AS, et al. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987;80:51–6. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryz SJ, Jr, Sadoff JC, Ohman D, et al. Characterization of the human immune response to a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. J Lab Clin Med. 1988;111:701–7. [PubMed] [Google Scholar]
- 5.Cryz SJ, Jr, Wedgewood J, Lang AB, et al. Immunization of noncolonized cystic fibrosis patients against Pseudomonas aeruginosa. J Infect Dis. 1994;169:1159–62. doi: 10.1093/infdis/169.5.1159. [DOI] [PubMed] [Google Scholar]
- 6.Cryz SJ, Jr, Lang A, Rudeberg A, et al. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst Mitt. 1997;98:345–9. [PubMed] [Google Scholar]
- 7.Schaad UB, Lang AB, Wedgwood J, et al. Safety and immunogenicity of Pseudomonas aeruginosa polyvalent conjugate A vaccine in cystic fibrosis. Lancet. 1991;338:1236–7. doi: 10.1016/0140-6736(91)92103-9. [DOI] [PubMed] [Google Scholar]
- 8.Lang AB, Schaad UB, Ruedeberg A, et al. Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Pediatr. 1995;127:711–7. doi: 10.1016/s0022-3476(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 9.Lang AB, Rüdeberg A, Schöni MH, et al. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J. 2004;23:504–10. doi: 10.1097/01.inf.0000129688.50588.ac. [DOI] [PubMed] [Google Scholar]
- 10.Johansen HK, Hougen HP, Cryz SJ, Jr, et al. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–46. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- 11.Moser C, Johansen HK, Song Z, et al. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS. 1997;105:838–42. [PubMed] [Google Scholar]
- 12.Moser C, Hougen HP, Song Z, et al. Early immune response in susceptible and resistant mice strains with chronic Pseudomonas aeruginosa lung infection determines the type of T-helper cell response. APMIS. 1999;107:1093–100. doi: 10.1111/j.1699-0463.1999.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 13.Moser C, Kjaergaard S, Pressler T, et al. The immune response to chronic Pseudomonas aeruginosa lung infection in cycstic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108:329–35. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumoboj KK, Kirchner HL, Kimmel R, et al. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier. J Infect Dis. 2003;187:1629–38. doi: 10.1086/374785. [DOI] [PubMed] [Google Scholar]
- 15.Kumboj KK, King CL, Greenspan NS, et al. Immunization with Haemophilus influenza Type b-CRM197 conjugate vaccine elicits a mixed Th1 and Th2 CD4+T cell cytokine response that correlates with the isotype of antipolysaccharide antibody. J Infect Dis. 2001;184:931–5. doi: 10.1086/323342. [DOI] [PubMed] [Google Scholar]
- 16.Wuorimaa T, Kayhty H, Eskola J, et al. Activation of cell-mediated immunity following immunization with pneumococcal conjugate or polysaccharide vaccine. Scand J Immunol. 2001;53:422–8. doi: 10.1046/j.1365-3083.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino Y, Morishima T, Kimura H, et al. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J Immunol. 1999;163:5735–40. [PubMed] [Google Scholar]
- 18.McElhaney JE, Pinkoski M, Meneilly GS. Changes in CD45 isoform expression vary according to the duration of T-cell memory vaccination. Clin Lab Diagn. 1995;2:73–81. doi: 10.1128/cdli.2.1.73-81.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zepp F, Knuf M, Habermehl P, et al. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–84. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+T cells upon NK T cell or T cell activation. J Immunol. 2000;165:4305–11. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]