Abstract
Myasthenia gravis (MG) is commonly regarded as the prototype of an antibody-mediated, organ-specific autoimmune disease. Antibodies against the acetylcholine receptor (AChR) on the muscle endplate trigger its typical clinical manifestations of weakness and fatiguability. T–B cell interactions are thought to play a crucial role in the pathogenesis of MG. OX40 (CD134), a costimulatory molecule that is expressed on activated CD4+ T-cells, might contribute to the development or pathogenesis of immune-mediated diseases such as rheumatoid arthritis and graft-versus-host disease. In the present study, we investigated the expression of OX40 on CD4+ T-cells from patients with MG and healthy individuals. Results from 36 MG patients and 28 healthy controls revealed that more freshly isolated CD4+ T-cells from MG patients expressed OX40 than cells from healthy individuals. High levels of antibodies against the AChR, thymic hyperplasia and onset at an early age were associated with elevated expression of OX40. Upon activation by various concentrations of anti-CD3 antibodies, CD4+ T-cells from MG patients showed a tendency toward higher levels of OX40 expression than cells from healthy individuals. Given the role of OX40 in the immune system, we conclude that OX40 might contribute to the development of MG.
Keywords: autoimmune disease, CD4+ T-cell, hyperplasia, myasthenia gravis, OX40 (CD134)
Introduction
Myasthenia gravis (MG) is an autoantibody-mediated disease and antibodies against the acetylcholine receptor (AChR) on the muscle endplate trigger its typical clinical manifestations. The autoantibody production is a T-cell dependent phenomenon both in human and experimental MG [1,2]. Thus, T–B cell interactions are essential events in the pathogenesis of MG. Among costimulatory pathways, there are two major families: the B7 family, including CD28 and CTLA-4, and the Tumour necrosis factor/Tumour necrosis factor receptor superfamily (TNF/TNFR). CD28 provides positive signals whereas CTLA-4 provides inhibitory signals to the T cell activation. We have earlier shown that MG patients had abnormally low expression of CTLA-4 on freshly isolated and stimulated T-cells in the peripheral blood [3]. Moreover, CTLA-4Ig was able to ameliorate experimental autoimmune MG [4]. In addition to the B7 family, TNF/TNFR superfamily members, such as CD27, OX40 (CD134), CD40L (CD154), 4–1BB (CD137), are equally important for the effective generation of many types of T cell responses. These molecules appear to provide signals that allow continued cell division initially regulated by CD28, and/or to prevent excessive cell death [5]. Blockade of CD40 ligand by anti-CD40L antibodies suppressed chronic experimental autoimmune MG [6].
OX40, a member of TNFR family, is a 50-kD cell surface glycoprotein expressed on activated CD4+ T-cells [7]. Engagement of OX40 enhanced proliferation and cytokine production by CD4+ T-cells as well as the long-term survival of CD4+ T-cells by promoting Bcl-xL and Bcl-2 expression [8]. In addition, OX40 ligation enhanced cell turnover of antigen-activated CD4+ T-cells in vivo[9], and led to TRAF2 (Tumour necrosis factor receptor-associated factor 2) and TRAF5- mediated NF-κB activation [10]. OX40 ligand (OX40L) is expressed on APCs, such as activated B-lymphocytes [11,12] and dendritic cells [13]. Blocking of OX40–OX40L interaction with polyclonal anti-OX40 antibody resulted in a profound decrease of antigen-specific, T-cell-dependent-antibody production [14]. Mice lacking OX40L showed impairment of APC function and a reduction in T cell proliferation and production of both Th1 and Th2 cytokines [15,16].
Due to its involvement in T–B cell interactions, the OX40 signal is strongly implicated in autoimmune disease. Signals from an agonistic antibody against OX40 could break an existing state of tolerance in CD4+ T-cells [17,18]. This suggests that a similar mechanism operating in vivo could lead to autoimmunity. Moreover, excess interactions of OX40/OX40L resulted in autoimmune-like inflammatory bowel disease (IBD) [19]. Expression of OX40 and/or OX40L has been demonstrated in tissues in several autoimmune disorders such as experimental autoimmune encephalomyelitis (EAE) [20,21], atherosclerosis [22], experimental IBD [23], human proliferative lupus nephritis [24], rheumatoid arthritis [25], human IBD [26], human inflammatory muscle disease [27] and human graft-versus-host disease (GVHD) [28]. Blocking the OX40:OX40L pathway ameliorated ongoing EAE [29], diabetes in NOD mice [30], murine models of asthma [31], collagen-induced arthritis [25], murine graft-versus-host disease [32] and experimental IBD [23].
In MG patients, OX40 was up-regulated in thymic tissues adjacent to germinal centres and might interact with OX40L in germinal centres to enhance anti-AChR antibody production in MG [33].
In the present study, we have tested CD4+ T-cells from peripheral blood mononuclear cells (PBMC), both freshly isolated and after stimulation with anti-CD3 antibody. The results indicate an involvement of OX40 in MG.
Materials and methods
Patients and healthy individuals
Thirty-six patients with clinically defined MG and 28 healthy age- and sex-matched controls (HC) were enrolled in the study. The diagnosis of the disease was based on a typical case history and clinical investigation, a positive response to edrophonium, a decremental response following repetitive nerve stimulation and the presence of antibodies against AChR. Samples from patients and healthy controls were taken between 0900h and 1400h. The analysis of samples was performed blinded. All MG patients were recruited in the MG centre at the Neurological Department in Karolinska Hospital. No patient was treated with immuno-suppressive drugs. Patient data are shown in Table 1. Patients were classified into different groups according to disease severity, thymic histology, age at onset, gender and levels of antibodies against the AChR. The Osserman-Oosterhuis classification was used to grade the disease [34]. Stage I, II and III indicate ocular MG, generalized disease and severe acute disease, respectively. Anti-AChR antibodies in sera from MG patients were determined by standardized radio-immuno-precipitation assay (RIA). The levels of the antibodies were given as arbitrary units [35].
Table 1.
The percentage (%) of CD4+OX40+ T-cells of total CD4+T-cells from MG patients (n = 36) and healthy controls (HC) (n = 28).
| No. of patients | CD4+OX40+T-cells | P-value versus HC | |
|---|---|---|---|
| MG | 36 | 7·7 (7·0–10) | 0·012 |
| Stage | |||
| I | 6 | 8·0 (3·1–15·9) | ns |
| II | 28 | 7·3 (6·5–9·6) | 0·022 |
| III | 2 | 12 (9·0, 15·1) | – |
| Thymic histopathologyy | |||
| Thymoma | 0 | – | |
| Hyperplasia | 16 | 9·1 (6·3–10·36) | 0·035 |
| Normal | 2 | 8·9 (6·0, 8·8) | ns |
| Unthymectomized | 18 | 7·2 (6·3–11·34) | ns |
| Gender | |||
| Female | 21 | 7·4 (6·5–10·4) | ns |
| Male | 15 | 7·9 (6·1–11·1) | ns |
| Age of onset | |||
| Early onset (< 40) | 22 | 8·4 (6·7–10·8) | 0·022 |
| Late onset (> 40) | 14 | 7·2 (5·8–10·6) | ns |
| Serum anti-AChR antibody (arbitrary unit) | |||
| 0 | 6 | 6·1 (2·6–12·3) | ns |
| > 0 (> 0–5 plus > 5) | 30 | 8·4 (7·1–10·3) | 0·008 |
| > 0–5 | 22 | 7·3 (6·2–10·1) | 0·045 |
| > 5 | 8 | 10·3 (6·7–13·7) | 0·009 |
| HC | 28 | 5·8 (4·8–7·3) | |
Subgroups of MG patients were classified by stage, thymic histopathology, age of onset, gender and levels of anti-AChR antibodies. The numbers of CD4+OX40+ T-cells in CD4+ T-cells are shown as the median and the 5th to 95th confidence intervals. P-values obtained by the Mann–Whitney test between healthy individuals, total numbers of MG patients and subgroups of MG patients are listed in the table.
Antibodies
The following monoclonal antibodies (mAbs) were purchased from Becton & Dickinson (Mountain View, CA, USA): FITC-labelled anti-OX40 (clone ACT35); PE-labelled anti-CD69 (clone FN50), anit-CD25 (clone M-A251), anti-CD28 (clone 28·2), anti-CTLA-4 (CD152, clone 14A2.H1); PerCP-labelled anti-CD4 (clone RPA-T4), anti-CD3 (clone HIT3a). Irrelevant isotype-matched mouse mAbs were FITC-IgG1 (clone JDC-1) and PE-IgG1 (clone G17-1).
Preparation and culture of mononuclear cells
Heparinized blood samples were collected from controls and MG patients. PBMC were separated by Ficoll density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). The cells were collected and washed three times with RPMI-1640 (Gibco, Paisley, UK) containing antibiotics.
Increasing levels of immobilized anti-CD3 antibodies were used to stimulate PBMC. Briefly, anti-CD3 antibody (OKT3) was coated on 24 well cell culture plates (Sarstedt, USA) and plates were incubated at 4 °C overnight. Plates were then washed three times with PBS before adding RPMI-1640 medium, supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml l-glutamine and 10% (V/V) FCS (Life Technologies, Rockville, MD, USA). PHA (Life Technologies) was used as a positive control.
Flow cytometry
Cell suspensions were triple stained with FITC, PE- and PerCP-conjugated mAbs. Samples were incubated with mAb for 30 min at room temperature in the dark. Only cell surface molecules were detected and no intracellular staining was done in the present study. The cells were washed three times with PBS and analysed in a Becton-Dickinson FACScalibur flow cytometer using CellQuest software (Becton-Dickison). For freshly isolated PBMC, only small lymphocytes were gated and analysed. For stimulated PBMC, a larger gate was set according to the forward and side scatter.
Statistical analysis
The nonparametric Mann–Whitney test was used to compare the expression of OX40 on CD4+ T-cells in patients with MG, subgroups of MG and HC. Reported P-values were two-tailed and considered statistically significant at P < 0·05.
Results
OX40 expression on freshly isolated PBMC from MG and HC
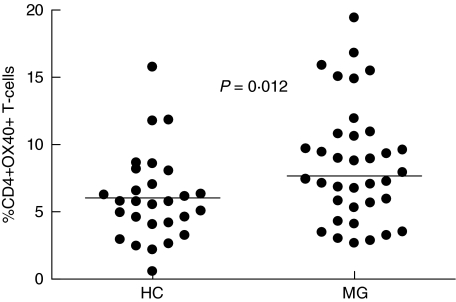
The percentage of CD4+OX40+ T-cells among blood CD4+ T-cells in all MG patients are shown in Table 1 and Fig. 1. Results from 36 MG patients and 27 healthy controls revealed that more CD4+OX40+ T-cells in freshly isolated PBMC from MG patients than from healthy individuals (P = 0·012, Table 1 and Fig. 1). There was no difference in the mean fluorescence intensity (MFI) of OX40 between patients and healthy controls (data not shown).
Fig. 1.
OX40+ expression on freshly isolated CD4+ T-cells from MG patients (n = 36) and healthy controls (HC) (n = 28) (P = 0·012). The vertical lines indicates the median levels of CD4+ T-cells expressing OX40.
Patients were divided into subgroups according to the stage, thymic histology, age of onset and levels of serum anti-AChR antibodies levels for additional analysis (Table 1). Patients with generalized disease, onset before 40 years of age, thymic hyperplasia and high levels of anti-AChR antibodies had more cells expressing OX40 (P = 0·022, 0·022, 0·035 and 0·008 versus HC, respectively). Patients without antibodies against the AChR antibodies had the same frequencies of OX40+ T cells as healthy controls (Table 1).
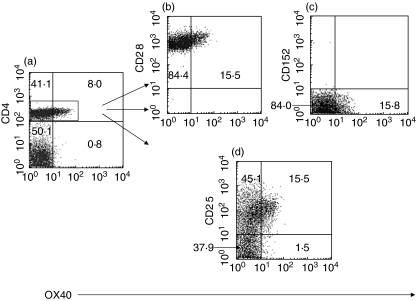
Figure 2 shows representative plots of OX40 expression on CD4+ T-cells from a MG patient. The expression of the surface molecules CD28, CTLA-4, CD25 and CD69 on CD4+ T-cells were analysed and shown in Fig. 2b–d). All CD4+OX40+ T-cells expressed CD28 (100%) but very few of them expressed CTLA-4(CD152) (< 1%) (Fig. 2b,c). CD25, normally expressed on activated T cells, was coexpressed on 91% of CD4+OX40+ T-cells (Fig. 2d), which is in agreement with previous studies [33]. In contrast, only 54·5% of CD4+OX40- T-cells from this patient expressed CD25 (Fig. 2d). CD69 could not be demonstrated on the surface of CD4+OX40 T-cells (data not shown). All CD4+OX40+ T-cells from patients and controls displayed the same pattern as the cells from the MG patient shown in Fig. 2. CD8+ cells expressing OX40+ were rare in both patients and controls (data not shown).
Fig. 2.
Representative examples of flow cytometry plots showing (a) CD4+OX40+ T-cells from one MG patient (15·5% of total CD4+T-cells expressed OX40) and surface expression of (b) CD28 (100%) (c) CD152 (CTLA-4) (< 1%) (d) CD25 on CD4+OX40+ T-cells. CD4+OX40+ T-cells exhibited elevated CD25 (91%) expression compared with CD4+OX40- T-cells (54·5%).
OX40 expression on activated CD4+ T-cells from MG and HC
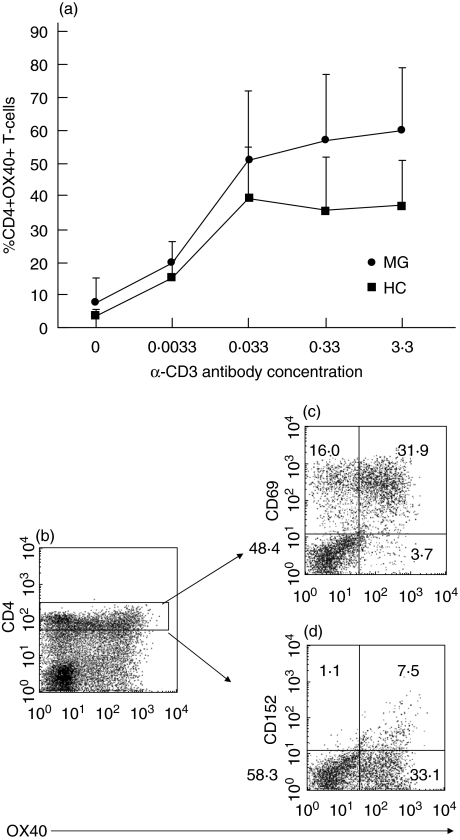
According to previous kinetic studies, OX40 expression on CD4+ T-cells was demonstrated within 24 h of stimulation with anti-CD3 antibodies and peaked between 48 and 72 h [7]. In this study, we stimulated PBMC with increasing concentrations of anti-CD3 antibodies for 24 and 96 h. Data from a few patients and healthy controls showed a similar kinetic pattern (data not shown). CD4+ T-cells from five MG patients showed a tendency towards elevated OX40 expression compared to cells from five healthy individuals after stimulation of 24 h (Fig. 3a). Consistent with previous findings, anti-CD3 antibody alone induced expression of OX40 on CD4+ T-cells [7] (Fig. 3b). PHA was used as a positive control. Stimulated CD4+OX40+ T-cells coexpressed the activation markers CD69 (89% of total OX40+ T-cells), and CD152 (18% of total OX40+ T-cells) (Fig. 3c,d). There was no difference after 96 h of stimulation (data not shown).
Fig. 3.
(a) Induction of OX40 expression on CD4+ T-cells after 24 h (a). PBMC from MG (•, n = 5) and HC (▪, n = 5) were stimulated in parallel with immobilized anti-CD3 antibody at the indicated coating concentrations for 24 hRepresentative examples of flow cytometry plots showing (b) CD4+OX40+ T-cells from one MG patient stimulated with anti-CD3 antibody for 24 h and surface expression of (c) CD69 (89%) (d) CD152 (CTLA-4) (18%) on CD4+OX40+ T-cells.
Discussion
Up-regulation of OX40 has been demonstrated in several types of autoimmune disease. T-cells in the synovial fluid of patients with active rheumatoid arthritis displayed the OX40 surface antigen [36]. OX40 was highly expressed in the gastrointestinal tissue of patients with IBD [37]. Biopsies from thymic hyperplastic areas from MG patients showed that OX40+ cells around the germinal centres (GC) were more frequent than in normal thymuses. A considerable number of OX40+ cells were present in the thymic tissues adjacent to thymomas from MG patients [33]. A patient who developed MG 25 months after receiving an allogeneic bone marrow transplant displayed prominently increased CD4+OX40+ T-cells in the peripheral blood one month before the onset of MG [38]. However, correlation of peripheral blood OX40+ T cells with chronic graft-versus-host disease (GVHD) in patients who received allogeneic haematopoietic stem cell transplantation was also observed [28]. The last two reports prompted us to question whether up-regulation of OX40 on PBMC was due to the effect of transplantation or of MG, and whether enhancement of OX40 expression is a typical feature of human MG. Indeed, our results demonstrated that the circulating CD4+OX40+ T-cell populations were increased in patients affected with MG when compared to healthy controls. There was no difference in the MFI between patients and controls, indicating that the number of OX40 molecules per cell was not changed. The CD4+OX40+ T-cells exhibited high levels of CD25, suggesting they include the activated, autoreactive T-cells [39]. There was no CD69 expression on these cells, indicating that they did not represent recently activated T-cells. In addition, no expression of CTLA-4 on the surface of CD4+OX40+ T-cells was detected, implying that these T-cells were not regulatory cells. This was further substantiated using CD25 and OX40 double staining, which showed that the OX40+CD25+ T-cells were never CD25bright, which was to be expected for naturally arising regulatory T cells [40].
One of the crucial events in the development of MG is activation of self-reactive T-cells and abundant IgG autoantibody production. Altered expression of costimulatory molecules, such as CTLA-4 [3], CD80 and CD86 [40] on CD4+ T-cells from peripheral blood, and CD95 on thymocytes [41] has been demonstrated in patients with MG. Costimulation through CD80 is believed to result in the development of Th1 immune responses characterized by cell-mediated tissue destruction, whereas CD86 promotes Th2 immune responses characterized by a strong humoral component [42]. The increase of these two molecules on T-cells from patients thus may lead to profound cytokine production and T-cell-dependent antibody production. CTLA-4 exerts inhibitory effects to T-cell activation [43]. Additionally, CD95 is involved in activation-induced cell death, particularly in negative selection [44]. The low expression of CTLA-4 on T-cells [3], but accumulated CD95 on thymocytes that occur in MG patients may permit the escape of autoreactive T-cells from negative selection and result in robust T-cell activation upon autoantigen. All data listed here indicated the activated background of MG will help us to understand the contribution of costimulatory molecules in the disease development of MG.
Co-stimulation through OX40 is crucial for the induction of an allo-reactive T-cell response both in humans and mice and involved in T-cell help for B-cells in the development of IgG responses [12,14,45]. We found that MG patients with anti-AChR antibodies had elevated level of CD4+OX40+ T-cells (Table 1), compared with healthy controls. Up-regulation of OX40 might induce activation of autoreactive T-cells and T-cell dependent autoantibody production. This speculation was further confirmed by animal experiments, which showed that constitutive OX40–OX40L interactions triggered autoimmune-like disease [19], and that an agonistic anti-OX40 antibody allowed anergic, autoreactive T-cells to acquire effector cell functions [17,18]. Nevertheless, considering the low percentage of AChR-specific T-cells in PBMC, we have to conclude that many CD4+OX40+ T-cells in patients may actually be activated bystanders. More detailed investigations relating the up-regulation of OX40 on CD4+ T-cells and MG disease development in both mice and human are required to address the issue.
The association between OX40 with generalized disease, hyperplasia and early age onset is an interesting finding of the present study. In generalized MG, thymic hyperplasia is the most frequent and typical pathological change. The thymus is characterized by numerous and prominent lymph follicles with germinal centres and a surrounding T-cell zone, containing autoreactive activated T-cells, and also B cells producing anti-AChR antibodies. High expression of OX40 is present in inflammation diseases such as IBD [36], rheumatoid arthritis [25] and atherosclerosis [22]. Our finding further confirmed the crucial role of OX40 in inflammation/immune activation.
Accumulating evidence indicates that reagents designed to block OX40–OX40L interactions in vivo could be a promising therapy for human autoimmune disease. Two different OX40-specific therapeutic interventions have successfully been used to treate animals with autoimmune diseases [21,29]. Unlike conventional immunotherapies, blockage/inhibition of OX40–OX40L interactions seems to specifically inhibit the disease-responsible effector T-cell function, and therefore does not cause widespread immunosuppression. Given the increased expression of the OX40 molecule on CD4+ T-cells from MG patients, and the possibility of its contribution to disease development, blocking the OX40-OX40L pathway may provide a new therapy for human MG.
Acknowledgments
Authors thank Dr Ricardo Giscombe and Dr Xiongbiao Wang (Karolinska Institutet, Stockholm, Sweden) for their excellent technical supports and Dr Patrick Dessi (Karolinska Institutet, Stockholm, Sweden) for critical review of this paper. This work was supported by grants from the European Comission, the Fifth Framework Programme (grant no QLG1-CT-2001–01918), the Swedish Research Council (grant no. 05646), the foundations of the Karolinska Institutet and the Palle Ferb foundation.
References
- 1.Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Ann NY Acad Sci. 1976;274:283–99. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- 2.Hohlfeld R, Kalies I, Kohleisen B, Heininger K, Conti-Tronconi B, Toyka KV. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986;36:618–21. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- 3.Wang XB, Kakoulidou M, Giscombe R, Qiu Q, Huang D, Pirskanen R, Lefvert AK. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130:224–32. doi: 10.1016/s0165-5728(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh KR, Linsley PS, Bacha PA, Bacha PA, Drachman DB. Immunotherapy of experimental autoimmune myasthenia gravis. selective effects of CTLA4Ig and synergistic combination with an IL2-diphtheria toxin fusion protein. J Neuroimmunol. 1998;87:136–46. doi: 10.1016/s0165-5728(98)00071-x. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. Costimulation of T cells by OX40, 4–1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–73. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Im SH, Barchan D, Maiti PK, Fuchs S, Souroujon MC. Blockade of CD40 ligand suppresses chronic experimental myasthenia gravis by down-regulation of Th1 differentiation and up-regulation of CTLA-4. J Immunol. 2001;166:6893–8. doi: 10.4049/jimmunol.166.11.6893. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 8.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 9.Weatherill AR, Maxwell JR, Takahashi C, Weinberg AD, Vella AT. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF)2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273:5808–14. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 11.Akiba H, Oshima H, Takeda K, et al. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–66. [PubMed] [Google Scholar]
- 12.Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–21. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 13.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed] [Google Scholar]
- 14.Stuber E, Strober W. The T cell–B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–89. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen AI, McAdam AJ, Buhlmann JE, et al. OX40-ligand has a critical costimulatory role in dendritic cell: T cell interactions. Immunity. 1999;11:689–98. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 17.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 18.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ. WeinbergAD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 19.Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–36. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 20.Ndhlovu LC, Ishii N, Murata K, et al. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–9. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg AD, Wegmann KW, Funatake C, et al. Blocking OX-40/OX−40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed] [Google Scholar]
- 22.Wang X, Ria M, Kelmenson PM, Eriksson P, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005. pp. 365–8. [DOI] [PubMed]
- 23.Higgins LM, McDonald SA, Whittle N, Crockett N, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–93. [PubMed] [Google Scholar]
- 24.Aten J, Roos A, Claessen N, Schilder-Tol EJ, Ten Berge IJ, Weening JJ. Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J Am Soc Nephrol. 2000;11:1426–38. doi: 10.1681/ASN.V1181426. [DOI] [PubMed] [Google Scholar]
- 25.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, Yagita H, Okumura K. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–23. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateyama M, Fujihara K, Ishii N, Sugamura K, Onodera Y, Itoyama Y. Expression of OX40 in muscles of polymyositis and granulomatous myopathy. J Neurol Sci. 2002;194:29–34. doi: 10.1016/s0022-510x(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 28.Kotani A, Ishikawa T, Matsumura Y, Ichinohe T, Ohno H, Hori T, Uchiyama T. Correlation of peripheral blood OX40+ (CD134+) T cells with chronic graft-versus-host disease in patients who underwent allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3162–4e. doi: 10.1182/blood.v98.10.3162. [DOI] [PubMed] [Google Scholar]
- 29.Nohar C, Akiba H, Nakajima A, et al. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–15. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Orozco N, Chen Z, Poirot L, et al. Paradoxical dampening of anti-islet self-reactivity but promotion of diabetes by OX40 ligand. J Immunol. 2003;171:6954–60. doi: 10.4049/jimmunol.171.12.6954. [DOI] [PubMed] [Google Scholar]
- 31.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazar BR, Sharpe AH, Chen AI, et al. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–8. doi: 10.1182/blood-2002-10-3048. [DOI] [PubMed] [Google Scholar]
- 33.Onodera J, Nagata T, Fujihara K, Ohuchi M, Ishii N, Sugamura K, Itoyama Y. Expression of OX40 and OX40 ligand (gp34) in the normal and myasthenic thymus. Acta Neurol Scand. 2000;102:236–43. doi: 10.1034/j.1600-0404.2000.102004236.x. [DOI] [PubMed] [Google Scholar]
- 34.Oosterhuis HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry. 1989;52:1121–7. doi: 10.1136/jnnp.52.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefvert AK, Bergstrom K, Matell G, Osterman PO, Pirskanen R. Determination of acetylcholine receptor antibody in myasthenia gravis: clinical usefulness and pathogenetic implications. J Neurol Neurosurg Psychiatry. 1978;41:394–403. doi: 10.1136/jnnp.41.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacomelli R, Passacantando A, Perricone Parzanese I, Rascente M, Minisola G, Tonietti G. T lymphocytes in the synovial fluid of patients with active rheumatoid arthritis display CD134-OX40 surface antigen. Clin Exp Rheumatol. 2001;19:317–20. [PubMed] [Google Scholar]
- 37.Stuber E, Buschenfeld A, Luttges J, Von Freier A, Arendt T, Folsch UR. The expression of OX40 in immunologically mediated diseases of the gastrointestinal tract (celiac disease, Crohn's disease, ulcerative colitis) Eur J Clin Invest. 2000;30:594–9. doi: 10.1046/j.1365-2362.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 38.Kotani A, Takahashi A, Koga H, et al. Myasthenia gravis after allogeneic bone marrow transplantation treated with mycophenolate mofetil monitored by peripheral blood OX40+ CD4+ T cells. Eur J Haematol. 2002;69:318–20. doi: 10.1034/j.1600-0609.2002.02789.x. [DOI] [PubMed] [Google Scholar]
- 39.Streeter PR, Zhang X, Tittle TV, Schon CN, Weinberg AD, Maziarz RT. CD25 expression distinguishes functionally distinct alloreactive CD4 CD134 (OX40) T-cell subsets in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:298–309. doi: 10.1016/j.bbmt.2003.12.302. [DOI] [PubMed] [Google Scholar]
- 40.Teleshova N, Matusevicius D, Kivisakk P, Mustafa M, Pirskanen R, Link H. Altered expression of costimulatory molecules in myasthenia gravis. Muscle Nerve. 2000;23:946–53. doi: 10.1002/(sici)1097-4598(200006)23:6<946::aid-mus16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Moulian N, Bidault J, Truffault F, Yamamoto AM, Levasseur P, Berrih-Aknin S. Thymocyte Fas expression is dysregulated in myasthenia gravis patients with anti-acetylcholine receptor antibody. Blood. 1997;89:3287–95. [PubMed] [Google Scholar]
- 42.Kuchroo VK, Das MP, Brown JA. B7–1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 43.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 44.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95) /FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 45.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]