Abstract
Persistent, localized Staphylococcus aureus infections, refractory to antibiotic treatment, can result in massive tissue destruction and surgical intervention is often the only therapeutic option. In that context, we investigated patients with S. aureus-induced infection at various sites, apparent as either olecranon bursitis, empyema of the knee joint or soft tissue abscess formation. As expected, a prominent leucocyte infiltrate was found, consisting predominantly of polymorphonuclear neutrophils (PMN) (up to 75%) and to a lesser extent of T lymphocytes and natural killer (NK) cells. In line with their bactericidal capacity, PMN expressed the high-affinity receptor for IgG, CD64 and the lipopolysaccharide (LPS) receptor CD14; moreover, the oxygen radical production in response to the bacterial peptide f-MLP was enhanced, while chemotactic activity was greatly reduced. The more intriguing finding, however, was that a portion of PMN had acquired major histocompatibility complex (MHC) class II antigens and CD83, indicative of a transdifferentiation of PMN to cells with dendritic-like characteristics. Of note is that a similar transdifferentiation can be induced in PMN in vitro, e.g. by gamma interferon or by tumour necrosis factor alpha. Co-cultivation of transdifferentiated PMN with autologous T lymphocytes resulted in prominent T cell proliferation, provided that S. aureus enterotoxin A was added. Taken together, persistent S. aureus infection induces PMN to acquire characteristics of dendritic cells, which in turn might promote the local immune response.
Keywords: inflammation, neutrophils, Staphylococcus aureus, transdifferentiation
Introduction
Infiltration into infected sites of leucocytes, primarily polymorphonuclear neutrophils (PMN), is a hallmark of bacterial infection. While the process of emigration from the blood vessel into the infected tissue has been studied extensively [1–3], the fate of the emigrated PMN is less well understood. From histomorphological studies, experimentally induced infections and in vitro experiments, it was deduced that the phagocytosis of bacteria − or even stimulating receptors involved in phagocytosis − initiates the programmed cell death of PMN [4–6]. Subsequently, infiltrating macrophages ‘clear’ the site by taking up the apoptotic PMN. It is generally accepted that the phagocytosis-induced apoptosis of PMN and subsequent clearance by macrophages is a prerequisite for the resolution of infection-associated inflammation, while the failure of PMN to undergo apoptosis creates a pathological situation [4–7]. The unrestricted release of cytotoxic and proteolytic entities from PMN, which may destroy the surrounding tissue as well as the release of proinflammatory mediators produced by PMN having escaped apoptosis, could sustain an inflammatory reaction and participate in the progress of an acute inflammation to chronicity, such as the development of sepsis, multi-organ failure and in local tissue destruction, respectively [7–9].
To assess whether PMN at sites of bacterial infections could indeed transform from their ‘protective’ to a ‘proinflammatory’ status, leucocytes from patients with persistent, destructive localized Staphylococcus aureus-induced tissue infections requiring surgery were studied. Surgery provided a unique possibility to obtain cells from the infected site and to investigate them ex vivo.
In line with their role in bacterial defence, PMN had up-regulated the high-affinity receptor for IgG, CD64, and the so-called lipopolysaccharide (LPS) receptor CD14. Moreover, up-regulation of superoxide production was seen. A portion of PMN had acquired a ‘dendritic-like’ phenotype, and the ability to present to T lymphocytes staphylococcal enterotoxin in vitro.
Patients, material and methods
Patients and collection of specimen
After approval by the ethic committee of the University of Heidelberg Hospital and after having obtained informed consent, 12 patients (11 male, one female; aged between 45 and 65 years) with a localized acute tissue infection, no other apparent illness and in good physiological condition were recruited into the study (six patients with olecranon bursitis, three patients with abscess formation and three with empyema of the knee joint). According to the patients’ reports the infection persisted for 1–2 weeks without any antibiotic or steroid therapy. The peripheral blood cell count was normal or mildly raised; the C-reactive protein (CRP) level ranged between 80 and 240 mg/l (normal value < 8 mg/l). In all patients S. aureus was identified as the causative agent. None of the patients had methicillin-resistant S. aureus; the bacteria were not differentiated further, e.g. for the ability to secrete toxins.
To recover cells from the infected site, during surgery the focus and the surrounding tissue were rinsed with up to 50 ml sterile saline. The fluid, referred to as ‘lavage’, was aspirated and transferred to sterile tubes containing heparin (500 µl in 50 ml; NH4-heparin, 5000 IE/ml, Braun, Melsungen, Germany) and 0·1% sodium azide. Drainage tubes were placed from which, on consecutive days, leucocytes were recovered. Peripheral blood was taken from each patient (7 ml in heparinized syringes, Sarstedt, Nümbrecht, Germany) immediately prior to surgery and on consecutive days (up to 5 days) and also 3–4 weeks thereafter.
Peripheral blood was taken from 20 healthy age- and sex-matched volunteers by venipuncture using heparinized syringes.
Recovery and characterization of cells from the lavage or drainage fluids
All analyses were performed within 3 h to avoid artefacts due to ex vivo storage. Cells were collected by centrifugation (10 min, 1500 g). Erythrocytes were lysed using hypotonic NaCl (0·2 M). The leucocytes were suspended in 0·01 M phosphate-buffered saline, containing 1% bovine serum albumin and 0·1% sodium azide. The leucocytes were viable as judged by trypan blue exclusion and microscopic evaluation.
Cytofluorometry
The following conjugated antibodies (all obtained from Immunotech, Marseille, France) were used: anti-CD66b-FITC; anti-CD14-PE, anti-CD62L-PE and anti-CD64-PE; anti-HLA-DP-DQ-DR to detect MHCII antigens; anti-CD3-FITC, anti-CD4-FITC and anti-CD8-PE, anti-CD56-FITC and anti-CD19-FITC. The antibody to CD83 was purchased from Immunotech, the antibody to interferon (IFN)-γ from Serotec (purchased from Biozol, Dusseldorf, Germany) and the antibody to IFN-γ from Serotec (Dusseldorf, Germany). To detect binding of the unconjugated antibodies a fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labelled antibody to mouse IgG, generated in goat was used (Immunotech). Cells derived from the lavage or the drainage were adjusted to 2–5 × 105. Of whole blood, 100 µl were used, and erythrocytes were lysed using FACS-lysing solution (Becton Dickinson, Heidelberg, Germany). Double staining was performed using CD66b-FITC as a marker for PMN, and the PE-labelled antibodies to detect the activation associated antigens. Fluorescence was measured with FacsCalibur (Becton Dickinson) and CellQuest as software. The data are expressed as percentage of positive cells or as mean fluorescence intensity (MFI), respectively. For detection of intracellular antigens the cells were mixed with permeabilizing solution (100 µl per 500 µl; Becton Dickinson); after 20 min incubation time at room temperature, the cells were washed with phosphate-buffered saline (PBS) containing 1% bovine serum albumin, 0·1% sodium azide. To the cell pellet, the respective antibodies or mouse IgG as isotype control were given (30 min, room temperature), and following washing with FACS-buffer, a FITC-labelled antibody to mouse IgG. After 30 min incubation in the dark, the cells were washed twice in FACS-buffer and resuspended in 1% paraformaldehyde. For detection of intracellular antigens FacsPerm (Becton Dickinson) was used and the protocol supplied by the manufacturer.
Data of all patients are summarized as statistical Box-and-Whiskers plots; differences between mean values of groups were calculated using anova.
Functional assays
Superoxide generation
was measured by its ability to reduce cytochrome C, as described in [10]. In brief, PMN (1 × 105) were suspended in cytochrome C [1 mg/ml in Hanks's balanced salt solution (HBSS)] and stimulated with either phorbol myristate acetate (PMA) (phorbol ester 1 µg/ml) or f-Met-Leu-Phe (10−8 M) (both obtained from Sigma). Reduction of cytochrome C within 30 min was measured as a shift in optical density read at 550 nm. Production of was calculated using 21·6 as a molar extinction coefficient.
Chemotaxis
A modified Boyden chamber assay was used, employing a 200 µm filter with 5 µ pore size, and using activated human serum as source of complement C5a [11] or interleukin (IL)-8. Random migration and chemotaxis was measured as leading front (in µm), being the distance from the top of the filter to a level where at least five cells could be detected. The reading was conducted using a Omnicon Alpha Image Analyser (Bausch and Lomb, Heidelberg, Germany). At least two parallel filters were prepared; and on each filter 10 different areas were evaluated.
Isolation of PMN or T lymphocytes from peripheral blood
Heparin-blood was layered on PolymorphPrep (Nycomed, Oslo, Norway); following centrifugation, the PMN fraction was harvested and washed repeatedly in PBS (pH 7·4). For the functional assays (superoxide production of chemotaxis), the cells were suspended in HBSS. For the antigen-presentation test, the PMN were purified further using anti CD15-coated magnetic beads (AutoMACS, Miltenyi Biotec, Bergisch-Gladbach, Germany), yielding > 99% PMN. Cells from the lavage were washed in PBS, resuspended in HBSS and then isolated further as described above. The T lymphocytes were harvested from the mononuclear cell fraction and further purified using anti-CD3-, anti-CD4- or anti-CD8-coated magnetic beads, respectively (AutoMACS),
T cell proliferation assay
Isolated T cells were seeded into 96-well culture dishes (2 × 104−1 × 105/µl/well; 12 parallel samples) and co-cultivated with PMN (1 × 105/100 µl) in the presence or absence of S. aureus enterotoxin A (10–100 ng) for 72 h. [3H]-Thymidine (0·037 Mbq/well) was then added and incorporation of radioactivity into DNA was measured after 24 h.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from 1 × 107 PMN using a RNAeasy kit by Quiagen (Hilden, Germany) following the manufacturer's instructions precisely. RNA was transcribed using a Gibco RT Superscript II kit. MHC class II and CD83-specific products were generated as described by Iking-Konert et al. [12] and separated by a 1·5% agarose gel, stained with SybrGreen (Molecular Probes, Leiden, the Netherlands) and analysed by FLA2000 (Fuji, Japan) using Image Gauge version 3·0 (Fuji) as software. The marker was Boehringer DNA Marker VI [range 154–2176 base pairs (bp)] in a 30 µg/ml concentration.
Results
Recovery and identification of the cellular infiltrate
From the 12 patients undergoing surgery because of localized soft tissue infection leucocytes were obtained by rinsing the infected site intraoperatively (0·5–2 × 107). The cells were viable as judged by trypan blue exclusion and microscopic examination (data not shown). Without substantial variation among the patients, the majority of leucocytes recovered from the infected site were PMN (CD66b and CD15 positive; 50–70% of leucocytes). T lymphocytes (CD3 positive) amounted to 20–30% and natural killer (NK) cells (CD56 positive, CD3 negative) to 10–15%. Monocytes (CD14 highly positive, CD66b negative) were less than 5% and B cells (CD19 positive) less than 1%. Of note was that viable leucocytes could also be recovered from the drainage fluids for up to 3 days after surgery; PMN were predominant.
From one patient with olecranon bursitis (patient 1) in addition to the leucocytes obtained from the lavage, cells from the opened bursa could also be obtained (Fig. 1). In the remaining patients with bursitis, this distinction was not possible because of massive tissue deterioration. The bursa contained predominantly PMN (> 90%).
Fig. 1.
Analysis of surface antigens on polymorphonuclear neutrophils (PMN) of patient 1 by cytofluorometry: PMN were identified by CD66b expression; within that gate, expression of CD14, CD62L and CD64 was measured in blood samples taken immediately prior to surgery, collected intraoperatively from the lavage or from the opened bursa. For comparison, peripheral blood obtained 28 days following surgery is shown (the thick lines show binding the specific antibody; the thin lines show the respective isotype controls).
Phenotypic characterization of the PMN
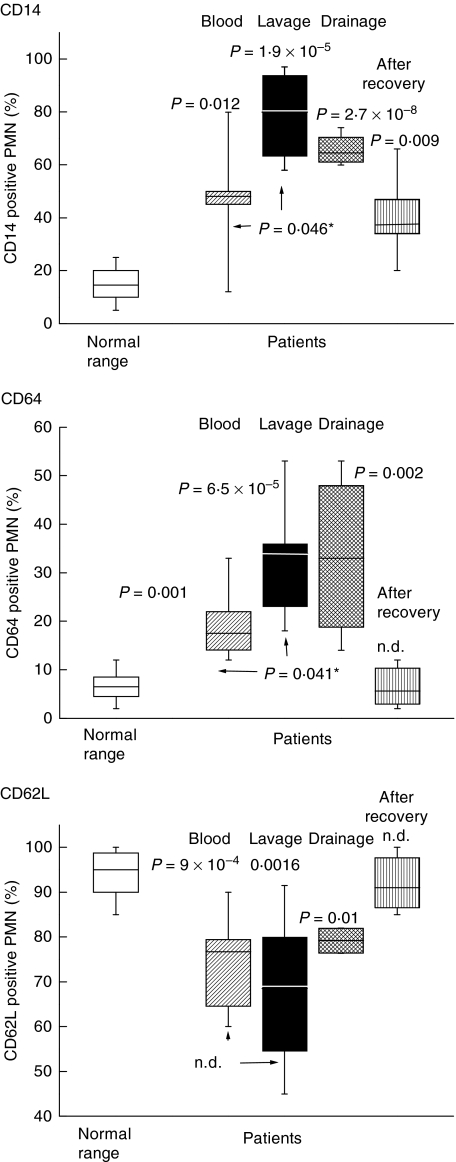
To characterize further the infiltrate PMN, the expression of activation-associated surface receptors was measured. Compared to the PMN of the peripheral blood of healthy donors, up-regulation of CD14 and CD64 and a loss of CD62L was seen (data for patient 1 are shown in Fig. 1; data of all patients are summarized in Fig. 2). Of note is that a similar activation pattern was also seen with PMN from the drainage fluid, and with the peripheral blood PMN of the patients taken immediately prior to surgery. Within days after surgery, the surface receptor pattern of the peripheral blood PMN normalized, as revealed by serial studies conducted for four patients (a representative experiment is shown in Table 1); by 2–4 weeks after surgery, in all patients the values were within the normal range (Fig. 2).
Fig. 2.
Expression of surface antigens of polymorphonuclear neutrophils (PMN) of all the patients (n = 12): The data obtained by cytofluorometry (as shown in Fig. 1) were summarized as statistical Box-and-Whisker blots with the box containing 50% of the values. The horizontal bar represents the median. Each panel summarizes the data for one of the receptors. Shown is the normal range (derived from 20 donors) (open box); expression on PMN of the peripheral blood taken prior to surgery (striped box); of the lavage (black box); of the drainage fluid obtained at day 2 post-surgery (hatched box); and in the peripheral blood 2–4 weeks after surgery (vertical stripes). Differences between the mean values calculated for the respective group and the normal range were determined by a t-test for unpaired samples; P-values are given; n.d. indicates that the groups were not different; the values shown as p* refer to differences between cells of the peripheral blood taken prior to surgery and cells of the lavage.
Table 1.
Surface receptor expression on polymorphonuclear neutrophils (PMN) of patient 1: follow-up study.
| Before surgery | Post-surgery day 1 | Post-surgery day 2 | Post-surgery day 3 | Post-surgery day 4 | Normal range2 | |
|---|---|---|---|---|---|---|
| Peripheral blood PMN | ||||||
| CD14 | 79·31 | 70·4 | 66·4 | 47·3 | 22·1 | 5–25 |
| CD64. | 15·21 | 16·7 | 18·1 | 10·1 | 10 | 0–10 |
| CD62L | 62·41 | 84·1 | 82·5 | Not done | Not done | 85–100 |
| Drainage fluid | ||||||
| CD14 | 471 | 65 | 43 | No cells | ||
| CD64 | 211 | 53 | 19 | No cells | ||
| CD62L | 76·51 | 94·8 | 82 | No cells | ||
% positive PMN;
data derived from 20 healthy donors.
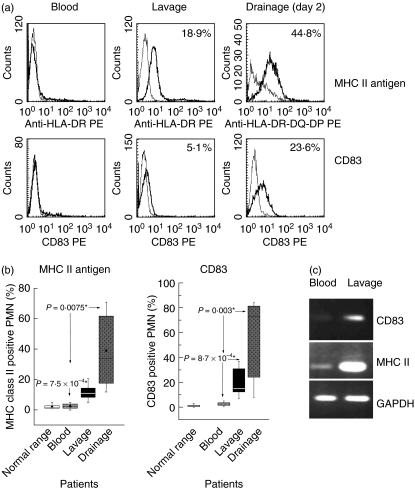
MHC class II antigens and CD83 are not expressed on the PMN of healthy donors; unlike other receptors, they are not stored intracellularly, but are synthesized de novo upon stimulation of PMN, for example by IFN-γ. Similarl to healthy donors, the peripheral blood PMN of the patients did not express MHC class II; in contrast, PMN of the lavage had acquired MHC class II antigens. Up to 20% (mean 12·8 ± 6·9) of the PMN were positive for MHC class II. An even higher percentage of the PMN recovered from the drainage fluid expressed MHC class II: on day 1 post-surgery between 23% and 81·9% of MHC class II-positive PMN were seen, and on day 2 between 14·7 and 73·1% (examples and summary of all data are shown in Fig. 3). Analogous data were obtained for CD83: a considerable portion of the PMN of the lavage, but not of the peripheral blood, expressed CD83. Again, CD83 positive PMN were more abundant in the drainage fluid obtained 1 or 2 days post-surgery (Fig. 3). In line with de novo protein synthesis, in the PMN of the lavage or drainage fluids MHC class II and CD83-specific RNA could be detected by RT-PCR (Fig. 3c).
Fig. 3.
Expression of major histocompatibility complex (MHC) class II antigens and of CD83 on polymorphonuclear neutrophils (PMN): (a) with an antibody recognizing HLA-DP-DQ-DR, expression of MHC class II antigen was found on PMN from the lavage (middle panel), the drainage fluid (right panel) but not on cells derived from the peripheral blood (left panel) (the thick lines show the specific antibody; the thin lines the isotype controls; data for patient 2 are shown). Similarly, expression of CD83 was seen on PMN derived from the lavage or the drainage fluid, but not on peripheral blood PMN. (b) Data for all patients are summarized (as detailed in the legend for Fig. 2). (c) Reverse transcription-polymerase chain reaction (RT-PCR) was performed with PMN derived from the peripheral blood or from the lavage of the same patient (data for patient 4 are shown) with primers specific for either CD83, MHC class II or GAPDH as housekeeping gene.
Functional analysis of the infiltrated PMN
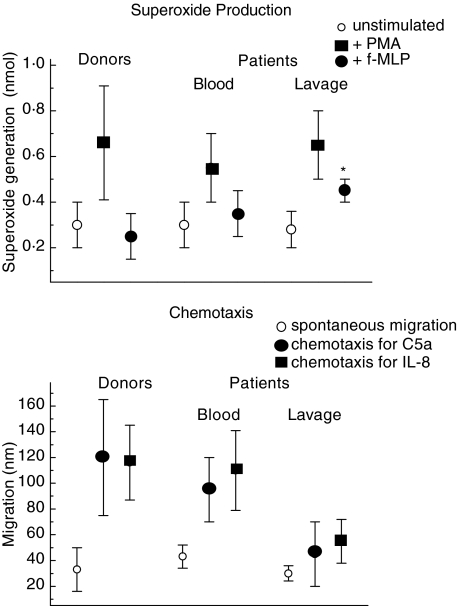
The PMN were not apoptotic as judged by propidium iodide and annexin V staining. Moreover, they were functionally active: when stimulated with phorbol ester, the PMN produce superoxides. There was no important difference between PMN from the lavage or the blood taken immediately prior to surgery. In contrast, stimulation with fMLP resulted in superoxide production by the lavage-derived PMN only (Fig. 4).
Fig. 4.
Superoxide production and chemotaxis of polymorphonuclear neutrophils (PMN). Upper panel: the superoxide production in response to phorbol myristate acetate (PMA) (black squares) or f-MLP (black circles) of PMN was measured isolated from the lavage or the blood of the patients taken immediately prior to surgery, or the blood of healthy donors (n = 20), respectively. Open circles show spontaneous superoxide production. Shown are the mean values ± s.d.; * indicates that the mean value is different when compared to that of the PMN derived from the blood of the patients (P = 0·025) or of the donors (P = 0·001). Lower panel: the chemotaxis towards C5a (black circles) or interleukin (IL)-8 (black squares) was tested of blood-derived PMN (taken immediately prior to surgery) or from lavage of the patients. For comparison, migration of healthy donors (n = 20) was tested (the open squares show the spontaneous random migration). Shown are the mean values ± s.d.; * indicates that the mean value is different when compared to that of the PMN derived from the blood of the patients (P = 0·020) or of the donors (P = 0·001), respectively.
The PMN were also tested for their chemotactic activity. Using C5a or IL-8 as chemoattractant, the chemotaxis of the peripheral blood PMN was within the normal range; the chemotaxis of the PMN recovered from the lavage was greatly reduced (Fig. 4).
Activation of T lymphocytes by PMN
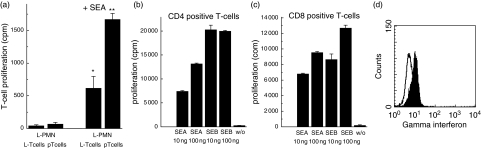
Because PMN stimulated to express MHC class II antigens in vitro are able to present to T cells antigens or superantigens, we tested whether the PMN retrieved from the infected site were able to activate T cells. In a first set of experiments, the PMN derived from the lavage were co-cultivated with autologous T cells, derived either from the peripheral blood or from the lavage. In none of these combinations was T cell proliferation observed. When S. aureus enterotoxin A was added, T cell proliferation was achieved (shown are T cells from the lavage, Fig. 5). Of note is that, with the enterotoxin, the PMN were able to induce proliferation of CD4+ and of CD8+ T cells. Under these conditions, the majority (up to 80%) of the lavage-derived CD8+ T cells also produced of IFN-γ (Fig. 5). Moreover, the presentation of S. aureus enterotoxin was not restricted to autologous T cells, but was also possible with T cells of a another heterologous donor (Fig. 5).
Fig. 5.
Proliferation of T lymphocytes using polymorphonuclear neutrophils (PMN) as antigen-presenting cells: (a) PMN derived from the lavage of patient 12 (L-PMN, 1 × 105) were co-cultivated with either lavage-derived T cells (L-T cells) or T cells isolated from the peripheral blood (pT cells, 2 × 104) (taken immediately prior to surgery) in the absence (two left columns) or presence (two right columns) of staphylococcal enterotoxin (SE) A (10 ng/ml); proliferation was measured by incorporation of [3H]-thymidine (cpm). Values represent the mean ± s.d. of 12 parallel wells. *Indicates that proliferation is different from that seen in the absence of SEA (P = 1·5 × 10−8); **proliferation of pT cells is enhanced compared to L-T cells (P = 1·2 × 10−8). (b) CD4 positive T cells (obtained from the lavage of patient 11) were cultivated in the presence of heterologous PMN and either SEA or SEB in the concentrations indicated; proliferation of T cells was measured at day 4. (c) A similar experiment using CD8 positive T cells. The values represent the mean ± s.d. of 12 parallel wells [please note that in the experiments shown in (b) and (c) five times more cells were used]. (d) CD8 positive T cells (derived from the lavage of patient 11) were cultivated in the presence of autologous PMN and SEA (10 ng/ml). After 24 h, synthesis of IFN-γ was measured by cytofluorometry of permeabilized cells (line IgG isotype control; filled peak anti-IFN-γ).
Discussion
Localized infections requiring surgery provide unique access to various anatomical sites such as joints or superficial abscesses, and thus allows the analysis of infiltrated cells both phenotypically and functionally. The present study, comprising patients with S. aureus infection, showed that despite the different anatomical sites the composition of the cellular infiltrate did not vary: PMN were found predominantly, T lymphocytes to a lesser extent and, rarely, B lymphocytes or monocytes. Moreover, the phenotypical and functional properties of the PMN were similar. Up-regulation of activation-associated surface receptors including CD14 and CD64 was seen, and loss of CD62L, enhanced superoxide production in response to a weak stimulus (f-MLP) as well as reduced chemotaxis towards the bona fide chemokines C5a or IL-8.
The up-regulation of CD14 and CD64 is in keeping with the role of PMN in the defence against bacterial infections [13,14] as is the conditioning response of PMN for superoxide generation, also known as ‘priming’ [15,16]. Moreover, from in vitro studies it is known that numerous proinflammatory cytokines down-regulate the chemotactic capacity of PMN concomitantly with a loss of CD62L [17,18]. Although chemotaxis is crucial for the infiltration of PMN into infected sites, it is no longer required once the PMN have arrived. The mechanism of chemotaxis inhibition is not yet known; one aspect might be the loss or the down-regulation of adhesion molecules such as CD62L.
The question arises as to why, despite the presence of highly activated PMN, infection persists. S. aureus are known to use various means to escape phagocytosis [19,20]. Moreover, S. aureus can also form biofilms, which are thought to protect the bacteria against immune defence [21]. Whatever the mechanism might be, failure to clear the bacteria has important consequences: not only does the infection persist, but so do the PMN. It is widely accepted that phagocytosis provides the signal for the PMN to undergo apoptosis [1–4], the latter being a prerequisite for their clearance by macrophages and thus for resolution of the infection-associated inflammation. Failure of PMN to undergo apoptosis is thought to create pathological situations such as tissue destruction, the progress of an acute inflammation to chronicity, or the development of sepsis and organ failure [1–8,22]. In our patients the failure of PMN to undergo apoptosis might induce the release of their cytotoxic and proteolytic entities, thereby contributing to the local destructive inflammatory process. This process is perpetuated further by the persistence of the infection, which represents a permanent stimulus for PMN infiltration. Persistent infection has yet another consequence: the PMN acquired the characteristics of dendritic cells, notably surface expression of MHC class II antigens and of CD83, a widely used marker molecule for dendritic cells. Expression by PMN of MHC class II and CD83, a phenomenon termed ‘transdifferentiation’, can be also induced in vitro by cultivating the PMN with either IFN-γ or TNF-α, and also results in the ability of those PMN to present superantigens to T cells [12,23–27]. Moreover, up-regulation of these antigens was also observed in chronic inflammatory disease [28–30]. At variance with these data, we could now show that persistent bacterial infection also induces the transdifferentiation of PMN.
Co-culture experiments with PMN recovered from the lavage with T lymphocytes from either the lavage or the peripheral blood, however, did not result in T cell proliferation. While this does not rule out that PMN had bound antigenic peptides to their MHC class II molecules, nor that PMN might have the ability to present peptide antigens in vivo, formal proof is still lacking.
Of note is that PMN ex vivo elicited IFN-γ synthesis and the proliferation of CD4+, and also of CD8+ T lymphocytes when S. aureus enterotoxins (SE) as superantigens were present. SE have been studied mainly in the context of acute reactions, such as toxic shock syndrome or food poisoning [31,32]; whether they play a role in the progression of infection to a destructive inflammatory disease has not yet been analysed in great detail, nor is there any information as to whether ‘superantigens’ lead to the activation and expansion of T cell clones in peripheral tissue. There is, however, increasing evidence for ‘effector lymphoid tissue’ in protective immunity and activation and expansion of T cell clones in response to bacterial infection [33–35]. Such effector T cells could provide cytokines with the ability also to modulate, among other cells, PMN.
In summary, persistent S. aureus-mediated local infection induces the local activation and transdifferentiation of PMN to cells with dendritic-like characteristics, the latter in contrast to the acute inflammatory response.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (WA 1623/1–4).
References
- 1.Melnikoff MJ, Horan PK, Morahan PS. Kinetics of changes in peritoneal-cell populations following acute inflammation. Cell Immunol. 1989;118:178–91. doi: 10.1016/0008-8749(89)90367-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 3.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 4.Watson RM, Redmond HP, Wang LH, Condron C, Bouchier-Hayes D. Neutrophil undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 5.Zhang B, Hirahashi J, Cullere X, Mayadas T. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis. J Biol Chem. 2003;278:28443–54. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi SD, Voyich JM, Somerville GA, et al. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J Leukoc Biol. 2003;73:315–22. doi: 10.1189/jlb.1002481. [DOI] [PubMed] [Google Scholar]
- 7.Savill J, Haslett C. Fate of neutrophil. In: Hellewell PG, Williams TJ, editors. Immunopharmacology of Neutrophils. San Diego, CA: Academic Press; 1994. pp. 295–314. [Google Scholar]
- 8.Härter L, Mica L, Stocker R, Trentz O, Keel M. Mcl-1 correlates with reduced apoptosis in neutrophils from patients with sepsis. J Am Coll Surg. 2003;197:964–73. doi: 10.1016/j.jamcollsurg.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 10.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenneis HA, Schmidt A, Blaas-Mautner P, Wörner I, Ludwig R, Hänsch GM. Chemotaxis of polymorphonuclear neutrophils (PMN) in patients suffering from recurrent infection. Eur J Clin Invest. 1993;23:693–8. doi: 10.1111/j.1365-2362.1993.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 12.Iking-Konert C, Csekö C, Wagner C, Stegmaier S, Andrassy K, Hänsch GM. Transdifferentiation of polymorphonuclear neutrophils. acquisition of CD83 and other functional characteristics of dendritic cells. J Mol Med. 2001;79:464–74. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- 13.Fjaertoft G, Hakansson L, Ewald U, Foucard T, Venge P. Neutrophils from term and preterm newborn infants express the high affinity Fcgamma-receptor I (CD64) during bacterial infections. Pediatric Res. 1999;45:871–6. doi: 10.1203/00006450-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Wagner C, Deppisch R, Denefleh B, Hug F, Andrassy K, Hänsch GM. Expression patterns of the lipopolysaccharide receptor CD14, and the Fc gamma receptors CD16 and CD64 on polymorphonuclear neutrophils: data from patients with severe bacterial infections and lipopolysaccharide exposed cells. Shock. 2003;19:5–12. doi: 10.1097/00024382-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kharazmi A, Nielsen H, Bendtzen K. Modulation of human neutrophil and monocyte chemotaxis and superoxide responses by recombinant TNF-alpha and GM-CSF. Immunobiology. 1988;177:363–70. doi: 10.1016/S0171-2985(88)80004-4. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj MS, Kew RR, Webster RO, Hyers TM. Priming of human neutrophil functions by tumor necrosis factor. enhancement of superoxide anion generation, degranulation, and chemotaxis to chemoattractants C5a and F-Met-Leu-Phe. Inflammation. 1992;16:241–50. doi: 10.1007/BF00918813. [DOI] [PubMed] [Google Scholar]
- 17.Kownatzki E, Kapp A, Uhrich S. Modulation of human neutrophilic granulocyte functions by recombinant human tumor necrosis factor and recombinant human lymphotoxin. Promotion of adherence, inhibition of chemotactic migration and superoxide anion release from adherent cells. Clin Exp Immunol. 1988;74:143–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Grutkoski PS, D’Amico R, Ayala A, Simms HH. Tumor necrosis factor-alpha-stimulated polymorphonuclear leukocytes suppress migration and bactericidal activity of polymorphonuclear leukocytes in a paracrine manner. Crit Care Med. 2002;30:591–7. doi: 10.1097/00003246-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 20.Mayer-Scholl A, Averhoff P, Zychlisnky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7:62–6. doi: 10.1016/j.mib.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–26. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 22.Sendo F, Tsuchida H, Takeda Y, et al. Regulation of neutrophil apoptosis − its biological significance in inflammation and the immune response. Hum Cell. 1996;9:215–22. [PubMed] [Google Scholar]
- 23.Klebanoff SJ, Olszowksi S, van Voorhis WC, Ledbetter JA, Walterdorph AM, Schlechte KG. Effects of gamma interferon on human neutrophils: protection from deterioration on storage. Blood. 1992;80:225–34. [PubMed] [Google Scholar]
- 24.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 25.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hänsch GM. Polymorphonuclear neutrophils (PMN) as accessory cells for T-cell activation. MHC class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iking-Konert C, Wagner C, Denefleh B, et al. Up-regulation of the dendritic cell marker CD83 on polymorphonuclear neutrophils (PMN): divergent expression in acute bacterial infections and chronic inflammatory disease. Clin Exp Immunol. 2002;130:501–8. doi: 10.1046/j.1365-2249.2002.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro S, Wang JM, Gong WH, Yan D, Kamohora H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 28.Hänsch GM, Radsak M, Wagner C, et al. Expression of major histocompaibility antigens on polymorphonuclear neutrophils in patients with Wegener's granulomatosis. Kidney Int. 1999;55:1811–8. doi: 10.1046/j.1523-1755.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, Hänsch GM. Post-traumatic osteomyelitis. analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–10. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 30.Wagner C, Kaksa A, Müller W, et al. Polymorphonuclear neutrophils in posttraumatic osteomyelitis: cells recovered from the inflamed site lack chemotactic activity, but generate superoxides. Shock. 2004;22:108–15. doi: 10.1097/01.shk.0000132488.71875.15. [DOI] [PubMed] [Google Scholar]
- 31.Fleischer B. Superantigens. APMIS. 1994;102:3–12. doi: 10.1111/j.1699-0463.1994.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 32.Kraukauer T. Immune response to staphylococcal superantigens. Immunol Res. 1999;20:163. doi: 10.1007/BF02786471. [DOI] [PubMed] [Google Scholar]
- 33.Van Panhuys N, Perret R, Prout M, Ronchese F, Le Gros G. Effector lymphoid tissue and its crucial role in protective immunity. Trends Immunol. 2005;26:242–7. doi: 10.1016/j.it.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 35.Yoon KS, Fitzgerald RH, Sud S, Song Z, Wooley PH : Experimental acute hematogenous osteomyelitis in mice. II. Influence of Staphylococcus aureus infection on T-cell immunity. J Orthop Res. 1999;17:382–91. doi: 10.1002/jor.1100170313. [DOI] [PubMed] [Google Scholar]