Abstract
The presence and the role of soluble gp130, the soluble form of a component of the interleukin (IL)-6 receptor complex, were investigated in inflammatory bowel disease. The serum concentrations of soluble gp130 were increased in ulcerative colitis (active disease, median, 93·5 ng/ml; interquartile range, 26–125 ng/ml; inactive disease, 81 ng/ml, 24·8–137·3 ng/ml) and to a lesser extent in Crohn's disease (active disease, 66 ng/ml, 44·4–87·6 ng/ml; inactive disease, 63 ng/ml, 43·5–82·5 ng/ml) compared to normal controls (43 ng/ml, 27–59 ng/ml). Paired analysis of serum samples showed a decrease of IL-6 and soluble IL-6 receptor concentrations in both diseases and an increase of soluble gp130 concentrations, especially in ulcerative colitis, just after the resolution of disease exacerbation. Size fractionation of the serum revealed that a part of the IL-6 co-eluted with soluble gp130 and soluble IL-6 receptor. The IL-6-induced proliferation of murine B9 hybridoma was enhanced by recombinant soluble IL-6 receptor, whereas the proliferation was inhibited by recombinant soluble gp130. These results indicate that soluble gp130 may function as a natural inhibitor of the IL-6 actions in inflammatory bowel disease.
Keywords: Crohn's disease, interleukin-6, soluble gp130, soluble interleukin-6 receptor, ulcerative colitis
Introduction
Although the causes of inflammatory bowel disease (IBD), i.e. ulcerative colitis (UC) and Crohn's disease (CD), remain unclear, cytokines undoubtedly play a major role in the initiation and perpetuation of IBD by their immunoregulatory and inflammatory activities [1,2]. Among the known cytokines, a prominent role for interleukin (IL)-6 in this disease has been described extensively. Previous studies have shown that the serum levels of IL-6 increased in patients with active IBD and decreased after successful treatment [3–7]. The IL-6 levels also correlated with the severity and extent of disease [3,7]. In other studies by our group [8] and others [9–12], increased levels of IL-6 mRNA and protein also have been demonstrated in colonic tissue obtained from patients with IBD. Furthermore, blocking of IL-6 actions with a monoclonal antibody against the IL-6 receptor (IL-6R) has been shown to attenuate inflammation in patients with CD [13], as well as animals with experimental colitis [14,15]. These data suggest that IL-6 plays an important role in the pathophysiology of IBD.
IL-6 exerts its multiple activities through interactions with specific receptors on the surface of target cells [16–18]. Two distinct types of cellular IL-6R have been identified by molecular cloning: an 80 kDa ligand-binding chain, known as IL-6R, and a 130 kDa signal-transducing chain, gp130. The gp130 is associated with the binding complex of IL-6 and IL-6R, resulting in the formation of high-affinity IL-6 binding sites and transduces the signal [19,20]. It was pointed out that a soluble form of gp130 (sgp130), which lacks transmembrane and intracytoplasmic domains but retains the ligand-binding extracellular domain, is normally present in human sera [21,22]. This molecule may be able to inhibit the activities of IL-6 by binding competitively to circulating complexes of IL-6 and sIL-6R, precluding their binding to membrane gp130 receptors. Therefore, additional studies of sgp130 may provide better insight into the regulation of IL-6. An important functional role of sIL-6R has been suggested in some IL-6-related diseases [23–26]. Little is known, however, about the role of sgp130 in human diseases, including inflammatory conditions of the gastrointestinal system. One study has reported on the sgp130 levels in sera and pleural effusions from patients with metastatic carcinoma, non-Hodgkin's lymphoma, tuberculosis and cardiac failure [27].
In this study, to understand better the regulation of IL-6 in IBD, we measured the serum sgp130 levels by an enzyme-linked immunosorbent assay (ELISA), evaluated their relationship with the serum IL-6 and sIL-6R levels and analysed the circulating immunoreactive forms of sgp130 by a fast protein liquid chromatography (FPLC) system. Furthermore, the in vitro effect of these two soluble IL-6 receptors on the activity of IL-6 was examined using the IL-6-dependent murine B9 hybridoma cell line.
Materials and methods
Ethics
This project was performed in accordance with the Helsinki Declaration and was approved by the Medical Ethics Committee of Kurume University Hospital. Informed consent was obtained from every participant.
Subjects
Twenty-seven patients with UC and 20 with CD were investigated. The diagnoses were based on characteristic clinical, endoscopic, radiological and histological features. Disease activity was assessed by the criteria of Truelove and Witts in UC [28] and the score of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) in CD [29]. The 27 patients with UC comprised 14 patients with active disease and 13 with inactive disease. There were 16 men and 11 women, with a median age of 30·4 years (range 18–66 years) and a median disease duration of 4·4 years (range 0·1–9·0 years). Fourteen patients had pancolitis, nine had left colon involvement and four had disease limited to the rectum. At the time of study, one patient was receiving corticosteroids only, three were receiving corticosteroids plus sulphasalazine, nine were taking sulphasalazine only and 14 had no specific treatment. The 23 patients with CD included 13 patients with active disease and 10 with inactive disease. Fourteen patients were men and nine were women, with a median age of 30·0 years (range 16–72 years) and a median disease duration of 4·2 years (range 0·5–12·0 years). The disease affected both the ileum and the colon in 15 patients, the colon in seven patients and the ileum in one patient. One patient was taking only corticosteroids, one both corticosteroids and sulphasalazine, four sulphasalazine alone and 17 received no specific treatment. Of the active disease patients, six with UC and seven with CD were also sampled just after their disease became inactive. The data from these paired samples were analysed separately.
Seventeen healthy, age- and sex-matched subjects served as the normal control group. The colitis control group comprised six patients with infectious colitis and two patients with ischaemic colitis.
Enzyme-linked immunosorbent assay (ELISA) for soluble gp130
A standard ELISA method was used to measure the sgp130 concentration [23,30]. In brief, a 96-well immunoplate was coated with mouse anti-human gp130 monoclonal antibody (Tosoh Corporation, Kanagawa, Japan). After blocking with 1% BSA/Tris buffer for 2 h at 37°C, the serum samples were added and incubated overnight at room temperature. The plates were washed, and biotinylated guinea pig anti-human gp130 polyclonal antibody (Tosoh Corporation) was added to each well and incubated for 1 h at 37°C. The plates were then washed and incubated with streptavidin–alkaline phosphatase conjugate (Bethesda Research Laboratories, Gaithersburg, MD, USA) at a dilution of 1: 4000 for 30 min at room temperature. After washing again, LUMI-PHOS (Lumigen Inc., Detroit, MI, USA) was added to each well. The luminescence intensity was measured with a luminescence reader (BLA-201; Aloka, Tokyo, Japan).
Determination of other cytokines and laboratory parameters
Immunoreactive IL-6 [3,8] and sIL-6R [26] concentrations were quantified by ELISAs (SRL Inc., Tokyo, Japan and Sumitomo Metal Bio-Science Inc., Kanagawa, Japan, respectively). C-reactive protein concentrations were measured by laser nephelometry (NA latex CRP kit; Hoechst Japan, Tokyo, Japan). The erythrocyte sedimentation rates were determined using a standard technique.
Gel filtration analysis
The circulating immunoreactive forms of sgp130 were analysed by a FPLC system (Pharmacia, Uppsala, Sweden). Each serum sample was fractionated by gel filtration through a Superose 6 HR 10/30 column equilibrated with 20 m m phosphate buffer, pH 7·5, and 50 m M NaCl. Each sample (90 µl) was eluted with a buffer flow rate of 0·3 ml/min. Fractions of 0·6 ml were collected and the immunoreactive IL-6, sgp130 and sIL-6R levels in each fraction were measured by ELISA. The column was calibrated with marker proteins of known molecular weight.
Hybridoma growth factor assay
IL-6 bioactivity was determined using the IL-6-dependent murine B9 hybridoma, as described previously [31]. The proliferation of B9 cells is dependent on IL-6 but not on other cytokines. The B9 cells were maintained in RPMI-1640 (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) with 10% fetal calf serum (Gibco Laboratories, Grand Island, NY, USA), 105 U/l penicillin, 100 mg/l streptomycin, 2000 mg/l l-glutamine in the presence of recombinant (r)IL-6 (0·25 U/ml; provided by Dr T. Taga, Kumamoto University). The cells were harvested, washed twice in the culture medium and preincubated further in the absence of IL-6 for 6 h at 37°C in an effort to minimize the residual binding of IL-6 to the cells. Subsequently, a cell suspension (1 × 105 cells/ml) in 50 µl of culture medium was cultured with various combinations of rIL-6, rsIL-6R (provided by Dr Taga, Kumamoto University) and rgp130 (R&D Systems, Minneapolis, MN, USA) in a final volume of 100 µl in a flat-bottomed microtitre plate (Nunc, Roskilde, Denmark). After incubation for 4 days, 25 µl of 3-(4,5)-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT) [6 mg/ml in phosphate-buffered saline (PBS); Sigma, St Louis, MO, USA] was added to each well and incubated for 4 h at 37°C. Next, 100 µl of 10% SDS dissolved in 1 m M NH4OH was added to each well. After incubation overnight at 37°C, the optical density was measured at 570 nm by an automatic microplate spectrophotometer. All assays were performed in triplicate. This in vitro experiment was repeated three times.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U-test or Wilcoxon's rank-sum test. A P-value of less than 0·05 was considered significant.
Results
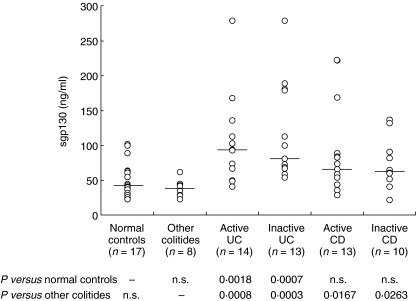
The individual serum concentrations of sgp130 in the patients with IBD and the control subjects are shown in Fig. 1. Detectable sgp130 levels were found in all the serum samples. sgp130 levels were increased significantly in patients with UC (active disease, median, 93·5 ng/ml; interquartile range, 26–125 ng/ml, P = 0·0018; inactive disease, median, 81 ng/ml; interquartile range, 24·8–137·3 ng/ml, P = 0·0007) compared to the normal controls (median, 43 ng/ml; interquartile range, 27–59 ng/ml). sgp130 levels also tended to be increased in patients with CD (active disease, median, 66 ng/ml; interquartile range, 44·4–87·6 ng/ml; inactive disease, median, 63 ng/ml; interquartile range, 43·5–82·5 ng/ml) compared to the normal controls, although it did not reach statistical significance. There was no significant difference in the serum sgp130 levels between active and inactive disease in patients with both UC and CD. The serum sgp130 levels in the other forms of colitis (median, 38·5 ng/ml; interquartile range, 30·8–46·3 ng/ml) were comparable to those of the normal controls. In addition, no significant differences in the sgp130 concentrations were evident between the patients who received drug therapy and those who did not (data not shown).
Fig. 1.
Serum concentrations of soluble gp130 (sgp130) in patients with ulcerative colitis (UC) and Crohn's disease (CD), patients with other colitides and normal controls. The median is indicated by a horizontal line.
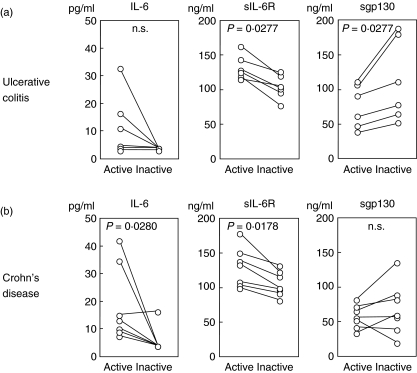
The serum concentrations of IL-6, sIL-6R and sgp130 in the paired samples obtained when the patients had active disease and when their disease became inactive are shown in Fig. 2. The high serum concentrations of IL-6 and sIL-6R decreased just after the disease activity entered an inactive phase. On the other hand, simultaneous measurement of the serum sgp130 concentration showed that it increased significantly in an inactive phase especially in patients with UC (P = 0·0277).
Fig. 2.
Comparison of serum concentrations of interleukin (IL)-6, soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in patients with inflammatory bowel disease before and after successful treatment. Paired serum samples were obtained from six patients with ulcerative colitis (a, UC) and seven with Crohn's disease (b, CD) during active phase and just after the resolution of disease exacerbation.
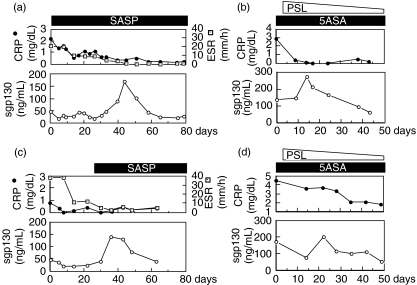
Figure 3 shows the serial changes of the serum sgp130 levels in four patients (two with UC and two with CD followed longitudinally over a long period). Eventually, the disease activity in these patients was controlled by treatment. The peak concentrations of sgp130 were found consistently as the disease resolved according to clinical and laboratory signs, and declined slowly thereafter.
Fig. 3.
Time-course of serum soluble gp130 (sgp130) concentrations in patients with inflammatory bowel disease. Four patients (a–d) were sampled serially over intervals where their disease status changed from active to inactive. (a) A 32-year-old man with ulcerative colitis who had total colonic involvement treated by sulphasalazine. (b) A 67-year-old man with ulcerative colitis who had left colonic involvement treated by 5-aminosalicylates and prednisolone. (c) A 27-year-old man with Crohn's disease who had colonic involvement treated by sulphasalazine. (d) A 44-year-old man with Crohn's disease who had colonic involvement treated by 5-aminosalicylates and prednisolone. SASP, sulphasalazine; 5ASA, 5-aminosalicylates; PSL, prednisolone; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
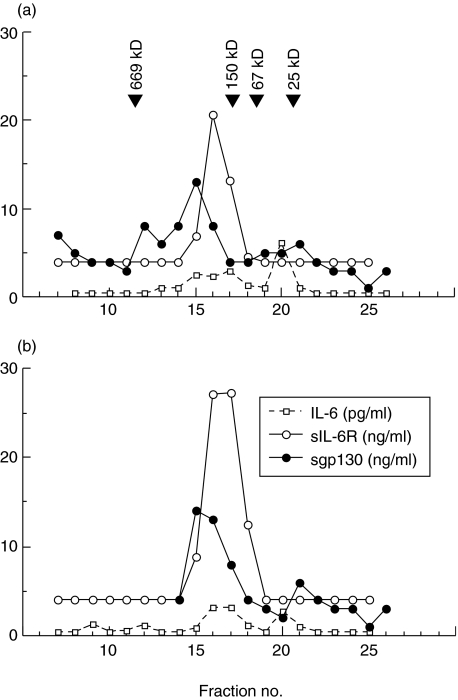
To analyse the possible association of sgp130 with IL-6 and sIL-6R in the circulation, we examined sera from five IBD patients by FPLC. The results shown in Fig. 4, two of five highly reproducible experiments, indicate that the sgp130 immunoreactive protein peaks at fraction 15 whereas the sIL-6R elutes at peak fraction 16–17. Immunoreactive IL-6 showed two major peaks, one eluting at fraction 16–17 and another at fraction 20. Clearly, some of the sgp130 eluted together with IL-6 and sIL-6R, indicating that these molecules may circulate partly as a complex. It is interesting that there is no sIL-6R at the expected molecular weight (approximately 80 kDa), suggesting that in patients with high levels of sgp130, this complexes all the circulating sIL-6R, together with some IL-6.
Fig. 4.
Fast protein liquid chromatography profile of serum obtained from a patient with ulcerative colitis (a) and from a patient with Crohn's disease (b). Serum samples were fractionated by molecular sieving over a Superose 6 HR 10/30 column. Each fraction was assayed by enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-6, soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130), and results were plotted as immunoreactive concentrations versus fraction number. Elution positions of the molecular markers, thyroglobulin (669 kDa), IgG (150 kDa), BSA (67 kDa) and chymotrypsinogen A (25 kDa) indicated by arrows. The results are representative for five patients studied.
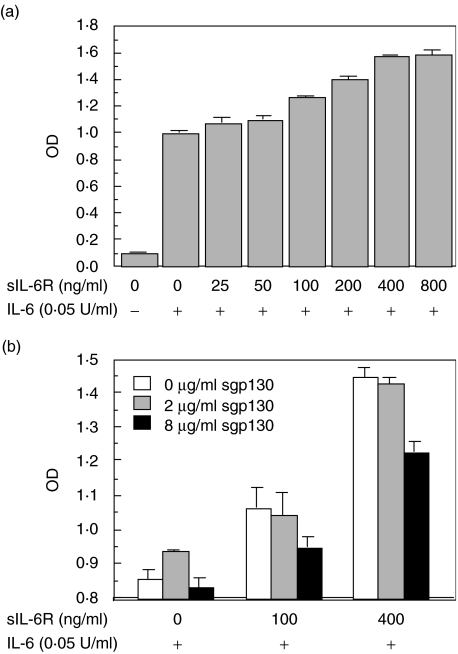
To clarify the functional role of sgp130, the effect of sgp130 on IL-6-induced proliferation of the murine B9 hybridoma was examined. The addition of rsIL-6R in the presence of rIL-6 led to an additional increase in the proliferation of B9 cells (Fig. 5a). In contrast, the addition of rsgp130 in the presence of both rIL-6 and rsIL-6R led to a gradual decrease in the proliferation of B9 cells (Fig. 5b).
Fig. 5.
Effect of soluble IL-6 receptor (sIL-6R) or soluble gp130 (sgp130) on interleukin (IL)-6-induced proliferation of murine hybridoma cell line B9. (a) The B9 cells were cultured with various concentrations of sIL-6R in the absence or presence of IL-6 for 4 days, and the cellular proliferation was determined by a colorimetric method using MTT dye as described in Materials and methods. (b) The B9 cells were cultured with various combinations of sIL-6R and sgp130 in the presence of IL-6 for 4 days, and the cellular proliferation was determined by a colorimetric method using MTT dye. Values represent the mean ± s.d. of triplicate cultures. The experiment was repeated three times and yielded comparable results.
Discussion
IL-6 possesses a variety of immune and inflammatory activities, which include induction of acute phase proteins from hepatocytes, and the activation and maturation of B cells and T cells [32,33]. These activities of IL-6 suggest that this molecule may play an important biological role throughout the disease course of IBD. The purpose of this study was to investigate the role of the soluble receptor sgp130 in patients with IBD.
First, we found an increase in the circulating levels of sgp130 in patients with UC and to a lesser extent in CD. Of particular note is the result showing the dynamic changes in the serum sgp130 concentrations as well as IL-6 and sIL-6R, especially in the paired samples. The serum levels of IL-6 and sIL-6R were elevated during the active phase of IBD and declined as the patients went into remission, similar to previous reports by our laboratory and others [3–7, 26]. Interestingly, a different pattern was occurred for sgp130. The serum sgp130 level increased just after the resolution of a disease exacerbation, particularly in UC. This pattern was confirmed further by the result in which the patients were followed longitudinally during the long period. In contrast, the data obtained from different individual showed only a modest change, especially in CD, indicating that circulating levels of sgp130 should be evaluated individually in each patient. The measurement of serum sgp130 probably does not have a role in specific diagnosis because it is likely to be raised in other disease conditions [27]. Nevertheless, its serial measurement may help to determine disease prognosis and confirm treatment efficacy.
To analyse the in vivo appearance of sIL-6R, sgp130 and IL-6 in the circulation, we examined the molecular form of these components. Of particular note is the finding that a substantial proportion of sgp130 co-elutes with IL-6 and sIL-6R at the same fraction, suggesting that these molecules may circulate partly as a complex. These changes in the molecular form of such components enable us to speculate that these two soluble factors may regulate tightly the IL-6-mediated immune process in IBD. Further study is needed to confirm our speculation.
At this point, the question raised is whether this natural sgp130 has a physiological role or is only a breakdown product. To answer this question, we attempted to determine whether sgp130 modulates IL-6 function using a proliferation assay with the IL-6-dependent murine B9 hybridoma. Interestingly, sgp130 inhibited the proliferation of B9 cells, suggesting that sgp130 may be the limiting factor of IL-6 function. Moreover, it has been pointed out previously that the DNA synthesis induced in BAF-130 cells by recombinant IL-6-supplemented serum was increased when the serum was deprived of sgp130 [22], indicating further the inhibitory effect of this product. Although murine B9 hybridoma cells would behave in a different manner compared to the clinical situation, these data suggest strongly that naturally occurring sgp130 may also contribute to terminating inflammation in vivo. Besides their direct importance in explaining IBD immunopathogenesis, quantitative assessments of serum sgp130, in addition to those of IL-6 and sIL-6R, may be important to characterize IL-6 function.
In contrast to the effect of this potential inhibitor sgp130, we substantiated the enhancing effect of sIL-6R on IL-6 activity in B9 cells. These data complement and extend previous findings in other cell types [19,34,35]. In IBD, we have already reported that excess amounts of sIL-6R circulate as a complex with IL-6 during the active phase [26]. Taken together, it is likely that these two different types of the soluble IL-6 receptor, sIL-6R and sgp130, may regulate IBD ingeniously.
The mechanism that leads to release of sgp130 in association with inflammation is currently unknown. In vitro investigation demonstrated that gp130 expression on human epithelial cell lines UAK and Hep3B are enhanced both at the mRNA and protein level by inflammatory cytokines, including IL-6, IL-1 and tumour necrosis factor-α. Furthermore, all these stimulants seem to act selectively on gp130, whereas IL-6R does not [36]. The above findings, together with the result of the high levels of inflammatory cytokines such as IL-6 or tumour necrosis factor-α during the active disease [8–12], suggest that such up-regulation of gp130 expression in response to these cytokines is not an in vitro event but a physiological phenomenon in vivo. Further studies will be needed to answer these questions.
It has been shown previously that gp130 is also involved in the signalling processes of leukaemia inhibitory factor, oncostatin M, ciliary neurotrophic factor and IL-11 [17,37–44]. Thus, it would be of interest to examine whether sgp130 may affect the biological functions of these cytokines as well as IL-6 in patients with IBD.
In this report, we described the dynamic changes of sgp130 in sera of IBD patients that could inhibit IL-6 actions in in vitro experiments. These data indicate that the IL-6-mediated immune process observed in IBD is regulated tightly by these two forms of soluble IL-6 receptors sIL-6R and sgp130. Of these, sgp130 may function as a natural inhibitor of the ligand actions. It must be kept in mind that simple determination of IL-6 concentrations from biological samples has limited value without knowledge of the exact interaction among IL-6, sIL-6R and sgp130.
Acknowledgments
We thank Drs Tadashi Nakasone, Hitomi Yoshizaki and Mitsuo Honda (National Institute of Infectious Diseases, Tokyo, Japan) for their excellent assistance.
References
- 1.Podolsky DK, Fiocchi C. Cytokines, chemokines, growth factors, eicosanoids, and other bioactive molecules in inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory bowel disease. 5. Philadelphia: WB Saunders; 2000. pp. 191–207. [Google Scholar]
- 2.Cominelli F, Arseneau KO, Pizarro TT. The mucosal inflammatory response. Cytokines and chemokines. In: Targan SR, Shanahan F, Karp LC, editors. Inflammatory bowel disease: from bench to bedside. 2. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2003. pp. 147–76. [Google Scholar]
- 3.Mitsuyama K, Sata M, Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn. 1991;26:20–8. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- 4.Mahida YR, Kurlac L, Gallagher A, Hawkey CJ. High circulating concentrations of interleukin-6 in active Crohn's disease but not ulcerative colitis. Gut. 1991;32:1531–4. doi: 10.1136/gut.32.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992;102:514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 6.Lobo AJ, Evans SW, Jones SC, et al. Plasma interleukin-6 in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1992;4:367–72. doi: 10.1111/j.1440-1746.1993.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 7.Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285–92. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuyama K, Sasaki E, Toyonaga A, et al. Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion. 1991;50:104–11. doi: 10.1159/000200747. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs KL, Sartor B, Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992;103:1587–9. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- 10.Stevens C, Walz G, Singaram C, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–26. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 11.Raab Y, Hällgren R, Gerdin B. Enhanced intestinal synthesis of interleukin-6 is related to the disease severity and activity in ulcerative colitis. Digestion. 1994;55:44–9. doi: 10.1159/000201122. [DOI] [PubMed] [Google Scholar]
- 12.Jones SC, Crabtree JE, Rembacken BJ, et al. Mucosal interleukin-6 secretion in ulcerative colitis. Effects of anti-inflammatory drugs and T-cell stimulation. Scand J Gastroenterol. 1994;29:722–8. doi: 10.3109/00365529409092500. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 16.Foxwell BMJ, Barrett K, Feldmann M. Cytokine receptors: Structure and signal transduction. Clin Exp Immunol. 1992;90:161–9. doi: 10.1111/j.1365-2249.1992.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–7. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 18.Taga T, Hibi M, Murakami M, et al. Interleukin-6 receptor and signals. Chem Immunol. 1992;51:181–204. [PubMed] [Google Scholar]
- 19.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 20.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 21.Yasukawa K, Futatsugi K, Saito T, et al. Association of recombinant soluble IL-6-signal transducer, gp130, with a complex of IL-6 and IL-6 receptor, and establishment of an ELISA for soluble gp130. Immunol Lett. 1992;31:123–30. doi: 10.1016/0165-2478(92)90138-e. [DOI] [PubMed] [Google Scholar]
- 22.Narazaki M, Yasukawa K, Saito T, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–6. [PubMed] [Google Scholar]
- 23.Honda M, Yamamoto S, Cheng M, et al. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148:2175–80. [PubMed] [Google Scholar]
- 24.Gaillard J-P, Bataille R, Brailly H, et al. Increased and highly stable levels of functional soluble interleukin-6 receptor in sera of patients with monoclonal gammopathy. Eur J Immunol. 1993;23:820–4. doi: 10.1002/eji.1830230408. [DOI] [PubMed] [Google Scholar]
- 25.De-Benedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A. Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest. 1994;93:2114–19. doi: 10.1172/JCI117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsuyama K, Toyonaga A, Sasaki E, et al. Soluble interleukin-6 receptors in inflammatory bowel disease; relation to circulating interleukin-6. Gut. 1994;36:45–9. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dore P, Lelievre E, Morel F, et al. IL-6 and soluble IL-6 receptors (sIL-6R and sgp130) in human pleural effusions: massive IL-6 production independently of underlying diseases. Clin Exp Immunol. 1997;107:182–8. doi: 10.1046/j.1365-2249.1997.d01-889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myren J, Bouchier IAD, Watkinson G, Softley A, Clamp SE, Dombal FT. The OMGE multinational inflammatory bowel disease survey 1976–82. A further report on 2657 cases. Scand J Gastroenterol. 1984;19(95):1–27. [PubMed] [Google Scholar]
- 30.Nakajima T, Yamamoto S, Cheng M, et al. Soluble interleukin-6 receptor is released form receptor-bearing cell lines in vivo. Jpn J Cancer Res. 1992;83:373–8. doi: 10.1111/j.1349-7006.1992.tb00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aarden LA, De Groot ER, Schaap OL, Lansdorp PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–16. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T. The biology of interleukin 6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 33.Kunimoto DY, Nordan RP, Strober W. IL-6 is a potent cofactor of IL-1 in IgM synthesis and of IL-5 in IgA synthesis. J Immunol. 1989;143:2230–5. [PubMed] [Google Scholar]
- 34.Mackiewicz A, Rose-John S, Schooltink H, Laciak M, Gorny A, Heinrich PC. Soluble human interleukin-6-receptor modulates interleukin-6-dependent N-glycosylation of alpha 1-protease inhibitor secreted by HepG2 cells. FEBS Lett. 1992;306:257–61. doi: 10.1016/0014-5793(92)81012-b. [DOI] [PubMed] [Google Scholar]
- 35.Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149:2021–7. [PubMed] [Google Scholar]
- 36.Snyers L, Content J. Enhancement of IL-6 receptor beta chain (gp130) expression by IL-6, IL-1 and TNF in human epithelial cells. Biochem Biophys Res Commun. 1992;185:902–8. doi: 10.1016/0006-291x(92)91712-y. [DOI] [PubMed] [Google Scholar]
- 37.Gearing DP, Comeau MR, Friend DJ, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–7. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 38.Ip NY, Nye SH, Boulton TG, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–32. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 39.Taga T, Narazaki M, Yasukawa K, et al. Functional inhibition of hematopoietic and neurotrophic cytokines by blocking the interleukin 6 signal transducer gp130. Proc Natl Acad Sci USA. 1992;89:10998–1001. doi: 10.1073/pnas.89.22.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Modrell B, Aruffo A, et al. Interleukin-6 signal transducer gp130 mediates oncostatin M signaling. J Biol Chem. 1992;267:16763–6. [PubMed] [Google Scholar]
- 41.Rose TM, Bruce AG. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci USA. 1991;88:8641–5. doi: 10.1073/pnas.88.19.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilton DJ, Gough NM. Leukemia inhibitory factor: a biological perspective. J Cell Biochem. 1991;46:21–6. doi: 10.1002/jcb.240460105. [DOI] [PubMed] [Google Scholar]
- 43.Stöckli KA, Lottspeich F, Sendtner M, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–3. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y-C, Yin T. Interleukin-11 and its receptor. Biofactors. 1992;4:15–21. [PubMed] [Google Scholar]