Abstract
Both the infection of human cytomegalovirus (HCMV) and the immunization of its recombinant glycoprotein (gB) in mice have been known to induce autoimmunity, resulting in symptoms similar to those of human systemic lupus erythematosus (SLE). Research has also found that the murine cytomegalovirus (MCMV)-specific monoclonal antibody (mAb) is able to react with a human U1-70K-like autoantigen. To investigate HCMV involvement in autoimmunity, we analysed the humoral responses to HCMV by autoimmune patients and normal adults. Our studies show unambiguously that sera from SLE patients exhibited an elevated IgG titre to HCMV when compared with those observed in controls and other connective tissue disease (CTD) patients (P < 0·001). The IgM titres to HCMV and IgG to HBV were evaluated, and no significant differences were noted among all testing groups. In addition to initiating T cell activity, as reported by many investigators, we found that the HCMV pp65 antigen (also known as lower matrix protein) was able to induce humoral responses in SLE patients. Immunoblot assays showed that 82·56% of sera from SLE patients reacted with the HCMV pp65 antigen, but only 11·11%, 23·53% and 31·17% of patients from normal control, rheumatoid arthritis (RA) and CTD patients, respectively, reacted to it. Unlike HCMV pp65, HCMV pp150 induced B cell activity in most collected sera (92·22%-98·04%). Finally, female NZB/W F1 mice immunized with plasmids encoding HCMV pp65 open reading frame (pcDNApp65) developed an early onset of autoantibody activity and more severe glomerulonephritis. Thus, we conclude that the HCMV pp65 antigen triggers humoral immunity in SLE patients and autoimmune-prone mice and that it could very well exacerbate the autoimmune responses in susceptible animals.
Keywords: autoantibody, autoimmunity, cytomegalovirus, pp65 lower matrix protein, systemic lupus erythematosus
Introduction
Human cytomegalovirus (HCMV) is a member of the herpes virus group and is a ubiquitous pathogen that causes latent, usually asymptomatic, infection throughout a patient's lifetime [1]. HCMV persists in hosts after primary infection and can be reactivated by immunosuppression. In addition to severe medical conditions in infant and immune-suppressed individuals, HCMV infection has been shown to induce autoimmunity through molecular interactions with the immune system [2–4].
It has been suggested that infection with HCMV can evoke autoimmunity [2,5,6]. HCMV infection is seen frequently in systemic lupus erythematosus (SLE) patients [4] and can induce diseases with autoimmune-like components. Examples of this include acute myocarditis and Sjögren's syndrome (SS) [5,7]. Another characteristic of autoimmunity found in humans and laboratory animals as a result of HCMV infection is the presence of circulating autoantibodies to erythrocytes, nuclear components or smooth muscles [8,9]. HCMV shares sequence homology with the host, and the immunological cross-reactivity between the host and the virus is a possible mechanism to the HCMV-induced tolerance break [10]. HCMV early RNA has also been found to induce anti-Ro (SSA) activity [11]. Similarly, its infection can cause the cell surface expression of Ro autoantigens [12] and anti-phospholipid antibodies [13]. Furthermore, immunization with HCMV gB (UL55) provokes anti-snRNP activity in mice [3,14]. The monoclonal antibody to HCMV structural protein against snRNP has also been reported [15].
The HCMV particle contains at least 33 structural proteins and the lower matrix phosphoprotein (pp65) antigen, which is a product of UL83 open reading frame (ORF), is the most abundant one. Although the anti-pp65 antibodies and the pp65 antigens are detected in immune-depressed patients with active viral infection [16], the antibody response to the pp65 antigen in normal infected individuals is not always detectable by immunoblotting [17]. It has been reported that the pp65 antigen is targeted by a cell-mediated immune response and that its vaccination can induce a pp65-specific CTL response [18–21]. Unlike the pp65 antigen, the HCMV pp150 is another viral tegument phosphoprotein that has been found to elicit a strong humoral response in most infected individuals [17].
Both the M83 and M84 ORFs of murine cytomegalovirus-encoded proteins are homologues to the HCMV pp65 antigen. The M83 and M84 share 17% and 21% of their identity, respectively, to their human counterparts [20,22]. The M84 ORF shares greater homology to pp65 and induces CTL responses. The M83 gene product is a 98-kDa protein, which is similar to HCMV pp65 in its late expression, phosphorylation and virion association. It has been demonstrated that a murine cytomegalovirus (MCMV)-neutralizing monoclonal antibody (mAb) cross-reacts to both the M83 ORF product-like, 113-kDa phosphorylated structural protein and human U-1 70K-like autoantigen. This mAb caused further tissue damage that resembles a mixed connective tissue disease (MCTD) [15]. Unfortunately, the relation between the MCMV M83 and the 113-kDa structural protein remains elusive.
In the present study, we conducted a preliminary serological analysis to investigate the humoral response of SLE, connective tissue diseases (CTD), rheumatoid arthritis (RA) patients and healthy donors to HCMV, hepatits B virus (HBV) and Epstein–Barr virus (EBV) among patients in Taiwan. The population of Taiwan is known to have high seropositive rates for HCMV, HBV (85–91%) and EBV (90%) [23,24]. While EBV infection and its association with SLE has been studied in great detail [25–28], little has been done to reveal the link between HCMV and SLE. Herein, we found a significant elevated IgG titre to the HCMV pp65 antigen in SLE patients in Taiwan. HCMV pp65-immunized NZB/W F1 mice also developed early onset of anti-dsDNA, anti-nuclear activities and more severe glomerulonephritis. In addition to NZB/W F1 mice, BALB/c and C57/B6 mice exhibited positive reactivity to nuclear components following immunization. These results suggest a possible association between the anti-HCMV pp65 antigen responses and a tolerance break among SLE patients and animals.
Materials and methods
Patients
This study, involving human subjects, was approved by the Tzu Chi University, National Science Committee and the National Blood Center or Taichung Veteran Hospital Review Boards. Patients were classified based on the classification criteria of the American College of Rheumatology as SLE (n = 86), rheumatoid arthritis (RA, n = 51), CTD (Sjögren's syndrome) (SS, n = 34), dermatomyositis (DM, n = 20), systemic sclerosis (SSc, n = 15) and progressive systemic sclerosis (PSS, n = 8). Normal sera were collected from qualified, sex- and age-matched adult blood donors (n = 90). The demographics, clinical status, disease duration and treatment history of the patients are presented in Table 1. Disease activity was defined as described previously [29–33].
Table 1.
Demographics of patients, state of disease activity and treatment received for patient and controls that were studied.
| Normal | SLE | SS | SSc | DMs | PSS | CTD | RA | |
|---|---|---|---|---|---|---|---|---|
| Age (years) (mean) | 18–67 (42·7) | 18–77 (41·1) | 17–78 (48·2) | 37–78 (55·2) | 27–51 (41) | 25–76 (55·1) | 18–80 (54·9) | |
| % Female | 91 | 91 | 91 | 87 | 75 | 75 | 80 | |
| Total specimen | 90 | 86 | 34 | 15 | 20 | 8 | 77 | 51 |
| State of disease (no. of patients) | N/A | Inactive (39) Active (47) | Inactive (22) Active (12) | Inactive (9) Active (6) | Inactive (13) Active (7) | Inactive (5) Active (3) | Inactive (21) Active (30) | |
| Duration mean ± s.d. (months) | N/A | 36·9 ± 46·9 | 22·0 ± 25·6 | 21·9 ± 15·7 | 40·6 ± 44·1 | 44·3 ± 34·6 | 92·6 ± 63·5 | |
| Treatment (total patients) | N/A | NSAID (4)/P (64)/pL (74) | NSAID (3)/P (12)/pL (31) | NSAID (1)/P (6)/pL (12) | NSAID (2)/P (8)/pL (16) | NSAID (0)/P (2)/D(5) | NSAID (51)/P(42)/pl (46) |
Prednisolone (P), plaquenil (pl), d-penicillamine (D). Active systemic lupus erythematosus (SLE) was defined as SLEDAI (SLE Disease Activity Index) more than 2 at the time of investigation. Active rheumatoid arthritis (RA) was defined as RA patients who had more than six swollen and six tender joints for at least 3 months. Active Sjögren's syndrome (SS) was defined as being manifest in patients who had extraglandular manifestations, including vasculitis, non-erosive arthritis or fibrosing alveolitis at the time of investigation. Active dermatomyositis (DM) was defined as the presence of active cutaneous manifestations with or without clinical myositis at the time of investigation. Active progressive systemic sclerosis (PSS) was defined as being present when the index is more than 3 by the whole series activity index, as proposed by the European Scleroderma Study Group (EScSG). For immunoblot, sera were tested on purified human cytomegalovirus (HCMV) blots, as described in the Materials and methods section. The results are representative of at least two similar experiments. P < 0·001 (compared to normal control).
Cell culture and purification
HCMV, HBV and EBV were collected, as described [34], but with some modification. Briefly, the HCMV AD 169 strain was purchased from the American Type Culture Collection (ATCC) and was grown on MRC-5 cells. The MRC-5 cells and medium were collected when the 100% cytopathic effect had occurred or when the HCMV-infected cells detached from culture dishes. HBV was collected from a supernatant of MS-G2 cells which was a gift from Dr Shi-yen Lo of Tzu Chi University. EBV was collected from a B958 cell culture following induction with tetradecanoyl phorbol acetate and sodium butyrate. The B958 cell line was a gift from Lin-chun Lin of Tzu Chi University. For virus purification, HCMV-, HBV- or EBV-infected cultures were frozen, thawed and refrozen several times, and viral particles were purified following a few rounds of low- and high-speed gradient centrifugations. For viral denaturation, viral particles were placed in a 1% SDS buffer prior to enzyme-linked imunosorbent assays (ELISA).
Mice
NZB and NZW mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA), and BALB/c and C57/B6 mice were purchased from the National Experimental Animal Center, mated and maintained in a specific pathogen-free animal facility in Tzu Chi University. The mice were examined daily, and any mouse that showed evidence of having entered a rapid terminal decline was sacrificed, as described previously [35]. Organ samples were collected and placed in buffered formalin and processed for subsequent histological analysis, and the haematoxylin- and eosin-stained tissues were examined using light microscopy.
Plasmid constructs, immunization and sera collection
Plasmid-encoding pp65 was a gift from Dr Zaia [16], and the plasmid encoding M83 and M84 genes were gifts from Dr Morello [22]. Briefly, the CMVpp65 gene comprising intronA/CMVpp65 was removed from pBluescript with Spe1 and EcoR1 and inserted in the Nhe1 and EcoR1 site of pcDNA 3·1+ vector (Invitrogen, San Diego, CA, USA). All the plasmid DNAs were transformed in DH5α competent cells, grown in appropriate media and isolated using Qiagen Maxi Kit (Qiagen, Valencia, CA, USA). Sequencing was performed on the plasmid isolates to verify the constructs.
A total of 25 6-week old female NZB/W F1 mice were divided randomly into pp65 (n = 10), M83 (n = 5), M84 (n = 5) and plasmid only (n = 5) groups. All the mice were inoculated intramuscularly five times at 2-week intervals with 80 mg of pcDNA3·1 plasmids encoding either HCMV pp65 (pcDNApp65), MCMV M83 (pcDNAM83) or M84 (pcDNAM84) or without insertion in 100 ml of sterile saline in one thigh. The mice were bled via the retro-orbital vein 1 day prior to each assay and at 2–4-week intervals. Unused sera were stored in a −20°C and −80°C freezer.
A total of 18 6-week-old female C57BL/6 and BALB/c mice were divided randomly into pp65 (n = 3/each), M83 (n = 3/each) and M84 (n = 3/each) groups. All mice were inoculated and tested, as described in NZB/W F1 immunization.
Affinity purification of HCMV pp65 antigen
The HCMV pp65 and the pp150 antigens were purified from viral particles following electrophoresis and blotting. As described [36], antigens on the PVDF membrane were excised and eluted individually using an elution buffer [1% sodium dodecyl sulphate (SDS), 0·5% Triton X100, pH 7·4]. When required, SDS in the buffer was removed via dilution or acetone precipitation. The eluted proteins were subjected to MALDI-TOF-MS to confirm their purity.
ELISA
The titre for the anti-dsDNA antibody of NZB/W F1 mice was measured in accordance with ELISA, as described previously [37]. In brief, each well of the 96-well plates (Costar, Cambridge, MA, USA) was coated with calf thymus DNA (Sigma-Aldrich, St Louis, MO, USA), air-dried at room temperature and washed in Tris-buffered saline (TBS, pH 7·4). After being blocked with skimmed milk (5%), 25 µl of 400–1000× phosphate-buffered saline (PBS) diluted sera were added, and this preparation was incubated for 2 h at room temperature. Subsequently, each well was washed and incubated with proper secondary antibody with horseradish peroxidase conjugated. To visualize the interaction of the antigen and antibody, o-phenylenediamine dihydrochloride (OPD) was used as a substrate, and absorbance at 450 nm for each well was measured with a microplate reader.
Titres for the anti-HCMV, anti-nuclear and anti-pp65 antibodies were measured by ELISA as described [38]. In brief, each of the 96 wells was coated with a nuclear protein extract or the purified HCMV pp65 antigen overnight at 4°C and was then washed in TBS, pH 7·4, several times. After being blocked with skimmed milk (5%), ELISA was performed as described in the anti-dsDNA antibody ELISA.
Anti-dsDNA assays (Crithidia luciliae assay)
In addition to using an ELISA for the anti-dsDNA activity, the anti-dsDNA antibody was also measured with FLRRO nDNA kits (MBL, Nagoya, Japan). The assay was performed as described by the manufacturer. At least two different dilutions (1: 10 and 1: 20) for each serum were tested. It was defined as positive only when positive for both concentrations. When it was positive for a lower dilution but negative for a higher dilution, it was described as a weak positive.
Immunoblot and anti-nuclear antibodies (ANA)
Immunoblot was performed as described previously [35]. Briefly, cell lysates or the antigens of interest were electrophoresed and transferred onto polyvinylidine fluoride (PVDF) membrane and blotted with the sera of interest. The interaction between antigens and antibodies was visualized via 4-chloro-1-naphthol (4 CN) developed colour or by following enhanced chemoluminescence (ECL) methods (Amersham, Piscataway, NJ, USA), as described by the manufacturer.
We assessed the ANA-specificities (Sm, RNP, SS-A, SS-B) with Western blot immunoassay (Immco Diagnostics, Inc., Buffalo, NY, USA). This assay was performed as described by the manufacturer.
Renal histology and necropsy
The severity of glomerulonephritis was scored as described previously [35,39]. Briefly, the number of specific abnormalities in 100 glomeruli in a 4 mm-thick haematoxylin and eosin-stained cross-section of each kidney was recorded. The glomerular abnormalities were scored: mesangial stalk thickening and hypercellularity, glomerular base membrane thickening, generalized glomerular hypercellularity and crescent formation.
Statistical analysis
The differences in the titres and frequency of positive test results were compared using Student's t-test and Fisher's two-tailed exact test, respectively, using the prism program (version 4·0, Graphpad) on Macintosh computers. P-values < 0·05 were considered significant.
Results
Antiviral activity for collected sera
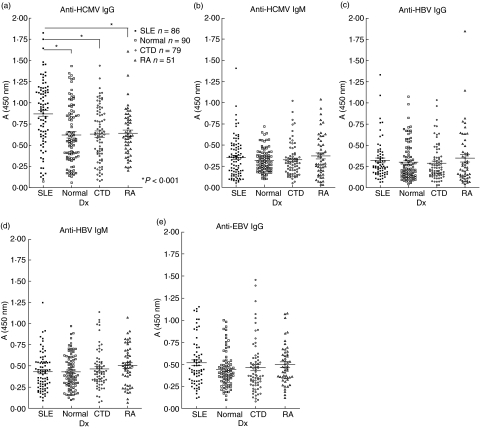
Our ELISA analysis revealed that sera from SLE adult patients exhibited a more elevated IgG titre to the HCMV viral particles than did those from normal blood donors and non-SLE CTD and RA patients (P < 0·001, Fig. 1a). To confirm whether reinfection or active infection contributed to this anti-HCMV activity, the IgM titres to HCMV of all collected sera were measured, but no significant differences were found (Fig. 1b). The differences in the titres of both IgG and IgM from HBV were insignificant in all testing groups (Fig. 1c, d). We also found elevated EBV activity in SLE sera, but the titre was not statistically significant (Fig. 1e) compared to health controls.
Fig. 1.
Detection of IgG and IgM anti-human cytomegalovirus (HCMV) and anti-hepatitis B virus (HBV) in sera from systemic lupus erythematosus (SLE) (n = 86), connective tissue diseases (CTD) (n = 77), rheumatoid arthritis (RA) (n = 51) patients and normal controls (n = 90). Sera were tested by enzyme-linked immunosorbent assay (ELISA) against purified HCMV (a,b), HBV (c, d) and EBV (e) viral particles. (a,c,e) IgG reactivity to the viruses; (b,d) IgM reactivity to the same virus. *P < 0·001 (Student's t-test, compared to normal controls).
Qualitative analysis of anti-HCMV pp65 and pp150 reactivity
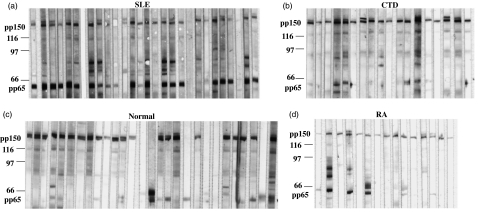
Immunoblot was used to identify antigenic peptides in HCMV and the anti-pp150 activity was used as a reference to define HCMV seropositivity, as both our studies and the published results [17] showed that close to 100% HCMV-infected individuals developed an antibody activity to the pp150 antigen. Those who were seropositive (SP) were grouped and analysed for reactivity to pp65. We found that the HCMV pp65 antigen was recognized by the majority of SLE sera (88%) but less frequently by CTD and RA sera (32% and 24%, respectively). Reactivity to the pp65 antigen by normal controls was rare (12%, Fig. 2 and Table 1, 2). The anti-pp65 reactivity was often found in patients with active disease, irrespective of their clinical manifestation (Table 2). All collected patient sera with active disease showed significantly elevated titres of IgG than normal controls, but the differences were insignificant for sera collected from patients with inactive disease. It is noteworthy that, even in the inactive disease state, SLE sera still have a considerably higher positive rate to the pp65 antigen (77%) than do CTD, RA and normal sera (Table 2).
Fig. 2.
A representative immunoblot analysis showing IgG reactivity to human cytomegalovirus (HCMV) antigens among systemic lupus erythematosus (SLE) patients (a), connective tissue diseases (CTD) (b), normal controls (c) and rheumatoid arthritis (RA) (d) patients. Markers, the HCMV pp150 and pp65 are labelled on the left.
Table 2.
The frequencies of antibody to human cytomegalovirus (HCMV) pp65 antigen in HCMV seropositive patient and controls.
| SLE | CTD | RA | Normal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-pp65 | + | – | IgG | + | – | IgG | + | – | IgG | ||
| Active (%) | 41 (87) | 6 (13) | Elevated* | 10 (36) | 18 (44) | Elevated* | 8 (27) | 22 (73) | Elevated* | ||
| Inactive (%) | 30 (77) | 9 (23) | 13 (27) | 36 (73) | 4 (17) | 17 (83) | |||||
| HCMV seropositive (%) | 81 (94) | 5 (6) | 73 (95) | 4 (5) | 50 (98) | 1 (2) | 85 (92) | 5 (8) | |||
| Anti-pp65 positive (%) | 71 (88) | 23* (32) | 12 (24) | 10 (12) | |||||||
P < 0·05 (versus normal sera). For immunoblot, sera were tested on purified human cytomegalovirus (HCMV) blots, as described in the Materials and methods section. Seropositive is defined as antibody reactivity to any of the viral antigens at immunoblot assays. The results are representative of at least two similar experiments. P < 0·001 (compared to normal control).
Quantitative analysis of anti-HCMV pp65 and pp150 activities
One significant feature of SLE sera was the highly elevated anti-pp65 activity, as measured by ELISA. To avoid background and interference from other viral antigens, both the HCMV pp65 and the pp150 antigens were blot-purified from isolated HCMV virions. Results showed that SLE patients exhibited IgG reactivity to the HCMV pp65 antigen almost twice as high as those from other patients or normal controls (Fig. 3). Some CTD patient sera, such as SS and RA, also exhibited enhanced anti-HCMV pp65 activity but these elevations were not statistically significant (P = 0·068, data not shown). The IgM titres to the HCMV pp65 antigen by different patient groups were analysed, and no significant differences were found (data not shown). The HCMV pp150 tegument protein was recognized by the majority of HCMV seropositive sera regardless of their clinical conditions [17]. Most SLE sera had more elevated IgG titres to the pp150 antigen than those from normal controls and CTD (P = 0·045 and P = 0·007, respectively, Fig. 3). The anti-HCMV pp150 IgM titres of all sera were analysed, and no statistical differences were found (data not shown).
Fig. 3.
Detection of IgG anti-human cytomegalovirus (HCMV) pp65 and pp150 in sera from systemic lupus erythematosus (SLE) (n = 86), connective tissue diseases (CTD) (n = 77), rheumatoid arthritis (RA) (n = 51) patients and normal controls (n = 90). Sera were tested by enzyme-linked immunosorbent assay (ELISA) against blot purified HCMV pp65 (a) and 150 (b). The error bars show the s.e.m. *P < 0·001, **P = 0·0014 (Student's t-test, compared to normal controls).
Slot-blot analysis
Slot-blot analysis was performed to compare the antigenicity of pp65 under denatured and renatured states. The majority of normal sera did not react to the pp65 antigen on immunoblot despite increased serum concentration (Fig. 2, data not shown), but exhibited low reactivity to the pp65 antigen in the ELISA tests. To investigate this discrepancy, blot purified pp65 was either denatured with SDS or renatured with 6 M guanidine hydrochloride prior to this assay. The results showed that approximately 86% and 23% of the HCMV seropositive SLE and normal sera, respectively, recognized the denatured form of pp65. Most seropositive SLE sera (91%) and normal controls (68%) showed activity to the renatured pp65 antigen, although the titre was notably lower for the latter (data not shown).
Induction of a specific anti-HCMV pp65, anti-dsDNA and anti-nuclear antibody
To investigate the possible connection between the immune response to the pp65 antigen and autoimmunity, mice were immunized with a DNA vaccine encoding the pp65 antigen. The results showed that pcDNApp65- or pcDNAM83-immunized mice developed a significant antibody reactivity to the HCMV pp65 antigen in both ELISA and immunoblot at 4 and 6 weeks, respectively, post-primary immunization (Fig. 4, but immunoblot data not shown). Both pcDNAM84 and plasmid-immunized mice did not elicit a detectable antibody response to the HCMV pp65 antigen (Fig. 4).
Fig. 4.
Mean IgG development against the human cytomegalovirus (HCMV) pp65 (± s.e.m.) antigen in sera from mice (NZB/W) immunized with plasmids encoding HCMV pp65, murine cytomegalovirus (MCMV) M83, M84 and vector only. Sera were tested by enzyme-linked immunosorbent assay (ELISA) against the blot purified HCMV pp65 antigen. The error bars show s.e.m. P-values are listed at the top (Student's t-test, compared to vector control). Tests were conducted in triplicate.
NZB/W F1 mice that received pcDNApp65 developed an early onset of both anti-nuclear components and anti-dsDNA antibodies (Fig. 5). Both Crithidia luciliae assay and ELISA were undertaken to compare qualitatively and semiquantitatively the anti-dsDNA titres among different treatment groups. The pcDNApp65-immunized mice exhibited anti-dsDNA activity in ELISA assay at 4 weeks post-primary immunization (Fig. 5a), and the activity increased in the next 4 weeks. For the Crithidia luciliae assay, seven of 10 sera from this group showed positive stains at 1: 20 dilution. In addition, anti-nuclear antibody titre from this group reached statistical significance at 8 weeks post-primary immunization (Fig. 5b). The pcDNAM83-immunized mice developed anti-dsDNA activity in ELISA at 6 weeks, and the titre increased at 8 weeks, although this increase in titre was not statistically significant (Fig. 5a). For the Crithidia luciliae assay, three of five sera from this group showed positive stains at 1: 10 dilution at 8 weeks post-immunization (data not shown). Anti-nuclear activity from the same mice was detected at 8 weeks after primary immunization (Fig. 5, P = 0·047).
Fig. 5.
Mean IgG development against dsDNA and nuclear components (± s.e.m.) in sera from mice (NZB/W, C57BL/6, BALB/c) immunized with plasmids encoding human cytomegalovirus (HCMV) pp65, murine cytomegalovirus (MCMV) M83, M84 and vector only. Sera were tested by enzyme-linked immunosorbent assay (ELISA) against dsDNA or nuclear extract. (a,c) Anti-dsDNA activities by NZB/W F1 or C57BL/6 and BALB/c, respectively. (b) Anti-nuclear reactivity by NZB/W F1 mice. The error bars show s.e.m. P-values are listed at the top (Student's t-test, compared to vector control). Tests were conducted in triplicate.
In addition to the NZB/W mice, C57BL/6 and BALB/c mice also developed anti-dsDNA reactivity following immunization with pcDNApp65 (Fig. 5a), as shown in ELISA and Crithidia luciliae stain. Both C57BL/6 and BALB/c mice that received pcDNApp65 developed an elevated anti-pp65 (data not shown) and anti-dsDNA activity in ELISA at 4 and 8 weeks post-primary immunization, respectively, and the titre increased in the next 4 weeks. For the Crithidia luciliae assay, two BALB/c mice (n = 5) were positive (1: 20), one was weakly positive (1: 10) and the other was negative at 12 weeks post-immunization (data not shown). In the C57BL/6 (n = 4) mice only one serum was positive, two were weakly positive and one was negative (data not shown) for the Crithidia luciliae stain. Unlike the NZB/W mice, the pcDNAM83 plasmid was unable to induce a detectable autoreactivity in either of the two assays.
With the pcDNAM84- or plasmid-immunized mice, both NZB/W F1 and normal strains did not develop a detectable autoantibody activity. The proteinurea was tested for both BALB/c and C57BL/6 mice, but no pathological changes were found at 12 weeks post-primary immunization (18 weeks of age). Most NZB/W F1 mice, regardless of their previous treatment, developed signs of proteinurea by 18 weeks, but the differences were statistically insignificant.
Renal pathology
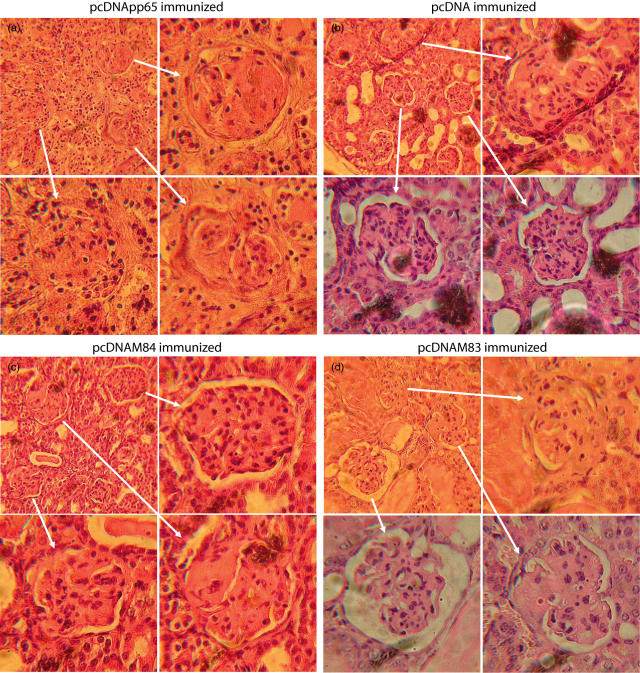
NZB/W F1 mice were sacrificed either at the exhibited terminal signs of disease or at the end of the project (28 weeks of age). The pcDNApp65-immunized mice had more severe glomerulonephritis than mice immunized with other plasmids (Figs 6, 7, P < 0·001). The pcDNAM83-immunized mice also had a higher score for nephritis, but the differences were statistically insignificant when compared to either the M84- or plasmid- immunized animals. The pcDNApp65-immunized mice exhibited severe signs of sclerosis (Fig. 7) and slower growth than pcDNAM84 and controls (data not shown). Despite significant signs of renal disease, the pcDNApp65-immunized animals did not show early mortality when compared to other treatment groups (data not shown). We did not observe significant glomerulonephritis in control animals (BALB/c and C57BL/6) and the increased anti-pp65 titres are not directly correlated to anti-dsDNA activity.
Fig. 6.
The effect of human cytomegalovirus (HCMV) pp65 and murine cytomegalovirus (MCMV) M83 and M84 immunization on glomerular lesion counts. Mice received pp65, M83, M84 and the plasmid had a mean renal severity score of 61, 60, 54 and 50, respectively (a). The error bars show s.e.m. P-values are listed at the top (Student's t-test, compared to vector control).
Fig. 7.
Histological staining of renal tissues from mice that received DNA vaccinations.
Discussion
There is controversy over the connection between infection of HCMV and SLE, and high seropositive rates to HCMV, HBV and EBV (Fig. 2 and Tables 1 and 2) [23,24] in Taiwan further dilute the possible connection. Some researches have shown a negative relationship between HCMV infection and autoimmunity and stated that infection could not increase autoimmunity in a random population. These studies often focused on studying the infection rate rather than how the immunity responded to HCMV in populations with possible genetic differences (SLE versus normal), as we have performed. We found that the anti-HCMV IgG activity was significantly elevated in SLE patients, but their reactivity to HBV and EBV was not different. The SLE, CTD and RA patients with active disease showed elevated IgG, but this elevation was insignificant in patients with inactive diseases. Even though their disease state was inactive, SLE patients exhibited more elevated anti-HCMV activity than non-SLE patients and normal controls. Unlike HCMV, neither HBV nor EBV was capable of inducing elevated immunity in SLE patients (Fig. 3). Non-SLE patients (CTD and RA) exhibited insignificant changes in their IgG titres to HCMV, HBV and EBV (Fig. 1), despite almost identical medical treatment for these patients (Table 1). These observations suggested that the elevated B cell reactivity to HCMV by SLE patients was less likely to be a result of viral reinfection/reactivation.
The elevated anti-HCMV pp65 activity that was found in SLE patients was unlikely to be caused by polyclonal activation of B cells as a result of chronic viral infection and inflammation. Both EBV and HBV infection could cause chronic infection, but immune responses to these viruses were not enhanced in all patient sera. Polyclonal activation of B-lymphocytes due to active disease has been reported for SLE, CTD and RA, but only SLE sera showed enhanced IgG activity to the HCMV pp65 antigen. Also, non-SLE sera showed elevated IgG for an active disease as SLE sera, but their reactivity to HCMV or the HCMV pp65 antigen was not enhanced significantly. Moreover, James et al. have studied viral infection in SLE patients, and polyclonal activation to specific viruses was not found [40]. These results indicated that this B cell activity to the HCMV pp65 antigen found in SLE sera was unlikely to be an outcome of polyclonal activation by infection. The discovery of the enhanced anti-EBV activity among SLE patients concurred with previous studies [40], but the differences were not statistically significant. The insignificant response to EBV by SLE patients could have been the result of a high seropositive rate in Taiwan.
Biased Th1 versus Th2 is one possible cause of autoimmunity but a diverted immune response to a specific antigen in the opposite direction, such as from T to B cells, in an autoimmune disease was not reported. The HCMV pp65 antigen is a very well-known T cell antigen [41,42]. In addition, it has been reported that the humoral response by viral-infected individuals against the pp65 antigen appears to be variable and is not always detectable with immunoblot [17]. Contrary to these studies, B cell activity on the part of SLE patients to the HCMV pp65 antigen was enhanced dramatically. Both slot-blot and immunoblot assays suggested that SLE sera reacted with additional epitopes that were not (or less) immunogenic to controls. Severe immunosuppression, such as in transplant or AIDS patients, may experience reactivation of HCMV and an elevation in IgG specific to the p65 antigen [1], but such incidences were not reported for autoimmune patients even in those under therapy or in the active disease state. Additionally, according to clinicians, none of our SLE patients developed signs of HCMV infection. Therefore, the highly selected IgG activity to the HCMV pp65 antigen is more like a feature of SLE patients than an outcome induced by immunosuppressive treatment for SLE patients.
The SLE sera showed higher IgG titre to the HCMV pp150 than normal controls, but the difference was less significant than the anti-HCMV pp65 reactivity (P = 0·045 versus P < 0·001). The HCMV pp150 is a strong humoral immunogen in terms of eliciting B cell responses at the time of infection, and close to 100% of HCMV seropositive individuals exhibited anti-pp150 activity (Fig. 2, Table 2[18]). Epitope spreading is one possible cause of this elevated anti-pp150 activity in SLE sera, because a highly elevated anti-pp65 response may enhance the reactivity to the pp150 antigen via spreading. Viral reactivation may also induce elevated activity to the HCMV pp150, but this is a less plausible scenario because in our study the anti-pp150 reactivity was enhanced much less than the anti-pp65 reactivity.
The development of an early onset of autoimmunity and glomerulonephritis in NZB/W F1 mice following immunization suggested a possible pathogenic role with the anti-pp65 response. Genetics plays an essential component in autoimmunity, particularly in its clinical manifestation. Therefore, NZB/W F1 mice were selected as our primary subject to verify the consequences following an immune response to the HCMV pp65 antigen. It has been well established that the immunization of pcDNApp65 could initiate strong CTL responses in mice [43], while the immunization of pcDNAM83 or pcDNAM84 induces humoral and cellular reactivity, respectively [44]. Our results showed that the immunization of either pcDNApp65 or pcDNAM83 induced humoral activity to the pp65 antigen in NZB/W F1 mice, and this cross-reactivity could probably be attributed to homology between the two antigens [20]. The pp65 antigen was found to be ‘less’ immunogenic than the M83 antigen with regard to inducing IgG activity to themselves. One possible explanation is that the CTL-dominated responses by the pp65 antigen could downplay the humoral response to this antigen through Th1 cytokines. It is noteworthy that the pcDNApp65 plasmid is more potent in inducing anti-dsDNA activity. The early onset of the anti-dsDNA activity by NZB/W F1 mice could be related to the renal abnormality we observed in these mice. The pcDNApp65 immunization also induced anti-dsDNA activity in normal strains of mice, but the clinical symptoms were not obvious. The plasmid itself was unlikely to be the cause of the autoimmunity because either plasmid alone or the pcDNAM84 was unable to reproduce the anti-dsDNA activity. It has been reported that a surrogate peptide can induce anti-dsDNA activity in mice, but we did not identify such an epitope on the pp65 antigen [45]. This autoimmune exacerbating effect by pcDNApp65 indicated possible autoimmune-inducing epitopes on the pp65 antigen. However, cross-examination of the sequence homology between the pp65, M83, M84 and the human genome via GenBank did not reveal any possible sequence. The mortality rates of the immunized animals were statistically indifferent among treatment groups (data not shown). One possibility is the low sample size (n = 5), except in the case of the pcDNApp65-treated animals (n = 10). We also noted that pcDNApp65-immunized NZB/W F1 and C57/B6 mice exhibited slower growth rates than other treatment groups (data not shown).
The immune response to the pp65 antigen may have initiated a process that triggers the tolerance break in subjects with a particular genetic background. It was reported that healthy human subjects with previous HCMV infection and HCMV vaccination did not develop autoimmunity [46]. Other studies also verified that the pp65 antigen activated a CTL response in a normal population [21,41]. We found strong B cell activity against the HCMV pp65 antigen in SLE patients, and immunization of the pp65 antigen induced early onset of autoimmunity on NZB/W F1 mice. An imbalance of Th1 and Th2 in those autoimmune patients has long been speculated [47]. It is possible that the pp65 antigen constitutes epitopes that are able to induce a Th1-dominant response in non-autoimmune subjects, but SLE-prone subjects may preferentially direct the anti-pp65 response towards Th2 due to genetic make-up. The T cells are known to suffer more intense selection pressure than B cells. Therefore, a T cell-dominated response to the HCMV pp65 antigen in a normal population may exert a protective effect in order to avoid a risky autoimmune-prone Th2 response. This biased response by SLE patients could be the consequence of genetic make-up, but it is not clear which genes are involved. It is of great interest to determine whether SLE patients could develop proper CTL activity to the pp65 antigen as well, and subsequent studies in this regard are in progress.
In conclusion, we report that compared to normal and CTD controls, SLE patients exhibited a significant humoral activity in response to the HCMV pp65 antigen. Immunoblotting and ELISA results revealed increased B cell activity to the HCMV pp65 antigen by SLE sera. NZB/W F1 mice injected with the pcDNApp65 plasmid also developed early onset of autoimmunity and a higher score for glomerulonephritis. The pcDNApp65-immunized C57BL/6 and BALB/c mice also produced autoantibodies to nuclear components. Thus, the HCMV pp65 antigen is not only an inducer to a CTL activity, but also a humoral immunogen among SLE patients. The immune response to this antigen by SLE patients and NZB/W F1 mice may contribute to the subsequent development of autoimmunity.
Acknowledgments
The authors would like to thank Dr Mats Ohlin, Lund University, Sweden, for the monoclonal anti-HCMV pp65 antibody, Dr J. A. Zaia, Beckman Research Institute of the City of Hope, Duarte for the pp65 plasmid and Dr D. H. Spector, University of California, San Diego for the M83 and M84 plasmids. This study was supported by grant NSC922320B320033, Taiwan, the Republic of China.
References
- 1.Nichols WG, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25–40. doi: 10.1016/s1386-6532(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 2.Sekigawa I, Nawata M, Seta N, Yamada M, Iida N, Hashimoto H. Cytomegalovirus infection in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2002;20:559–64. [PubMed] [Google Scholar]
- 3.Newkirk MM, van Venrooij WJ, Marshall GS. Autoimmune response to U1 small nuclear ribonucleoprotein (U1 snRNP) associated with cytomegalovirus infection. Arthritis Res. 2001;3:253–8. doi: 10.1186/ar310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider JR, Ollier WE, Lock RJ, Brookes ST, Pamphilon DH. Human cytomegalovirus infection and systemic lupus erythematosus. Clin Exp Rheumatol. 1997;15:405–9. [PubMed] [Google Scholar]
- 5.Fairweather D, Lawson CM, Chapman AJ, et al. Wild isolates of murine cytomegalovirus induce myocarditis and antibodies that cross-react with virus and cardiac myosin. Immunology. 1998;94:263–70. doi: 10.1046/j.1365-2567.1998.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratta P, Canavese C, Ciccone G, et al. Correlation between cytomegalovirus infection and Raynaud's phenomenon in lupus nephritis. Nephron. 1999;82:145–54. doi: 10.1159/000045391. [DOI] [PubMed] [Google Scholar]
- 7.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin Exp Immunol. 1989;78:454–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen P, Molgaard J, Andersen I, Andersen HK. Smooth-muscle antibodies and antibodies to cytomegalovirus and Epstein–Barr virus in leukaemias and lymphomata. Acta Pathol Microbiol Scand. 1976;84:86–92. doi: 10.1111/j.1699-0463.1976.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayes K, Alford C, Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis. 1987;156:615–21. doi: 10.1093/infdis/156.4.615. [DOI] [PubMed] [Google Scholar]
- 10.Fujinami RS, Nelson JA, Walker L, Oldstone MB. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: a means for graft rejection and immunosuppression. J Virol. 1988;62:100–5. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magro CM, Allen J, Pope-Harman A, et al. The role of microvascular injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin Pathol. 2003;119:556–67. doi: 10.1309/0B06-Y93E-GE6T-Q36Y. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J. Cytomegalovirus infection induces expression of 60 KD/Ro antigen on human keratinocytes. Lupus. 1995;4:396–406. doi: 10.1177/096120339500400511. [DOI] [PubMed] [Google Scholar]
- 13.Gharavi AE, Pierangeli SS, Espinola RG, Liu X, Colden-Stanfield M, Harris EN. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545–52. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- 14.Curtis HA, Singh T, Newkirk MM. Recombinant cytomegalovirus glycoprotein gB (UL55) induces an autoantibody response to the U1–70 kDa small nuclear ribonucleoprotein. Eur J Immunol. 1999;29:3643–53. doi: 10.1002/(SICI)1521-4141(199911)29:11<3643::AID-IMMU3643>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Chapman AJ, Farrell HE, Thomas JA, et al. A murine cytomegalovirus-neutralizing monoclonal antibody exhibits autoreactivity and induces tissue damage in vivo. Immunology. 1994;81:435–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Zaia JA, Forman SJ, Ting YP, Vanderwal-Urbina E, Blume KG. Polypeptide-specific antibody response to human cytomegalovirus after infection in bone marrow transplant recipients. J Infect Dis. 1986;153:780–7. doi: 10.1093/infdis/153.4.780. [DOI] [PubMed] [Google Scholar]
- 17.Jahn G, Scholl BC, Traupe B, Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987;68:1327–37. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- 18.Gallez-Hawkins G, Lomeli NA, X LL, et al. Kinase-deficient CMVpp65 triggers a CMVpp65 specific T-cell immune response in HLA-A*0201.Kb transgenic mice after DNA immunization. Scand J Immunol. 2002;55:592–8. doi: 10.1046/j.1365-3083.2002.01099.x. [DOI] [PubMed] [Google Scholar]
- 19.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–70. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 20.Morello CS, Cranmer LD, Spector DH. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65) J Virol. 2000;74:3696–708. doi: 10.1128/jvi.74.8.3696-3708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyulai Z, Endresz V, Burian K, et al. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB. 150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J Infect Dis. 2000;181:1537–46. doi: 10.1086/315445. [DOI] [PubMed] [Google Scholar]
- 22.Morello CS, Cranmer LD, Spector DH. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83) J Virol. 1999;73:7678–93. doi: 10.1128/jvi.73.9.7678-7693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu SC, Chin LT, Wu FM, et al. Seroprevalence of CMV antibodies in a blood donor population and premature neonates in south-central Taiwan. Kaohsiung J Med Sci. 1999;15:603–10. [PubMed] [Google Scholar]
- 24.Tsai WS, Chang MH, Chen JY, Lee CY, Liu YG. Seroepidemiological study of Epstein–Barr virus infection in children in Taipei. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1989;30:81–6. [PubMed] [Google Scholar]
- 25.McClain MT, Harley JB, James JA. The role of Epstein–Barr virus in systemic lupus erythematosus. Front Biosci. 2001;6:E137–47. doi: 10.2741/mcclain. [DOI] [PubMed] [Google Scholar]
- 26.James JA, Harley JB, Scofield RH. Role of viruses in systemic lupus erythematosus and Sjogren syndrome. Curr Opin Rheumatol. 2001;13:370–6. doi: 10.1097/00002281-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman KM, Kirby MY, Harley JB, James JA. Peptide mimics of a major lupus epitope of SmB/B′. Ann NY Acad Sci. 2003;987:215–29. doi: 10.1111/j.1749-6632.2003.tb06051.x. [DOI] [PubMed] [Google Scholar]
- 28.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 29.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. The Committee on Prognosis Studies in SLE. [DOI] [PubMed] [Google Scholar]
- 30.Prevoo ML, van ‘’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 31.Valentini G, Della Rossa A, Bombardieri S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis. 2001;60:592–8. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentini G, Bencivelli W, Bombardieri S, et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis. 2003;62:901–3. doi: 10.1136/ard.62.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentini G, Silman AJ, Veale D. Assessment of disease activity. Clin Exp Rheumatol. 2003;21:S39–41. [PubMed] [Google Scholar]
- 34.Wolfram B, Hartmut H, Ulrich HK. A mouse model for cytomegalovirus. In: Coligan EJ, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current protocols in immunology. New York: John Wiley & Sons, Inc.; 2001. pp. 19.7.1–7.3. [Google Scholar]
- 35.Chang M, Walker SE, Hoffman RW. Immunization with a bacterial ATP-binding cassette transporter fragment suppresses autoimmunity and prolongs survival in MRL/lpr lupus-prone mice. Lupus. 2000;9:655–63. doi: 10.1191/096120300667608330. [DOI] [PubMed] [Google Scholar]
- 36.Szewczyk B, Pilat Z, Bienkowska-Szewczyk K, Summers DF. Elution of glycoproteins from replicas of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Electrophoresis. 1998;19:220–3. doi: 10.1002/elps.1150190214. [DOI] [PubMed] [Google Scholar]
- 37.Chang M, Wang RJ, Yangco DT, Sharp GC, Komatireddy GR, Hoffman RW. Analysis of autoantibodies against RNA polymerases using immunoaffinity-purifed RNA polymerase I, II, and III antigen in an enzyme–linked immunosorbent assay. Clin Immunol Immunopathol. 1998;89:71–8. doi: 10.1006/clin.1998.4591. [DOI] [PubMed] [Google Scholar]
- 38.Fredriksen K, Brannsether B, Traavik T, Rekvig OP. Antibodies to viral and mammalian native DNA in response to BK virus inoculation and subsequent immunization with calf thymus DNA. Scand J Immunol. 1991;34:109–19. doi: 10.1111/j.1365-3083.1991.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 39.McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol. 1991;147:3780–7. [PubMed] [Google Scholar]
- 40.James JA, Neas BR, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein–Barr virus exposure. Arthritis Rheum. 2001;44:1122–6. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin-Taylor E, Pande H, Forman SJ, et al. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–10. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 42.Boppana SB, Britt WJ. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology. 1996;222:293–6. doi: 10.1006/viro.1996.0424. [DOI] [PubMed] [Google Scholar]
- 43.Pande H, Campo K, Tanamachi B, Forman SJ, Zaia JA. Direct DNA immunization of mice with plasmid DNA encoding the tegument protein pp65 (ppUL83) of human cytomegalovirus induces high levels of circulating antibody to the encoded protein. Scand J Infect Dis Suppl. 1995;99:117–20. [PubMed] [Google Scholar]
- 44.Morello CS, Ye M, Spector DH. Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication. J Virol. 2002;76:4822–35. doi: 10.1128/JVI.76.10.4822-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall BC, McPherson RA, Greidinger E, Hoffman R, Adler SP. Lack of autoantibody production associated with cytomegalovirus infection. Arthritis Res. 2002;4:R6. doi: 10.1186/ar429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]