Abstract
Due to the fact that many cellular proteins are extensively glycosylated, processing and presentation mechanisms are expected to produce a pool of major histocompatibility complex (MHC) class I-bound protein-derived peptides, part of which retain sugar moieties. The immunogenic properties of the presented glycosylated peptides in comparison to their non-glycosylated counterparts have not been determined clearly. We assessed the cellular immunogenicity of MUC1 (mucin)-derived peptides O-glycosylated with a Tn epitope (GalNAc) using HLA-A*0201 single chain (HHD)-transfected cell lines and transgenic mice. For part of the compounds Tn moiety did not interfere with the HLA-A*0201 binding. Moreover, part of the glycopeptides elicited effective cytotoxic responses, indicating recognition of the glycopeptide-HLA-A*0201 complex by the T cell receptor (TCR) and subsequent cytotoxic T lymphocyte (CTL) activation. The CTLs exhibited a substantial degree of cross-reactivity against target cells loaded with glycosylated and non-glycosylated forms of the same peptide. The studied (glyco)peptides showed cellular immunogenicity in both MUC1-HHD and HHD mice and induced effective lysis of (glyco)peptide-loaded target cells in CTL assays. However, the elicited CTLs did not induce selective lysis of human MUC1-expressing murine cell lines. Moreover, immunization with (glyco)peptide-loaded dendritic cells (DCs) did not induce significant immunotherapeutic effects. We conclude that Tn glycosylated MUC1-derived peptides can be presented by MHC class I molecules, and may be recognized by specific TCR molecules resulting in cytotoxic immune responses. However, the studied glycopeptides did not offer significant benefit as targets for cytotoxic immune response due apparently to (a) cross-reactivity of the elicited CTLs against the glycosylated and non-glycosylated forms of the same peptide and (b) low abundance of glycopeptides on tumour target cells.
Keywords: cytotoxic T lymphocytes, glycopeptides, glycosylation, tumour immunity
Introduction
Immunotherapy specific for tumour-associated antigens (TAAs) has become a major field of investigation for the treatment of cancer. The processing, presentation and cellular immunogenicity of TAAs in vitro and in vivo has been assessed in numerous studies. The limited success of peptide-based epitopes applied for mounting cytotoxic immune responses in clinical settings provided a drive for the search of additional, potentially more specific and efficient approaches that could be applied as targets for specific immune modulation and therapy.
During the last decade it has been shown that the repertoire of antigenic structures recognized by T cells is highly diverse and is not limited to peptides only, but also includes glycopeptides, lipids and glycolipids [1]. The extensive glycosylation of the major part of cellular proteins implies that following their processing, glycosylated peptides may be formed and presented on major histocompatibility complex (MHC) class I and II molecules resulting in glycopeptide-specific immune responses.
The principal issues regarding the processing, presentation and immunogenicity of the glycopeptides in comparison to their non-glycosylated counterparts have been studied by several research groups. Synthetic glycopeptides, derived mainly from MUC1 that is heavily glycosylated and overexpressed in a wide range of epithelial tumours [2–5], were instrumental tools for studying the possibility of glycopeptide presentation by the MHC molecules and the resulting immunogenicity. Synthetic MUC1-derived glycopeptides were shown to elicit glycopeptide-specific CD8+ T cell responses in cultured splenocytes or following immunization of experimental animals [6,7]. Accommodation of the glycopeptidic ligands by the MHC peptide-binding groove that is required for mounting these CD8+ T cell responses was assessed using crystallography. In most cases it was shown that the carbohydrate moiety protrudes outside the peptide-binding groove towards the T cell receptor (TCR) [8]. The possible role of glycosyl moieties as anchor groups has been demonstrated recently for a MUC1-derived peptide bearing Thomsen–Freidenreich (T) antigen (Gal-GalNAc) [9].
Despite the significant scientific efforts to elucidate the details of glycopeptide presentation and immunogenicity, the more clinically orientated aspects of this field remain largely unestablished. It is not determined whether the abundance of the glycosylated peptides presented on the sell surface, and the fine specificity of their recognition by the TCR compared to the non-glycosylated peptides, permit specific recognition and make them a suitable target for anticancer immune responses in experimental animals and humans.
To assess these issues, we studied a panel of MUC1-derived glycosylated and non-glycosylated peptides applying ‘humanized’ HLA-A*0201-transfected cell lines and transgenic mice. The glycosylation pattern of the MUC1-derived glycopeptides and the length and conformation of the glycosyl side chain is expected to be critical for the interaction with cytotoxic T cells and the resulting immune response [10] Thus, we studied Tn (GalNAc) glycosylated peptides, Tn being one of the most abundant moieties present on MUC1 in cancer cells [11,12], and such peptides are expected to be presented on MHC class I molecules by MUC1-expressing cells. To assess the potential clinically orientated aspects of the elicited cytotoxic immune responses, immunogenicity of the MUC1-derived (glyco)peptides was studied in mice that are transgenic for human MHC class I (HLA-A*0201, HHD mice) and human MUC1 (MUC1-HHD mice).
Methods
Mice
HHD mice
HLA-A*0201/Db-β2 monochain transgenic, H-2Db–/– ×β2m–/– double-knock-out mice (the resulting phenotype: HHD+, Kb–, Db–) [13].
MUC1-HHD mice
HHD mice transgenic for the human MUC1 gene (MUC1+/–); were obtained by breeding HHD and MUC1 transgenic mice and back-crossing to HHD mice. The resulting mice (HHD+, Kb–, Db–) are homozygous for HHD and heterozygous for MUC1. The experimental animals were bred at the Weizmann Institute of Science. All experiments were conducted in accordance with Weizmann Animal Facility and National Institutes of Health guidelines.
Cell lines
EL4-HHD is an HHD transfectant of the murine T lymphoma EL4. RMA-S-HHD-B7·1 is a TAP-2-deficient RMA-S lymphoma clone of C57Bl/6 origin transfected with HHD and the murine B7·1 co-stimulatory molecule. D122-HHD (designated as DH) is an HHD transfectant of the poorly immunogenic, low Kb-expressor D122 clone of 3LL Lewis lung carcinoma. D122-HHD-pcDNA3·1 (designated as DH-mock) is a mock transfectant clone of D122-HHD. D122-HHD-MUC1Y-4 (designated as DH-MUC1Y) is a D122-HHD clone transfected with the short version (MUC1Y) of human MUC1 sequence (lacking tandem repeats). D122-HHD-22TR MUC1 2-4-11 and D122-HHD-22TR MUC1 2-4-6 (designated as DH-MUC1REPlow and DH-MUC1REPhigh, respectively) are transfectants of D122-HHD with the long version of human MUC1 sequence (with 22 tandem repeats). EL4-HHD and RMAS-HHD-B7·1 cells were maintained in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal calf serum (FCS), combined antibiotics and 500 µg/ml of Geneticin (both from Life Technologies, Rockville, MD, USA).
D122-HHD cells were maintained in DMEM supplemented with 10% FCS, 2 mm l-glutamine, 1% sodium pyruvate, 1% non-essential amino acids (all obtained from Sigma) and combined antibiotics. The D122-HHD transfectants (DH-mock, DH-MUC1Y, DH-MUC1REPlow and DH-MUC1REPhigh) were maintained in the medium applied for D122-HHD cells supplemented with 300 µg/ml Hygromycin B (Calbiochem, San Diego, CA, USA).
Peptide synthesis
Non-glycosylated peptides (see Table 1) were synthesized on an ABIMED AMS 422 multiple peptide synthesizer (Abimed, Langenfeld, Germany), employing the a-N-fluorenylmethoxy-cartonyl (Fmoc) strategy following the commercially available manufacturer's protocols. Glycopeptides (see Table 1) were synthesized manually applying similar protocol using glycosylated amino acids {Fmoc-L-Ser[Tn(Ac4)]-OH, Fmoc-L-Thr[Tn(Ac4)]-OH, obtained from IRIS Biotech, Marktredwitz, Germany} as building blocks for synthesis. For both peptide and glycopeptide synthesis, the peptide chain assembly was conducted on a 2-chlorotrityl chloride resin (Novabiochem, Laufelfingen, Switzerland). Crude compounds were purified to homogeneity by reversed-phase high performance liquid chromatography (HPLC) on a semipreparative silica C-8 column (250 × 10 mm; Lichnosorb RP-8; Merck, Darmstadt, Germany). Elution was accomplished by a linear gradient established between 0·1% trifluoroacetic acid (TFA) in water and 0·1% TFA in 70% acetonitrile in water (v/v). Composition of the products was determined by amino-acid analysis (automatic amino acid analyser; Dionex, Sunnyvale, CA, USA) after extraction acid hydrolysis. Molecular weight was ascertained by mass spectrometry (VG Tofspec; Laser Desorption Mass Spectrometry; Fisons, Manchester, UK).
Table 1.
The studied glycopeptides.
| Non-glycosylated peptides1 | O-glycosylation on serine residue2 | O-glycosylation on threonine residue2 | |||
|---|---|---|---|---|---|
| A7 | NLTISDVSV | S-A7 | NLTISDVSV | T-A7 | NLTISDVSV |
| B5 | SLSYTNPAV | S-B5 | SLSYTNPAV | – | – |
| E6 | ALASTAPPV | S-E6 | ALASTAPPV | T-E6 | ALASTAPPV |
| D6 | LLLTVLTVV | – | – | – | – |
| PAP3 | ILLWQPIPV | – | – | – | – |
A7, B5, E6 and D6 are derived from the MUC1 sequence. PAP3 is an irrelevant peptide derived from prostatic acid phosphatase.
O-gycosylation with Tn epitope (GalNAc) was applied.
Human MUC1 gene cloning, expression in pcDNA3·1/Hygro(+) and transfection into D122-HHD cells
The short version of human MUC1 (MUC1Y) was a kind gift from Professor D. Wreshner (Tel Aviv University, Israel) [14], and the long version of human MUC1 that includes 22 tandem repeats (22TR) was a kind gift from Professor O. Finn (University of Pittsburg, USA) [15]. Each version of MUC1 was transferred into a pcDNA3·1/Hygro(+) vector (Invitrogen, Groningen, the Netherlands) by polymerase chain reaction (PCR) using primers containing HindIII and XhoI restriction sites. For MUC1Y, the stop codon was eliminated and replaced with an HA tag sequence followed by a stop codon. The inserted sequences were verified by sequencing.
Plasmid integrity was confirmed by transient transfection into 293T cells followed by Western blot analysis using mouse anti-HA (for MUC1Y) and H23 monoclonal antibodies (mAbs) (for 22TR) and FACS analysis using H23 mAb (for 22TR).
D122-HHD cells were transfected with pcDNA3·1/Hygro(+)-MUC1 expression vectors using the Lipofectamine reagent (Invitrogen, Life Technologies) in accordance with the manufacturer's protocol. Stable transfectants were selected and cloned in the presence of 300 µg/ml Hygromycin B (Invitrogen, Life Technologies). The presence of MUC1 transcript and proteins in the selected D122-HHD-MUC1 clones was confirmed by reverse transcription-polymerase chain reaction (RT-PCR), Western blot analysis using anti-HA or H23 mAbs (for DH-MUC1Y or DH-MUC1REP clones, respectively) and FACS analysis using H23 mAb (for 22TR).
Peptide loading and measurement of peptide binding by stabilization of cell surface MHC
Peptide binding to HHD single chain was measured by stabilization of HHD on RMA-S-HHD-B7·1 cells using an indirect FACS analysis. Cells, 5 × 105/sample, were incubated overnight at 26°C in Opti-MEM I medium with (glyco)peptides or control peptides (20 nmol/ml) or vehicle, followed by incubation for 3 h at 37°C (not applied for PAP3 and vehicle controls). HHD expression on the cell surface was determined by direct FACS analysis using αhuman leucocyte antigen (HLA-ABC)-fluorescein isothiocyanate (FITC) antibody, FACScan apparatus and Cellquest Software (Becton Dickinson, San Jose, CA, USA).
Generation of murine bone marrow-derived dendritic cells
The procedure described by Lutz et al. [16] was applied with some minor modifications. Bone marrow from femurs and tibiae from 4 to 6-week-old HHD male mice was flushed with phosphate-buffered saline (PBS). The bone marrow cells (5 × 106) were cultured in 100 mm bacteriological Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) in 10 ml DC medium (RPMI-1640 supplemented with 2 mM l-glutamine, 50 µM 2-mercaptoethanol, combined antibiotics (all from Sigma) 10% FCS and 40 ng/ml recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (Prospec, Rehovot, Israel). On day 3, an additional 10 ml of DC medium containing 40 ng/ml GM-CSF were added to each plate. On day 6, half the culture supernatant was replaced with fresh DC medium containing 40 ng/ml GM-CSF. On day 8 non-adherent cells were collected, centrifuged at 300 g for 5 min at room temperature (RT), resuspended in 10 ml fresh DC medium containing 20 ng/ml GM-CSF and seeded into 100-mm tissue culture plastic dishes (Falcon, Germany). On day 9, 10 ml fresh DC medium containing 20 ng/ml GM-CSF was added together with lipopolysaccharide (LPS) (Sigma, Rehovot, Israel, to a final concentration of 1 µg/ml). On day 10, non-adherent cells were harvested and used for vaccination. FACS analysis indicated that the harvested cells exhibited typical characteristics of mature DCs (> 95% were CD11c+, CD80+low, CD86low, MHC IIhigh).
Vaccination
HHD mice were immunized intraperitoneally (i.p.) three times at 7-day intervals with 2 × 106 irradiated (5000 rads) peptide-loaded RMA-S-HHD-B7·1 or with 1·5 × 106 (glyco)peptide- pulsed (100 µM for 2 h at 37°C) dendritic cells.
In vitro cytotoxicity assays
HHD mice were immunized as described above. In all vaccination modes, spleens were removed on day 10 after the last immunization and cell suspensions were prepared. A third part of the splenocyte suspension was pulsed with 100 µM synthetic (glyco)peptides for 2 h at 37°C, and then added to the rest of the splenocytes. Cultures were incubated for 5 days in lymphocyte medium (RPMI medium supplemented with 25 mM HEPES pH 7·4, 10% FCS, 2 mM glutamine, 1 mM sodium pyruvate, 1% non-essential amino acids, 5 × 10−5 M 2-mercaptoethanol and combined antibiotics, all from Life Technologies). Viable effector lymphocytes were separated by Lympholyte-M (Cedarlane Laboratories Ltd, Hornby, Ontario, Canada) gradients, resuspended in lymphocyte medium and admixed at different ratios with 5000 35S-methionine-labelled target cells. Cytotoxic T lymphocyte (CTL) assays were carried out as described previously [17] in four to eight effector-to-target ratios, ranging from 100: 1 to 12·5: 1, or 100: 1–0·78: 1. Percentage of specific lysis was calculated as follows: % lysis = [counts per min (cpm) in experimental wells−cpm spontaneous release)/(cpm maximal release − cpm spontaneous release] × 100%.
Immunotherapy of tumour-bearing mice
HHD mice (8–10 per group) were challenged subcutaneously (s.c.) with 1 × 106 D122-HHD-MUC1 tumour cells (DH-MUC1Y or DH-MUC1REPlow clones). Twelve days later, when tumours reached 2–3 mm in diameter, mice were immunized by injecting peptide-loaded DCs, as described above. Each group was immunized three times at 7-day intervals. Tumour diameter and survival of mice were monitored.
Statistical analysis
The data obtained in the MHC stabilization assays and CTL assays were analysed by one-way anova. Analysis of tumour growth and survival differences was performed using the log-rank test.
Results
Glycosylation of peptide with Tn epitope does not necessarily interfere with peptide-MHC binding
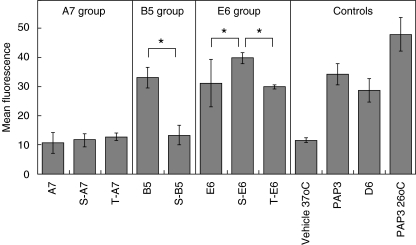
To assess the influence of glycosylation with Tn moiety on binding of the resulting glycopeptide to HLA-A*0201 molecules, we applied MHC stabilization assays using the murine TAP-2 deficient, HLA-A*0201-transfected RMA-S cells (RMA-S-HHD-B7·1) that exhibit low levels of endogenous peptide presentation. External loading of the studied compounds followed by incubation at 37°C and FACS determination of HLA-A*0201 provide an indirect mean to determine the affinity of the studied compounds because at these conditions only peptide-bound MHC class I molecules are stable and are present on the cell surface.
The outcome of the MHC stabilization assays indicates that peptide A7 possessed the least binding affinity for HLA-A*0201 (see Fig. 1), and glycosylation of A7 with a Tn moiety did not increase significantly the HLA-A*0201 binding. For B5, that exhibited moderate affinity to HLA-A*0201, glycosylation at the serine residue resulted in substantial decrease in HLA-A*0201 binding. On the other hand, both S-E6 and T-E6 showed moderate HLA-A*0201 binding that was higher than or comparable to that of the parent compound (for S-E6 and T-E6, respectively).
Fig. 1.
Glycosylation of peptide with Tn epitope does not necessarily interfere with peptide–major histocompatibility complex (MHC) binding. TAP2-deficient RMA-S-HHD-B7·1 cells were incubated overnight at 26°C in Opti-MEM I medium with (glyco)peptides or control peptides (20 nmol/ml) or vehicle, followed by incubation for 3 h at 37°C (apart from PAP3 control that was incubated at 26°C). Direct FACS analysis was performed using αHLA-ABC-FITC antibody. Mean ± s.d. data of three experimental sets are presented. *P < 0·05 by one-way anova analysis.
It should be noted that all the studied (glyco)peptides are characterized by limited affinity to HLA-A*0201, in comparison to the control compounds D6 and PAP3. Prolonged incubation of the studied (glyco)peptides, but not of D6 and PAP3, with RMA-S-HHD-B7·1 cells (> 12 h) was required in order to determine the differences in HLA-A*0201 binding (preliminary data with 3–4 h incubation periods are not shown).
The results of the MHC stabilization assays indicate that the small-size Tn group attached to the central part of the peptide, may be accommodated in some cases within the HLA-A*0201 peptide-binding groove, enabling the presentation of glycopeptide by the HLA-A*0201 molecules.
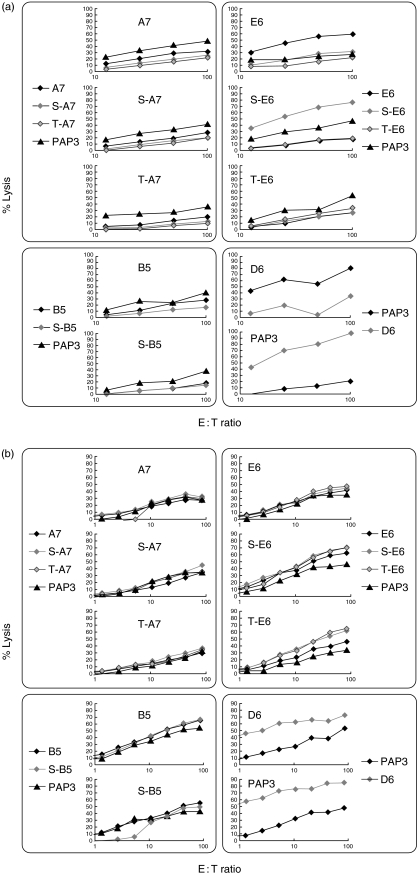
(Glyco)peptide-induced CTL responses in HHD mice
In order to determine the immunogenicity of the glycoepeptides compared to the non-glycosylated peptides, we applied the ‘humanized’ HHD mouse model. Immunization of the experimental animals with (glyco)peptide-loaded RMA-S-HHD-B7·1 cells induced limited CTL responses for most of the compounds studied (see Fig. 2a). Only immunization with E6 and S-E6-loaded cells resulted in expansion of CTL pools that lysed effectively the target cells loaded with the corresponding (glyco)peptide. Selective CTL responses were induced, as the resulting CTLs did not exhibit significant cross-reactivity towards the glycosylated and non-glycosylated forms of the same peptide. Interpretation of the experimental results was hampered by the high background of the control groups (killing of the PAP3-loaded target cells by the elicited CTLs; see, e.g. for S-E6 panel).
Fig. 2.
(Glyco)peptide-induced CTL responses in HHD mice. (a) (Glyco)peptide-loaded RMA-S-HHD-B7·1 cells induced limited cytotoxic T lymphocyte (CTL) responses. CTLs did not exhibit significant cross-reactivity to the glycosylated and non-glycosylated forms of the same peptide. (b) (Glyco)peptide-loaded dendritic cells (DCs) induced enhanced CTL responses. CTLs elicited against immunogenic peptides exhibited significant cross-reactivity to the glycosylated and non-glycosylated forms of the same peptide. HHD mice were immunized intraperitoneally three times at 7-day intervals with 1 × 106 (glyco)peptide-loaded RMA-S-HHD-B7·1 cells of mature HHD DCs. Spleens were removed on day 10 after the last immunization, and splenocytes were resensitized in vitro with immunizing (glyco)peptide. CTL assays were performed on day 15 with (glyco)peptide-loaded EL4-HHD cells as target cells. Mean experimental data are presented; the % CV values for the individual data points were below 10%. The labels on each individual plot indicate the immunizing peptide.
Both PAP3 (irrelevant peptide) and D6 (MUC1-derived peptide) controls were highly immunogenic in HHD mice (see Fig. 2a), indicating that the CTL clones that were able to recognize the studied peptides were not subject to clonal deletion, and the limited immunogenicity of the studied compounds derives most probably from inefficient immunization/interaction with TCR and not from the central immunotolerance.
Immunization of HHD mice with (glyco)peptide-loaded autologous DCs resulted in generally higher cytotoxic immunogenicity with lower background effects compared to that obtained after immunization with (glyco)peptide-loaded RMA-S-HHD-B7·1 cells (see Fig. 2b versus 2a). The experimental results showed a similar trend with high immunogenicity of the control compounds (PAP3 and D6), and no substantial cytotoxic responses for A7 and B5 peptides and their glyco-derivatives. On the other hand, the E6 peptide and its glyco-derivatives (S-E6 and T-E6) showed a substantial degree of cytotoxic immunogenicity with a high degree of cross-reactivity of the resulting CTLs towards the glycosylated and non-glycosylated forms of the same peptide.
Overall, the outcomes of immunization of HHD mice show that (glyco)peptides are more immunogenic when loaded onto DCs compared to the RMA-S-HHD-B7·1 cells (see E6 and its derivatives). The pattern of the cytotoxic immunogenicity of the studied compounds seems to reflect their affinity to the HLA-A*0201, with A7 and its glyco-derivatives being the low binders and the least immunogenic compounds, while higher-binding E6 and its glyco-derivatives being moderately immunogenic. Significant cross-reactivity of the elicited CTLs against the glycosylated and non-glycosylated forms of the same peptide indicates that cross-reactive pools of CTLs are activated in both cases and/or limited effect of the glycosyl moiety on the specificity of recognition of the glycopeptide-HLA-A*0201 complex by the TCR (for a single CTL clone).
Lack of specific lysis of MUC1-expressing cells by CTLs raised against the studied (glyco)peptides
In order to determine whether MUC1-derived (glyco)peptides could serve potentially as specific targets for cytotoxic immunotherapy in vivo, we performed CTL assays applying CTLs elicited by immunization of HHD mice with (glyco)peptide-loaded autologous DCs to MUC1-expressing D122-HHD tumour cell lines as target cells. No selective lysis of the MUC1-expressing clones versus the control mock-transfected clone was obtained (data not shown). The studied cell lines showed extensive HHD expression (all the cell lines) and MUC1 expression (DH-MUC1Y, DH-MUC1REPlow, and DH-MUC1REPhigh) on the cell surface (data not shown). Thus, MUC1-expressing cell lines were expected to present MUC1-derived peptides in the context of HHD molecules that could serve as targets for CTL-mediated cell killing. Lack of selective killing of MUC1-expressing clones by the CTLs may be related to the limited immunogenicity of the studied compounds, or to the low extent of presentation of MUC1-derived peptides.
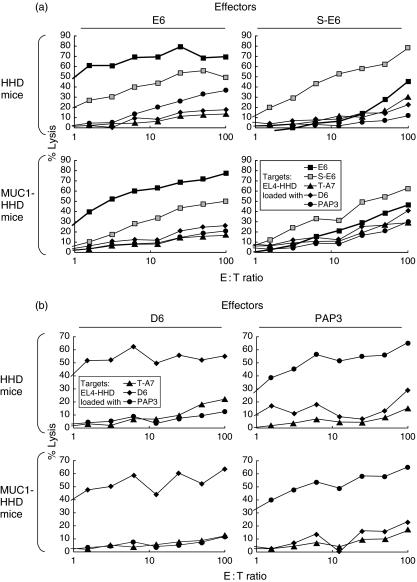
The (glyco)peptides showed comparable cellular immunogenicity in MUC1-HHD mice and HHD mice
Next, the influence of endogenic MUC1 expression on the resulting cellular immunogenicity of (glyco)peptides was studied in order to generate the experimental settings that resemble as close as possible the patterns of MUC1 expression by the tumour and normal tissues of cancer patients. To this end, we assessed the cellular immunogenicity of MUC1-derived glycopeptides in MUC1-HHD mice in comparison to HHD mice applying a panel of E6-derived compounds. MUC1-HHD mice were bred from MUC1 mice that show wide expression of MUC1 by epithelial tissues, and the patterns of MUC1 glycosylation by the tumour and normal tissues are expected to resemble those observed in MUC1-expressing carcinoma patients [18].
At higher effector-to-target ratios, E6-derived (glyco)peptides showed similar cellular immunogenicity in MUC1-HHD mice and HHD mice (see Fig. 3). At lower effector-to-target ratios, E6 and S-E6 showed higher cellular immunogenicity in HHD mice than in MUC1-HHD mice. On the other hand, both control peptides (irrelevant, PAP3; and MUC1-derived, D6) showed similar immunogenicity in MUC1-HHD mice and HHD mice at all the applied effector-to-target ratios. These outcomes indicate that MUC1 expression by normal tissues do not abolish the cytotoxic immunogenicity of MUC1-derived (glyco)peptides. The CTLs that are able to recognize the studied MUC1-derived (glyco)peptides do not undergo clonal deletion in MUC1-HHD mice and could be activated and proliferated following immunization with (glyco)peptide-loaded DCs.
Fig. 3.
The (glyco)peptides showed similar cellular immunogenicity and cross-reactivity in MUC1-HHD mice compared to the HHD mice.HHD or MUC1-HHD mice were immunized intraperitoneally three times at 7-day intervals with 1 × 106 (glyco)peptide-loaded mature HHD dendritic cells (DCs). Spleens were removed on day 10 after the last immunization, and splenocytes were resensitized in vitro with immunizing (glyco)peptide. Cytotoxic T lymphocyte (CTL) assays were performed on day 15 with (glyco)peptide-loaded EL4-HHD cells as target cells. Mean experimental data are presented; the % CV values for the individual data points were below 10%. The labels above each individual plot indicate the immunizing peptide.
MUC1Y-expressing tumour growth was unaffected by immunotherapy with (glyco)peptide-loaded DCs
In order to assess the clinical potential of the studied (glyco)peptides as targets for immunotherapy of established MUC1-expressing tumours, we studied the survival of tumour-bearing mice upon therapeutic vaccination with (glyco)peptide-loaded DCs. HHD mice were inoculated s.c. with tumorigenic doses of tumour clones expressing the short or the long version of MUC1 molecule (DH-MUC1Y, or DH-MUC1REPlow, respectively) and the immunizations were commenced after the tumours become palpable (2–3 mm diameter). Results of this experiment did not show significant tumour growth retardation or animal survival due to immunization with (glyco)peptide-loaded DCs compared to saline injections (data not shown).
Discussion
In this study we analysed systematically the effects of Tn moiety on the immunogenicity of specific MUC1-derived glycopeptides. The objective was to apply the experimental settings that represent the characteristics of glycopeptide-mediated immune response in MUC1-expressing tumour-bearing host characterized by HLA-A*0201-restricted presentation.
For this purpose, we have applied a panel of MUC1-derived peptides with Tn epitope moiety that is expressed abundantly on the MUC1 molecules in tumour cells and may potentially be applied as a target for tumour immunotherapy. Normal epithelia, on the other hand, express larger branched sugar moieties. The choice of the specific peptides was based on our previous studies of MUC1-derived peptides, that showed limited, medium and high immunogenicity for B5, A7 and E6 peptides, respectively [17]. The selected peptides included serine or threonine residues in non-anchor positions that were suitable for O-glycosylation. Glycosylation at these positions was expected to be tolerated from the point of view of the MHC binding (to HLA-A*0201 molecule), while the conformation of the sugar moiety pointing towards the TCR was expected to increase the probability for specific recognition of the presented glycopeptide by a pool of TCR molecules, in comparison to the non-glycosylated peptide, that is required for mounting of efficient specific CTL responses.
As expected, for most of the studied compounds, glycosylation with Tn (GalNAc) moiety at non-anchor positions within the peptide did not affect significantly the binding to the HLA-A*0201 molecule (see Fig. 1), implying that the Tn moiety pointed outwards of the peptide-binding groove. In a single case, for S-B5 glycopeptide, the binding to HLA-A*0201 molecule was reduced significantly in comparison to the non-glycosylated B5 peptide indicating that the residue is not readily accommodated within the peptide-binding groove.
The next step was to assess the immunogenicity of the glycopeptides versus the non-glycosylated peptides following immunization of experimental animals with (glyco)peptide-loaded antigen-presenting cells. These experimental settings were expected to provide the basic information regarding the differential activation of the class I-derived T cell repertoire to HLA-A*0201-loaded (glyco)peptides and the cross-reactivity of the resulting cytotoxic cells towards non-glycosylated and glycosylated peptides. For this purpose we utilized the HHD mouse model that combines HLA transgenesis (HLA-A*0201/Db-β2m single chain) with selective knock-out of murine H-2 (double knock-out deletion of the murine β2m and Db genes) and reduces the T cell repertoire to HLA-A*0201-restricted cells [13]. The functionality and relevance of the HHD model for identification of HLA-A*0201-restricted peptides has been demonstrated in previous studies by our and other research groups [17,19,20].
Immunization of HHD mice with (glyco)peptide-loaded RMA-S-HHD-B7·1 cells resulted in low cytotoxic immunogenicity for most of the experimental groups, while the same experimental setting using autologous DCs induced enhanced CTL responses (see Fig. 2). We concluded that the cytotoxic immunogenicity of the studied (glyco)peptides may be relatively limited (due apparently to a relatively low HLA-A*0201 binding) and requires the most efficient immunization settings. Because the DCs are better suited for efficient presentation and activation of cytotoxic T lymphocytes, the next experiments applied immunization with (glyco)peptide-loaded DCs.
Outcomes of CTL induction with (glyco)peptide-loaded DCs demonstrated that, for the panel of the studied peptides, moderate or higher affinity of the peptide to the HLA-A*0201 molecule was a prerequisite for effective induction of CTL responses. Peptides that showed limited affinity in the MHC stabilization assay (A7 and its glycoderivates, S-B5) were unable to mount effective CTL responses. On the other hand, peptides with moderate and high HLA-A*0201-binding affinity induced efficient CTL responses against EL4-HHD cells loaded with the immunizing peptide (see Fig. 2b).
For the immunogenic peptides, the elicited CTLs were characterized by a substantial cross-reactivity towards the glycosylated and non-glycosylated forms of the same peptide. This finding indicates that small-size Tn epitope may be insufficient for a significant discrimination between the different forms of the same peptide by the TCR, resulting in activation of cross-reactive pools of CTLs. This finding has profound implications for the purpose of cytotoxic immunotherapy of MUC1-expressing tumour cells. As a result, glycopeptides with Tn moieties may be insufficiently distinct to be applied as specific targets for mounting an anti-tumour immune response, and the elicited CTLs will have the ability to recognize the non-glycosylated peptides presented by the tumour cells, as well as by the normal MUC1-expressing cells.
The results of the cross-reactivity of the elicited CTLs are consistent with the results of previously studied model glycopeptides O-glycosylated with Tn versus T epitopes (monosaccharide GalNAc versus disaccharide Gal-GalNAc) [7]. Immunization with these glycopeptides elicited CTLs that showed significant cross-reactivity against Tn or T-glycopeptide-loaded EL4 target cells. Taken together, these results imply that a monosaccharide or disaccharide moiety attached to the peptide may be insufficient for selective recognition by a separate pool of the CTLs. Additional studies are required to assess whether such selective recognition can be attained by bigger sugar moieties. However, bigger and bulkier residues may decrease the affinity of the resulting glycopeptide to the MHC molecule, and prevent binding of the TCR molecule to the glycopeptide-MHC complex, thus abolishing the induction of CTL response.
At the next step we assessed whether endogenous MUC1 expression by the experimental mice affects the CTLs pools induced by immunization with (glyco)peptide-loaded DCs. As endogenous MUC1 is expressed by most of the epithelial tissues, this issue has a profound significance for mounting a specific cytotoxic antitumour immune response against MUC1-expressing tumours in clinical settings. To assess the effects of endogenous MUC1 expression in our experimental settings, we applied MUC1-HHD transgenic mice that were bred from the HHD and MUC1 transgenic mice. MUC1 transgenics were shown previously to express MUC1 in an organ-dependent fashion that is similar to that observed in human subjects [18]. The CTLs elicited in the MUC1-HHD mice were able to lyse efficiently the (glyco)peptide-loaded EL4-HHD target cells, while the killing efficiencies were somewhat reduced compared to the CTLs elicited in the HHD mice (see Fig. 3). This finding indicates that endogenous expression of MUC1 did not result in clonal deletion of the CTLs directed against the studied (glyco)peptides in the MUC1-HHD mice. The presence of MHC class I-restricted MUC1-specific CTLs in tumour-bearing MUC1 transgenic mice has been demonstrated previously by Mukherjee et al. [21]. The differences in killing efficiencies between the CTLs elicited in HHD and MUC1-HHD mice are consistent with the previous reports of peripheral CTL tolerance against MUC1-derived epitopes in MUC1-transgenic mice [21,22]. The CTL tolerance phenomenon did not affect the general patterns of cross-reactivity of the elicited CTLs towards the glycosylated and non-glycosylated forms of the same epitope that were similar in HHD and MUC1-HHD mice (see Figs 2 and 3a).
To elucidate the potential of the MUC1-derived glycopeptides as targets for specific antitumour responses we studied the lysis of MUC1-expressing tumour cells by the elicited CTLs in vitro, and established therapeutic MUC1-expressing tumour models in HHD mice. Clone D122-HHD, a low Kb-expressor derived from 3LL Lewis lung carcimoma [23] and transduced to express modified HLA-A*0201 molecules, was applied as a tumour model. The D122-HHD cells were transfected with the MUC1 cDNA (the short or long version of MUC1 molecule; MUC1Y or MUC1REP, respectively), and the resulting D122-HHD-MUC1 transfectants, that were tumorigenic upon s.c. challenge of 1 × 106 cells, were applied as targets for in vitro CTL assays and for active immunization experiments.
Unfortunately, no selective lysis of D122-HHD-MUC1 transfectants has been obtained in vitro using CTLs elicited against (glyco)peptides, and in vivo following active immunization using (glyco)peptide-loaded DCs (data not shown). The functionality of the CTLs has been demonstrated in the previous experimental sets by successful lysis of (glyco)peptide-loaded EL4-HHD target cells, although these cells present non-physiologically high amounts of (glyco)peptide-HLA-A*0201 complexes on their cell surface that are not representative of the amounts of the corresponding targets on the MUC1-expressing tumour cells in vivo. The HLA-A*0201 and MUC1 expression by the D122-HHD-MUC1 transfectants, that is a prerequisite for a successful HLA-A*0201-restricted presentation of MUC1-derived peptides, has been confirmed using reliable experimental techniques (see Methods and Results). Thus, inability of the CTLs to lyse D122-HHD-MUC1 transfectants in in vitro CTL assays and in active immunization experiments could be attributed to: (1) low abundance of the specific MUC1-derived peptides presented on D122-HHD-MUC1 transfectants, (2) activation of evasion mechanisms by the D122-HHD-MUC1 transfectants in vitro and in vivo due to MUC1 expression or via additional mechanisms and/or (3) limited functionality of the elicited CTLs that could effectively lyse the target cells with extremely high abundance of the target (glyco)pep-MHC complexes (external loading of the (glyco)peptides).
Unfortunately, we could not discriminate between these possibilities because the abundance of specific MUC1-derived peptides (i.e. E6, S-E6, T-E6, etc.) on the D122-HHD-MUC1 transfectants cannot be measured readily. This measurement can be performed generally using sophisticated analytical detection systems (LC-MS-MS), but it is extremely difficult to be attained due to the complexity of glycosylation patterns, low abundance of the specific glycopeptides and technical difficulties to discriminate between the specific glycosylated derivatives of the same peptide (e.g. Glc versus Gal-residues). Due to a combination of these reasons, the data regarding the actual presentation of the glycopeptides is very scarce. A specific study that assessed the fate of glycosylated residues during glycopeptide processing and presentation showed that presentation of MUC1-derived peptides on MHC class II following cross-presentation by DCs may proceed without removing the complex carbohydrate moieties [24]. In this study, a glycopeptide-specific hybridoma that recognizes a tumour-specific glycopeptide bearing a Tn antigen and fluorescently labelled lectin that specifically binds Tn antigen were applied for detection of cell-surface bound Tn containing structures. These methods could not be applied readily for the purpose of our study as D122-HHD-MUC1 transfectants are characterized by a high background of Tn expression on the cell surface due to the presence of extensively glycosylated transmembranal MUC1 molecules.
It seems that, secondary to HHD and MUC1 expression by the D122-HHD-MUC1 transfectants, the processing and presentation of MUC1 should have resulted in HLA-A*0201-restricted presentation of a certain amounts of specific non-glycosylated peptides that were assessed in this study (E6, B5, A7, D6). In addition, it is expected that the studied glycosylated peptides were also presented to a certain extent in HLA-A*0201-restricted fashion by the D122-HHD-MUC1 transfectants. However, lack of specific lysis by the elicited CTLs indicates that the studied glycosylated and non-glycosylated MUC1-derived peptides appear not to be the suitable targets for effective and specific anti-tumour therapy of MUC1-expressing tumours.
Based on the outcomes of this study, and on available knowledge on the antigen processing and CTLs activation mechanisms, it seems that glycosylated peptides in most cases would serve as inferior targets for anti-tumour therapy compared to the non-glycosylated peptides. Despite the fact that most of the proteins are heavily glycosylated and there are distinct patterns of glycosylation in tumour cells versus the normal cells, a major part of the presented peptides derives from the newly synthesized non-glycosylated protein molecules that did not pass through Golgi and endoplasmic reticulum where glycosylation takes place. An additional source of the presented peptides, degradation of the glycosylated proteins at the end on their life span, is expected to result in a low extent of MHC class I-restricted glycopeptide presentation compared to the corresponding peptide due to: (1) partial glycosylation of the amino acid backbone (i.e. only part of the potential glycosylation sites are actually glycosylated); and (2) part of the glycopeptide residues are expected to be cleaved during the glycopeptide processing. In addition, due to a highly variable glycosylation pattern, the presented glycopeptides are expected to bear different glycosylation moieties, and mounting an immune response against specific glycopeptide means that only a small fraction of the presented glycopeptides will serve as potential targets for the cellular immune response. Nevertheless, it is possible that in certain cases a specific glycopeptide may serve as a specific target for cytotoxic immune therapy in the case that the combination of MHC binding and specific cytotoxic immunogenicity for this glycopeptide will overweight the low efficiency of its presentation and will permit selective cytotoxic responses targeted against the cells presenting such peptide.
We conclude that Tn glycosylation of the studied peptides did not increase their cellular immunogenicity. Peptides glycosylated with a small-size Tn epitope did not offer significant benefits as targets for cytotoxic immune response due to a high degree of cross-reactivity of the elicited CTLs against the glycosylated and non-glycosylated forms of the same peptide. Glycosylation with larger-size (e.g. disaccharide or trisaccharide) sugar moieties may permit specific recognition of such glycopeptides by the CTLs. However, glycosylated peptides in most cases seem to be inferior targets for CTL-mediated specific cytotoxic immune responses due to the low abundance of MHC class I-restricted presentation of specific glycopeptides. On the other hand, Tn and related glycosyl groups could serve as a target for humoral immune responses, as have been demonstrated in numerous studies [25,26], but the clinical relevance and efficiency of antibody-based therapy against MUC1-expressing tumours is not clear.
Acknowledgments
We thank Prof. D. Wreshner and Prof. O. Finn for providing MUC1 constructs. This study was supported by grants from the Israel Cancer Association, Israel Science Foundation, the Israel Cancer Research Fund (to L. Esienbach) and a Clore Foundation postdoctoral grant (to D. Stepensky).
References
- 1.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–6. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 2.Graham RA, Burchell JM, Taylor-Papadimitriou J. The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother. 1996;42:71–80. doi: 10.1007/s002620050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–51. [PubMed] [Google Scholar]
- 4.Taylor-Papadimitriou J, Burchell JM, Plunkett T, et al. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–21. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 5.Hanisch FG, Muller S. MUC1: the polymorphic appearance of a human mucin. Glycobiology. 2000;10:439–49. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- 6.Haurum JS, Arsequell G, Lellouch AC, et al. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J Exp Med. 1994;180:739–44. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199:707–16. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glithero A, Tormo J, Haurum JS, et al. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity. 1999;10:63–74. doi: 10.1016/s1074-7613(00)80007-2. [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulos V, Yuriev E, Ramsland PA, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci U S A. 2003;100:15029–34. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speir JA, Abdel-Motal UM, Jondal M, Wilson IA. Crystal structure of an MHC class I presented glycopeptide that generates carbohydrate-specific CTL. Immunity. 1999;10:51–61. doi: 10.1016/s1074-7613(00)80006-0. [DOI] [PubMed] [Google Scholar]
- 11.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 12.Apostolopoulos V, Karanikas V, Haurum JS, McKenzie IF. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J Immunol. 1997;159:5211–18. [PubMed] [Google Scholar]
- 13.Pascolo S, Bervas N, Ure JM, et al. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knock-out mice. J Exp Med. 1997;185:2043–51. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zrihan-Licht S, Vos HL, Baruch A, et al. Characterization and molecular cloning of a novel MUC1 protein, devoid of tandem repeats, expressed in human breast cancer tissue. Eur J Biochem. 1994;224:787–95. doi: 10.1111/j.1432-1033.1994.00787.x. [DOI] [PubMed] [Google Scholar]
- 15.Magarian-Blander J, Hughey RP, Kinlough C, Poland PA, Finn OJ. Differential expression of MUC1 on transfected cell lines influences its recognition by MUC1 specific T cells. Glycoconj J. 1996;13:749–56. doi: 10.1007/BF00702339. [DOI] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 17.Carmon L, El-Shami KM, Paz A, et al. Novel breast-tumor-associated MUC1-derived peptides: characterization in Db-/- × beta2 microglobulin (beta2m) null mice transgenic for a chimeric HLA-A2.1/Db-beta2 microglobulin single chain. Int J Cancer. 2000;85:391–7. [PubMed] [Google Scholar]
- 18.Peat N, Gendler SJ, Lalani N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–60. [PubMed] [Google Scholar]
- 19.Carmon L, Bobilev-Priel I, Brenner B, et al. Characterization of novel breast carcinoma-associated BA46-derived peptides in HLA-A2.1/D (b)-beta2m transgenic mice. J Clin Invest. 2002;110:453–62. doi: 10.1172/JCI14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francini G, Scardino A, Kosmatopoulos K, et al. High-affinity HLA-A(*)02.01 peptides from parathyroid hormone-related protein generate in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol. 2002;169:4840–9. doi: 10.4049/jimmunol.169.9.4840. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee P, Ginardi AR, Madsen CS, et al. Mice with spontaneous pancreatic cancer naturally develop MUC-1-specific CTLs that eradicate tumors when adoptively transferred. J Immunol. 2000;165:3451–60. doi: 10.4049/jimmunol.165.6.3451. [DOI] [PubMed] [Google Scholar]
- 22.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 23.Eisenbach L, Hollander N, Greenfeld L, et al. The differential expression of H-2K versus H-2D antigens, distinguishing high-metastatic from low-metastatic clones, is correlated with the immunogenic properties of the tumor cells. Int J Cancer. 1984;34:567–73. doi: 10.1002/ijc.2910340421. [DOI] [PubMed] [Google Scholar]
- 24.Vlad AM, Muller S, Cudic M, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–46. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo-Man R, Bay S, Vichier-Guerre S, et al. A fully synthetic immunogen carrying a carcinoma-associated carbohydrate for active specific immunotherapy. Cancer Res. 1999;59:1520–4. [PubMed] [Google Scholar]
- 26.Vichier-Guerre S, Lo-Man R, Bay S, et al. Short synthetic glycopeptides successfully induce antibody responses to carcinoma-associated Tn antigen. J Pept Res. 2000;55:173–80. doi: 10.1034/j.1399-3011.2000.00167.x. [DOI] [PubMed] [Google Scholar]