Abstract
During ageing, autoimmune disorders and the higher susceptibility to infectious have been associated with alterations in the humoral immune response. We report that splenic B lymphocytes from aged mice exhibit lower level of apoptosis induced by B-cell antigen receptor (BCR) ligation in vitro. Respect to B cells from young mice the anti-µ stimulated aged B cells show similar Bcl-2 and Bcl-xL expression but differential kinetic of A1 degradation and a higher level of cFLIP and FAIM. Even though B cells from aged mice show minor Fas expression they exhibit the same susceptibility to anti-Fas induced apoptosis. Aged B cells also present upon BCR stimulation, a higher proliferative response and similar level of activation markers expression than B cells from young mice. These data agree with the observation that aged mice exhibit an increment of T2 and mature B cell subset which rapidly enters cell cycle upon BCR engagement. The diminished apoptosis after activation in aged mice could compromise homeostatic mechanism allowing the persistence of self and non-self antigen specific B cells.
Keywords: ageing, B lymphocytes, BCR, immunoregulation, programmed cell death, survival signals
Introduction
Ageing impacts the homeostatic function of many systems in the body, including the immune system. Thus, elderly individuals experience a higher incidence of several diseases such as autoimmune disorders and infections than younger individuals [1–3]. In this sense, deficiencies in the humoral and cell-mediated immune response have been reported during ageing [4,5]. Specifically, B cell function declines with age and this age-related changes in the B cell compartment has been associated with alteration in T cell-help and also with intrinsic B cell defects [2]. Humoral response disturbances include the production of a greater proportion of low-affinity antibodies [6,7], different VH gene usage [8] and incidence of autoreactive antibodies [9,10].
During the immune system evolution several mechanisms are evolved to ensure that B cells bearing potentially harmful antiself specificities are controlled from the system preventing the development of autoimmune diseases [11]. These mechanisms include: the functional silencing of the lymphocytes in a process termed anergy [12,13], the re-induction of gene rearrangement at the Ig heavy and light chain loci to generate edited receptors with non self reactivity [14,15] and finally the deletion of autoreactive B cells at specific points during development [16]. Although the precise mechanisms ensuring the negative selection remain undefined, it is clear that the induction of apoptosis, considered as a physiological cell death, is essential for the deletion of autoreactive cells in bone marrow as well as in periphery. Moreover, apoptosis is also the mechanisms involved in the control of the lymphocyte expansion playing a fundamental role in the homeostasis of the immune system [17].
Related to ageing, it has been reported that pre-B cells from 18-month-old mice presented, freshly isolated or after cultured, a higher degree of apoptosis than those 2-month-old mice [18]. The increased susceptibility to apoptosis of the pre-B cell from the old mice was associated with a decreased level of Bcl-xL mRNA. Furthermore, a study investigating whether the B cell survival was affected by ageing reported that LPS and IL-4 protect normal splenic B cells from apoptosis in an age-independent way [19]. In contrast, protection induced by dextran sulphate and IL-5 was shown to be very low in old normal mice [19].
The apoptosis can be triggered by two distinct but convergent signalling pathways that activate several cysteine aspartate proteases, called caspases, which finally lead to DNA fragmentation and cell death [20,21]. One of the apoptotic pathway (extrinsic) is triggered in the cell surface by the ligation of certain receptors belongings to the TNF receptor family, such as CD95 (Fas/APO) or TNF-R 1 and it is regulated by c-Flip and FAIM (Fas apoptosis inhibitory molecule) among other proteins. The other pathway (intrinsic) is originated by mitochondria disruption and is regulated by the interplay of pro- and anti-apoptotic members of Bcl-2 family [20].
In the case of B cells, the B cell antigen receptor (BCR)-ligation can trigger apoptosis. However, signalling through the BCR has different consequences on B cell fate depending on B cell developmental stage and factors such as strength and duration of the signal [22]. In vitro studies using B cells in different development stage have established that upon BCR stimulation, immature B cells die whereas mature B cells proliferate. BCR-mediated apoptosis has been clearly demonstrated in bone marrow-derived B lymphocyte after 18 h of soluble anti-Ig stimulation [23]. Watanabe et al. [24] have reported that when BCR are extensively ligated, using immobilized anti-Ig antibody, most mature B cells undergo apoptosis within 48 h, whereas only small fraction of mature B cells undergo apoptosis at this time by milder BCR ligation using soluble anti-Ig antibody.
Regarding to stimulation via BCR, in aged individuals the chronic antigenic exposure could drive to a preferential expansion of B lymphocytes. However, it has been proposed that the aged related qualitative and quantitative changes in antibody production might result from a failure of autoreactive cells to undergo cell death. In this work, we study the behaviour of the splenic B cells from Balb/c aged mice stimulated via BCR with F(ab)2 anti-µ antibody, simulating antigen mediated activation. The results show that BCR stimulated B cells from aged animals present lower levels of apoptosis and higher proliferative response than B cell from young mice.
With respect to apoptosis, the anti-apoptotic molecules Bcl-2 and Bcl-xL, are expressed in the same magnitude in B cells from both studied groups. However, F(ab)2 anti-µ stimulated B cells from aged show a differential kinetic of A1 degradation. In addition B cells from aged mice exhibit an increase level of c-Flip and FAIM and minor expression of Fas on their surface. This lower expression of Fas can not be explained by an inability of aged B cells to up-regulate activation markers since they are similarly expressed on B cells from young and aged mice.
Materials and methods
Animals
The experiments were performed using 3-month-old (young) and 18-month-old (aged) female BALB/c mice. Mice were obtained from the Comisión Nacional de Energía Atómica (CNEA), Argentina.
Cell preparations and culture
Splenocytes from young and aged mice were obtained by homogenization in a tissue grinder. Erythrocytes were lysed in red blood cell lysis buffer (Sigma-Aldrich, Saint Louis, MO, USA). For B cells purification, monocytes and T cells were depleted by magnetic cell sorting using rat IgG anti-mouse CD11b (Mac-1) Abs, followed by anti-rat IgG-coated magnetic bead and anti-Thy 1·2-coated magnetic beads (Dynal Biotech, Compiégne, France) as indicated in manufacturer's instructions. This procedure yielded an enriched B cell population containing >95% of CD19+ cells, < 2% CD3+ and < 3% Mac-1+ as determined by flow cytometry (FCM) analysis (data not shown). The cell viability was > 95% as determined by Trypan Blue exclusion. After that, B cells were adjusted to a final concentration of 2 × 106 cells/ml in complete RPMI medium (containing 10% FBS, 5 µM 2-mercaptoethanol and 40 µg/ml gentamicin) and cultured with medium alone or F(ab)2 anti-µ (10 µg/ml, Jackson ImmunoResearch Laboratoratories, PA, USA) for the times indicated.
Cultures and proliferation assay
All in vitro cultures were carried out in RPMI complete medium. Purified B cells (2 × 106/ml) from young or aged mice were cultured in triplicate in 96-well microculture plates at 37 °C in 5% CO2 with F(ab)2 anti-µ (10 µg/ml) or medium alone during different periods of time. Cells were harvested and proliferation was measured by incorporation of 1 µCi[3H]-thymidine (TdR)/well during the last 18 h of culture. Results are expressed as incorporation of radioactivity (cpm ± SD).
Flow cytometry analysis and apoptosis assay
Spleen mononuclear cells or B cells from young or aged mice freshly explanted or cultured with F(ab)2 anti-µ (10 µg/ml) or medium alone by different period of time were harvested, washed twice in ice-cold FCM buffer (HBSS, 1% FBS, 0·1% NaN3) and preincubated with anti-mouse CD32/CD16 monoclonal antibody (mAb) (Fc block, clone 2·4G2) at 4 °C for 30 min. Then, for surface staining, cells were incubated with the corresponding PE, FITC or biotin-conjugated antibodies (Ab) at 4 °C for 30 min and washed with FCM buffer. When biotin-labelled Abs were used, a third step involving an extra 30 min-incubation was performed with Cychrome-labelled Streptavidin (St-Cy). Data were acquired on a Cytoron Absolute® cytometer (Ortho Diagnostic System, Raritan, NJ, USA) and analysed using WinMDI 2·8 software (Joseph Trotter, Scripps Institute, CA, USA). FITC-labelled anti-mouse CD19 mAbs, PE-labelled anti-mouse CD19, Fas, CD23, mAbs and biotin-labelled anti-mouse CD24 mAbs as well as St-Cy were purchased from BD PharMingen (Palo Alto, CA, USA). In all cases, cell debris was eliminated through gating live cells from Forward Scatter versus Side Scatter dot plots.
For apoptotic cell detection propidium iodide (PI) staining was performed to analyse subdiploid DNA content as described previously by Nicoletti et al. [25]. Briefly, B cells from young or aged mice were cultured with medium alone or F(ab)2 anti-µ during different period of time. After that, 1 × 106 cells were harvested, washed twice times with HBSS and fixed overnight in 1 ml 70% ethanol at 4 °C. Cell pellets were gently resuspended in 1 ml hypotonic fluorochrome solution (50 µg/ml PI diluted in sodium citrate 4 mM plus 0·3% NP-40) and kept at 4 °C for 18 h in the dark. After that, the cells were washed twice with FCM buffer and acquired as indicated before.
Detection of early apoptosis by staining with Annexin-V
To detect early phases of apoptosis, access to phosphatidyl serine was measure using FITC-labelled Annexin-V staining performed according to manufacturer's instructions (BD Pharmingen). Briefly, purified B cells from young or aged mice were cultured with medium alone or F(ab)2 anti-µ during different period of time. The cells were harvested and 1 × 105 resuspended in 100 µl of 1 × Annexin V-binding buffer (10 mM HEPES/NaOH, pH 7·4, 140 mM NaCl, 2·5 mM CaCl2) plus 5 µl Annexin V-FITC and 10 µl of propidium iodide (50 µg/ml) for 15 min at room temperature in the dark. Then, 400 µl of 1 × Annexin V-binding buffer was added to each tube and analysed by flow cytometry.
SDS-PAGE and western blot
SDS-PAGE was performed in a Miniprotean II electrophoresis apparatus (Bio-Rad). Briefly, 50 µg of cellular lysates were diluted in sample buffer and resolved on a 15% separating polyacrylamide slab gel for Bcl-xL, Bcl-2 (BD Bioscience, CA, USA), c-Flip (Santa Cruz Biotechnology Inc., CA, USA), cIAP2 and P38 MAPK (Santa Cruz Biotechnology Inc.) detection. After electrophoresis, the separated proteins were transferred onto nitrocellulose membranes and probed with corresponding primary Ab. Blots were then incubated with peroxidase-conjugated secondary Ab and developed by using the ECL system. Prestained protein molecular weight markers (Sigma-Aldrich) were run in parallel. Protein concentration was estimated by the method of Bradford, using Bio-Rad Protein Assay (Bio-Rad, CA, USA). Bovine serum albumin was used as protein standard.
Fas-mediated apoptosis assay
Purified B cells from young and aged mice were cultured with F(ab)2 anti-µ (10 µg/ml) during 40 h. Then, the cells were washed and incubated with hamster IgG or hamster anti-Fas antibody (Jo2) (0·125 µg/ml, BD Bioscience) during 12 h. Then, B cells were fixed and stained with PI and the cells were subjected to hyplodiploid DNA content analysis by FCM as we have described above.
RNA isolation and RT-PCR
Total RNA was extracted from B cells using TRIzolTM reagents (Life Technologies) according to the manufacturer's recommendation and resuspended in 20 µl of DEPC-treated water. The synthesis of first strand cDNA suitable for PCR amplification was performed using First Strand cDNA Synthesis Kit (MBI Fermentas, Lithuania). Briefly, a reaction mixture containing 4 µg of total RNA (template), 0.5 µg of oligo(dT)18 primer, and nuclease-free deionized water up to 11 µl was prepared on ice. The mixture was incubated at 70 °C for 5 min and chilled on ice. Then, the following components were added: 4 µl of 5× reaction buffer, 20 U ribonuclease inhibitor, 2 µl 10 mM dNTP mix. This mixture was incubated at 37 °C for 5 min. Then, 40 U of M-MuLV reverse transcriptase were added and the mixture was incubated at 37 °C for 1 h. Finally, the reaction was stopped by heating at 70 °C for 10 min. The relative quantity of cDNA of each sample was first normalized after semiquantitative PCR for β-actin. PCR reaction mixture (25 µl) contained: 12·5 µl of 2× PCR Master Mix (Taq DNA Polimerase in reaction buffer 0,05 U/µl, MgCl2 4 mM, dNTPs 0·4 mM each) (MBI Fermentas, Lithuania), 0.2 µM forward and reverse primers, RNA template and deionized water up to 25 µl. PCRs were performed on a PE 9600 (Perkin Elmer). PCR reactions were conducted as follows: for A1, denaturation step 95 °C for 4 min and 35 cycles of 94 °C for 60 s, 55 °C for 60 s, 72 °C for 60 s. For FAIM: denaturation step 94 °C for 2 min and 35 cycles of 94 °C for 60 s, 55 °C for 60 s, 72 °C for 60 s. For β-actin: denaturation step of 94 °C for 2 min, followed by 22 cycles of 94 °C for 30 s, 62 °C for 30 s, 72 °C for 30 s.
The primers sequences were as follows A1: 5′-AAT TCC AAC AGC CTC CAG ATA TG-3′ and 5′-GAA ACA AAA TAT CTG CAA CTC TGG-3′; FAIM: CTG GAT GCC GAG GAC CTG AG-5′and GGT GTC ACT GAG TGA GCT CTG-3′; β-actin: 5′-CCA GGT CAT CAC TAT TGG CAA CGA-3′ and 5′-GAG CAG TAA TCT CCT TCT GCA TCC-3′.
Statistical analysis
The statistical significance was analysed using unpaired Student's t-test or when is indicated with one sample t-tests. Values were considered statistically significant when P ≤ 0·05. All experiments were repeated at least three times with similar results.
Results
Splenic B cells from aged animals are more resistant to BCR-mediated apoptosis
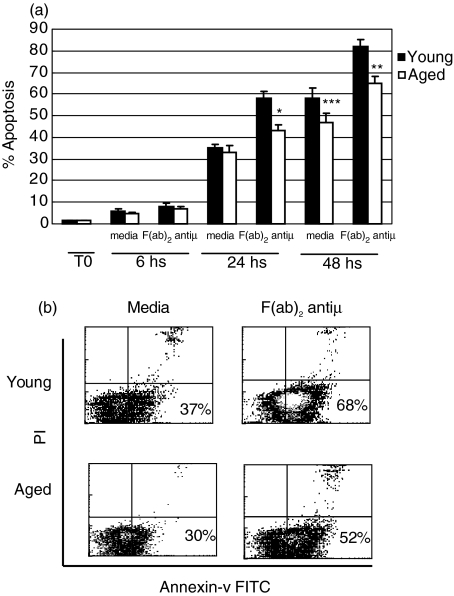
To assess whether BCR induced-apoptosis was affected by ageing, B lymphocytes from young or old mice were cultured with or without a F(ab)2 anti-µ Ab for different periods of time and the percentage of apoptotic B cells was determined by PI staining. As we can observe in Fig. 1a, there is no differences in the percentage of apoptosis between B cells from young and old mice neither when freshly explanted (0 h) nor after 6 h of culture with F(ab)2 anti-µ (8%versus 7%) or media alone (6%versus 8%). However, after 24 h post F(ab)2 anti-µ stimulus, the percentage of apoptotic B cells from aged mice was smaller than that observed with B cells from young mice culture under the same condition (43%versus 58%) (P < 0·04). This difference was determined to be more accentuated after 48 h of culture (65%versus 82%) (P < 0·035). In addition, we also noticed that the spontaneous apoptosis of B cells cultured with medium alone was similar for both groups during the 3 time points studied.
Fig. 1.
Apoptosis of B lymphocytes in young and old mice. (a) Purified B cells from young (▪) and aged (□) mice were cultured with medium alone or F(ab)2 anti-µ (10 µg/ml) during 6, 24 and 48 h. Cell nuclei from freshly (T0) explanted B cells or after culture were stained with PI and the cells were subjected to hyplodiploid DNA content analysis by FCM. The graph shows the percentages of apoptotic B cells. *P < 0·04 and **P < 0·035 indicate significant differences compared with stimulated-B cells from young animals and ***P = 0·13 indicate non-significant differences compared with nonstimulated B cells from young mice. (b) Purified B cells from young and aged mice were cultured with medium alone or F(ab)2 anti-µ (10 µg/ml) during 24 h. Then, the cells were stained with FITC-Annexin–V and PI. Two-colour density plot graphics show the percentage of Annexin-V+ cells within live gated population. One typical experiment from the three performed is shown.
These results were confirmed using Annexin-V and PI staining, detecting early phase of apoptosis. Thus, B cells from young and old mice were culture during 6, 12 and 24 h with F(ab)2 anti-µ or media alone and the percentage of apoptotic cells was evaluated by FITC-labelled Annexin-V and PI staining. We observed that after few hours (6 and 12 h) of culture, B cells from both group of mice show similar percentage of apoptotic cells (Annexin-V+ PI–). However, after 24 h of culture, B cell from young mice showed an increased percentage of apoptotic cells compared to young mice (68%versus 52%, P < 0·045) (Fig. 1b).
These findings indicate that at longer time points of culture, B cells from aged animals are more resistant to the soluble anti-µ-induced apoptosis than B cells from young mice.
B cells from aged animals differ in their ability to generate BCR-dependent survival signals
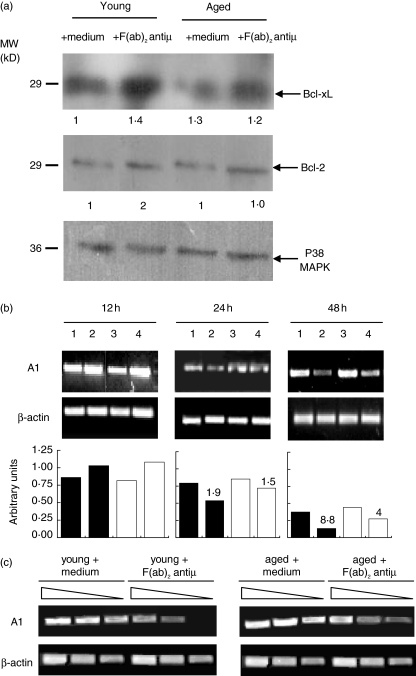
Trying to find out the mechanisms underlying aged mice B cells resistance to BCR-induced apoptosis, we studied the expression of antiapoptotic proteins involved in the resistance of B cell apoptosis such as Bcl-2, Bcl-xL [26] and A1 [27] in purified splenic B cells from young and aged mice. For this, B lymphocytes were cultured with or without F(ab)2 anti-µ Ab during 24 h, and then, Bcl-2 and Bcl-xL induction was assessed by Western blot analysis. As reported for B cells from young mice, aged-mice B cells increase the expression of Bcl-xL upon anti-µ stimulation; and the level of Bcl-xL induction seems to be identical between both groups. In addition, at time studied, Bcl-2 expression presented a slight increase after stimulation and the basal level of this protein is not different between B cells from young and aged mice (Fig. 2a).
Fig. 2.
Expression of Bcl-2, Bcl-xL and A1. (a) Purified B cells from young and aged mice were cultured with medium alone or F(ab)2 anti-µ (10 µg/ml) during 24 h. The expression of Bcl-2 and Bcl-xL was determined by Western blot. p38 MAPK expression was used to determine parity of loading. The densitometric values of Bcl-2 and Bcl-xl expression are shown in relative arbitrary units at the bottom of the figure. (b) Purified B cells from young and aged mice were cultured with medium alone or F(ab)2 anti-µ for 12, 24 and 48 h. RT-PCR was performed to detect A1transcripts. Number 1 and 3 represent B cells from young and aged mice, respectively, incubated with media alone, number 2 and 4 represent B cells from young and aged mice, respectively, stimulated with F(ab)2 anti-µ. The densitometric profile of A1 expression is shown in relative arbitrary units. (c) Purified B cells from young and aged mice were cultured with medium alone or F(ab)2 anti-µ for 48 h. Semi-quantitative PCR was performed to detect A1transcripts in serial dilutions of cDNA samples. In (b) and (c), β-actin was used as internal control of RNA integrity and equal loading. Immunoblotting for A1 could not be done due to the poor quality of commercially available Ab reagents. In (a–c) one typical experiment from the three performed is shown.
As we can observed in Fig. 2b anti-µ stimulated B cells from aged and young mice are able to up-regulate A1 expression at the same extent after 12 h of culture but they show a differential kinetic of A1 degradation at longer time culture. Thus, stimulated B cells from aged animals exhibit higher levels of A1 expression than B cells from young mice, after 24 and 48 h post stimulus. On the other hand, analysing the densitometric values obtained by anti-µ stimulated B cells after 24 and 48 h of culture and comparing them with the values obtained post 12 h of culture we observed that A1 mRNA levels of anti-µ stimulated B cells from young mice decreased 1·9 and 8·8 fold after 24 and 48 h, respectively, while A1 mRNA level of anti-µ stimulated aged B cells decreased 1·5 and 4·0 fold. We have found that the decrease in the A1 mRNA levels after 48 h of culture with anti-µ was significantly more accentuated in B cells from young mice compared to aged animals (P < 0·005).
In addition, the Fig. 2c depicts that B cells from aged mice cultured for 48 h without stimulus show comparable levels of A1 mRNA than those from young mice. In contrast, after 48 h of anti-µ stimulation, aged-mice B cells exhibit higher A1 mRNA level than their young mice counterpart cultured in the same conditions. In fact, only B cells from aged mice show detectable A1 mRNA transcripts even in the last dilution tested.
Taken together, these data demonstrate that after BCR-stimulation, B cells from aged animals are able to up-regulate and to keep higher levels of A1 transcripts than B cells from young mice. This higher expression of A1 may confer to aged-mice B cells a better protection against BCR-mediated apoptosis.
B cells from aged mice stimulated with F(ab)2 anti-µ exhibit a reduced Fas expression
Previous works have reported that activation stimuli, induces the up-regulation of the death receptor Fas on the B cell surface [28,29] making the cell more susceptible to apoptosis. In order to test whether B cells from aged animals up-regulate Fas expression in response to anti-µ stimulus, we compared by FCM, the percentage of Fas+ B cells from young and aged mice cultured during different periods of time with medium alone or stimulated with F(ab)2 anti-µ. We observed that upon 24 h of BCR stimulation, B cells from young and aged animals present a small percentage of Fas+ B cells. In both culture conditions tested, aged mice B cells show a minor percentage of Fas+ B cells compared to young mice B cells (5%versus 8% for medium alone and 9%versus 14% for F(ab)2 anti-µ). At longer times of stimulation (48 h) the percentage of Fas+ B cells is increased. This phenomenon is observed with B cells from aged as well as young animals; but interestingly, the percentage of Fas+ B cell in old mice is consistently lower than that observed in the young group (45%versus 67%) (Fig. 3) (P < 0·012). Likewise, after 48 h of culture either with media alone or anti-µ Ab, B cells from young mice present higher Fas expression than B cells from aged mice (mean fluorescence intensity: MFI 1415 versus 1258 for medium alone and 1546 versus 1375 for F(ab)2 anti-µ.
Fig. 3.
Fas expression on B cells from young and aged mice. Purified B cells from young and aged mice were cultured with F(ab)2 anti-µ (10 µg/ml) or media alone during 24 and 48 h. In all figures grey histograms represent B cells stained with FITC labelled anti-Fas Ab while staining with isotype control Ab is shown as thick black line histograms. One typical experiment from the three performed is shown. The statistical study was performed with One sample t-test.
Considering previous data that demonstrate that highly activated B cells express FasL and commit fraticide through Fas/FasL pathway [30,31]; we sought to evaluate if B cells from young and aged animals were able to increase the expression of FasL on their cell surface after BCR stimulation. No FasL induction was observed after 48 h of culture, but after 96 h of stimulation, B cells from young and aged mice express FasL at the same extent (data not shown).
We have also observed that upon anti-µ stimulation, B cells from aged mice reach the same activation level than B cells from young mice, as demonstrated by the similar MFI and percentages of CD19-activation marker (CD69, CD25, MHC Class II, CD80 or CD86) double positive cells (data not shown). Hence, B cells from aged mice are able to normally increase the expression of other activation markers after anti-µ stimulation; even when they showed a lower expression of Fas.
B cells from aged mice exhibit the same susceptibility to anti-Fas induced apoptosis but they express higher level of cFLIP and FAIM than B cells from young mice
Considering that Fas expression not necessarily correlate with Fas-mediated apoptosis susceptibility [32] and that B cells from aged-animals present lower Fas+ B cells than those from young mice, we examined the susceptibility of BCR-stimulated B cells from aged animals to anti-Fas killing. Thus, purified splenic B cells from young and aged mice were cultured with F(ab)2 anti-µ during 48 h, re-cultured with anti-Fas antibody or control hamster IgG during 12 h and stained with PI to determine the percentage of apoptotic cells by FCM. After culture with the anti-Fas Ab, B cells from either young or aged mice exhibit an increment in the percentage of apoptotic cells compared to B cells cultured with hamster IgG (67%versus 83% for young mice and 52%versus 70% for aged mice) (Fig. 4a). These data demonstrate that consistently with the percentage of Fas+ B cells, the percentage of dead cells after anti-Fas treatment was lower in B cells from aged animals compared with those from young mice. However, the increment of the Fas-mediated apoptosis respect to the control was similar in both groups studied (18% for aged-B cells versus 16% young mice B cells).
Fig. 4.
Fas–mediated apoptosis susceptibility and expression of antiapoptotic molecules. (a) Purified B cells from young and aged mice were cultured with F(ab)2 anti-µ (10 µg/ml) during 40 h. The cells were washed and incubated during 12 h more with hamster IgG (a,c) or anti-Fas Ab (Jo2) (0·125 µg/ml) (b,d). B cells were stained with PI and subjected to hyplodiploid DNA content analysis by FCM. M1 indicates the percentage of apoptotic B cells. The asterisks indicate significant differences *P < 0·04 compared with B cells from young animals cultured with anti-Fas antibody. (b) Purified B cells from young and aged mice were cultured with medium alone or F(ab)2 anti-µ (10 µg/ml) during 24 h. The expression of cIAP2 and cFlipL was determined by Western blot. p38 MAPK expression was used to determine parity of loading. Semi-quantitative RT-PCR was performed to detect FAIM. β-actin was used as internal control of RNA integrity and equal loading. The densitometric values of cIAP2, cFlipL and FAIM expression are shown in relative arbitrary units at the bottom of the figure. In A-B one typical experiment from the three performed is shown.
We next study the status of three antiapoptotic molecules such as cFLIP, FAIM and cIAP-2 in B cells from young and aged mice cultured 24 h with or without F(ab)2 anti-µ Ab. As we can observe in Fig. 4b. B cells from aged mice exhibit an increased expression of c-Flip when they were cultured without stimulus. After anti-µ stimulation c-Flip expression was greatly enhanced in B cells from young and aged animals but this up-regulation was significantly major in B lymphocytes from aged mice. Another strong difference in the B cell response was observed after 24 h of BCR stimulation; B cells from aged mice but not from young animals were able to express high levels of FAIM mRNA (Fig. 4b). In contrast, upon BCR-stimulus B cells from young mice presented a slight increase in the expression of cIAP-2 compared to their unstimulated counterparts, while B cells from aged mice does not change cIAP-2 expression. Likewise nonstimulated B cells from aged mice show higher levels of c-IAP-2 expression than B cells from young mice.
Taken together, these results demonstrate that B cells from aged animals differ in their ability to generate survival signals such as those mediated by cFlip and FAIM. The difference in the up-regulation of these molecules may explain why B cells from aged animals are more resistant to BCR-induced apoptosis but may not explain the susceptibility to Fas-mediated apoptosis.
B cells from aged mice show an increased proliferative response upon BCR-stimulation
We also evaluated whether the difference observed in the BCR-induced apoptosis between young and aged animals could be due to different proliferative capacity upon anti-µ stimulation.
The Fig. 5 depicts that the proliferative response, determined by [3H]-thymidine uptake, reached after 24 h of stimulation with F(ab)2 anti-µ was similar for B cells from both groups. Interestingly, the aged-mice B cells proliferate more vigorously than young-mice B cells after 48 h of stimulation (P < 0·02). This difference in the proliferative response was not a consequence of a differential expression of IgM since we observed by FCM that aged and young mice present same percentage of IgM+ B cells and the same level of surface IgM on B cell population (data not shown). In addition, after 12 and 24 h of culture, B cell from aged mice cultured with media alone present higher expression of an inducer of cell proliferation such as c-myc than B cell from young mice (data not shown).
Fig. 5.
Proliferative response of B cell from young and aged mice Purified B cells from young (▪) and aged mice (□) were cultured with medium alone or F(ab)2 anti-µ for 24 and 48 h and the[3H] thymidine uptake (cpm) was measured. Results represent the mean + SD. The data are representative of three independent experiments. The asterisks indicate significant differences *P < 0·02 compared with stimulated-B cells from young animals.
We also observed that B cells from young and aged mice secrete a high concentration of IL-10 after 48 h of anti-µ stimulation. However there was no difference on the levels of the cytokine secretion between B cells from young and aged mice, indicating that IL-10 is not mediating the higher B cell proliferation in aged mice (data not shown).
Aged and young Balb/c mice differ in the proportion of splenic B cell subsets
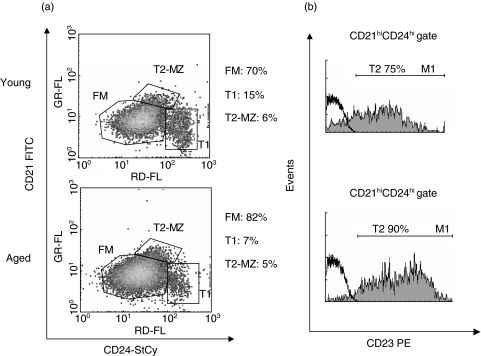
Splenic B cell compartment is composed by different B cell subsets that differ in their phenotype and ability to respond to BCR signalling [33]. Splenic B lymphocytes can be categorized in mature and immature or transitional cells. The mature B cell compartment involves the Follicular Mature (FM) B cells and the marginal zone (MZ) B cells while transitional (T) B cells are divided in T1 and T2. In response to BCR engagement, FM and T2 cells rapidly enter the cell cycle and resist cell death. In contrast, T1 B cells not only do not proliferate but also they die after BCR stimulation. MZ B cells are unable to respond to anti-µ stimulation [33]. Considering this and taking into account the different behaviour of B cells from aged and young mice to anti-µ stimulation, we further study the proportion of the different splenic B cells subset in both groups.
Based on CD24 and CD21 expression, the analysis of B cell subsets in aged mice revealed that FM B cells are slightly increased compared to young mice (82%versus 70%). In addition, we determined that in aged mice the percentage of T1 B cells is decreased (7%versus 15% in young mice) (P < 0·016) while the percentage of T2 plus MZ B cells is similar (5%versus 6% in young mice) (Fig. 6a). Based on the expression of CD23 in the CD21highCD24high population, we determined that aged mice present an increased percentage of T2 B cells (90%versus 75% in young mice) and consequently a decrease of MZ B cells compared to young mice (10%versus 25%) (Fig. 6b).
Fig. 6.
Splenic B cell subset from young and aged mice. Splenocytes from young and aged mice were stained with anti-mouse CD21, CD24, CD19 and CD23 mAbs. (a) Two-colour dot plot graphics show the percentage of T1, T2-MZ and FM B cells within CD19+ gated population. (b) Gray histogram represent CD23 surface expression within CD21high CD24high gated population. Isotype control Ab is shown as thick black line histograms. One typical experiment from the three performed is shown.
These data indicate that aged mice present a high percentage of B cells (FM and T2) able to proliferate upon BCR stimulus and a decrease in the percentage of B cells (T1) that die after BCR engagement. Taken together these results could explain the different behaviour of aged B cell to anti-µ stimulation.
Discussion
Considering that a highly orchestrated form of cell death is critical for maintaining the normal function of the immune system, an age-related disruption of B cell control mechanism would lead to B cell survival and consequently to self and non-self specific antibodies production and exacerbated cell proliferation. Despite the increasing interest in the relationship between programmed cell death and ageing, the role of B cell apoptosis during ageing remains obscure.
In this report we tested the ability of B cells from aged animals to undergo apoptosis after F(ab)2 anti-µ stimulation that simulates antigen–BCR interaction. The data presented herein indicate that after 24 h of culture with anti-µ Ab, B cells from aged mice show reduced levels of apoptosis compared to B cells from young mice, and this difference is increased by 48 h of culture. Our data are in line with reports showing that aged mice present an increase in the immunoglobulin serum level [34,35]. This increased resistance to programmed cell death of aged mice B cells can not be generalized to all kinds of apoptosis-producing agents since dexamethasone, cycloheximide or dibuyryl cAMP induce higher levels of apoptosis in B cells from aged animals than in those from young mice [19].
Trying to deepen into the mechanisms underlying the reduced apoptosis observed in B cells from aged mice we studied the status of several molecules playing different roles in the mitochondria-associated (intrinsic) and death receptor-associated (extrinsic) apoptotic pathways. It is well documented that several members of the Bcl-2 family can prevent cell death induced by BCR ligation; specifically, Bcl-2 or Bcl-xL overexpression is able to block anti-µ induced B cell apoptosis. Nevertheless, a higher expression of these antiapoptotic molecules or cIAP-2 is not responsible of the resistance of aged mice B cells to anti-µ induced apoptosis because they express the same level of these three proteins than young-mice B cells. Interestingly, F(ab)2 anti-µ stimulated B cells from aged and young mice, that up-regulate A1 antiapoptotic transcripts to the same extent after 12 h of culture, show a differential kinetic of A1 degradation at longer time culture. Hence, after 48 h of anti-µ stimulation, aged-mice B cells exhibit a significantly higher level of A1 transcript than young-mice B cells. Because A1 induction has been involved in follicular B cells survival signals [36] and BCR-mediated apoptosis protection induced by CD40 [37], our results regarding A1 expression could explain, at least in part, the improved resistance of aged mice B cells to anti-µ induced apoptosis.
Regarding the extrinsic apoptosis pathway, our studies about the expression of Fas on anti-µ stimulated B cells from aged and young animals show that aged mice B cells have reduced the level of Fas expression as well as a minor percentage of Fas+ B cell. These data are in line with that from Hsu et al. [38] demonstrating that, after 96 h of activation, the percentage of Fas+FasL+CD8+ cells is significantly lower in 18-month-old mice than in young mice. Nevertheless they present a diminished Fas expression in response to anti-µ, aged mice B cells are able to up-regulate other activation markers such as CD69, CD80, CD86 and CD25 at the same magnitude as B cells from young mice. In addition, aged mice B cells, after anti-µ stimulation, presented a higher proliferative response when compared to B cells from young animals. These results indicate that ageing did not affect the ability of B cells to become activated and proliferate.
Specific pathways mediating apoptotic induction by BCR are not fully understood. It has been postulated that BCR–mediated apoptosis and Fas-mediated apoptosis pathways are initially distinct but later they can converge [39]. Thus, it has been reported that the death receptor pathway is dispensable for BCR ligation-induced deletion of immature and mature resting B lymphocyte in vivo[40,41] and that BCR-induced apoptosis of B lymphoma-derived cells lines seems to be independent of Fas [42]. In addition, it has been demonstrated that the treatment with soluble anti-Ig Ab for 24 h is able to normally induce apoptosis in lpr B cells, indicating that Fas is not essential for apoptosis induced by mild BCR ligation. However, even when BCR-induced apoptosis appears to be normal in Fas-deficient MRL/lpr mice, autoantibodies are present in these mice, indicating the role of Fas in eliminating autoreactive B cells [43].
Based on this knowledge, we directly evaluated the susceptibility of anti-µ stimulated B cells from aged mice to anti-Fas Ab induced apoptosis. We observed that B cells from aged mice exhibit a lower percentage of apoptosis in response to Fas-crosslinking than those from young mice. After the culture with control hamster IgG or anti-Fas Ab, BCR-stimulated B cells from aged mice present lower levels of apoptosis than B cell from young mice treated in the same way. The susceptibility to Fas mediated killing was similar in B cells from both experimental groups studied indicating that during ageing B cells are protected from BCR mediated apoptosis but the susceptibility to Fas-induced death is not altered. However the finding that aged mice present less number of Fas+ B cells indicates that this pathway turn relevant at time to control an immune response.
We observed that aged mice B cells present after BCR-stimulation a higher expression of the antiapoptotic molecules c-Flip and FAIM, previously reported to confer resistance to Fas-mediated apoptosis induced by Ig-crosslinking, than those from young animals. However, in our system the expression of cFlip and FAIM is correlated with the resistance of aged B cells to BCR apoptosis but to Fas mediated apoptosis.
In summary, we demonstrate here that B lymphocytes from aged mice exhibit a decreased susceptibility to apoptosis after BCR-stimulation. The delayed A1 degradation as well as higher expression of c-Flip and FAIM, seems to contribute to the acquisition of the apoptosis-resistant phenotype. In addition, we have also observed that aged mice exhibit an increased percentage of splenic B cells subsets which are more resistant to BCR induced apoptosis such as T2 and FM B cells and a reduced percentage of T1 B cells that undergo apoptosis after BCR cross-linking [33]. These changes in the proportion of the splenic B cells could explain the different behaviour of aged B cells after anti-µ stimuli.
The diminished apoptosis after activation in aged mice could compromise homeostatic mechanism allowing the persistence of self and non-self antigen specific B cells. A further understanding of the mechanisms underlying antibodies-mediated diseases during ageing may eventually offer the potential for their prevention and therapeutic modulation.
Acknowledgments
This work was supported by Agencia Córdoba Ciencia, SECYT-UNC and CONICET PIP ♯ 02962 grants. E.A.R. thanks CONICET for the fellowships granted. C.L.M., A.G, and M.C.P.P are members of the Scientific Career of CONICET.
References
- 1.Kay MM, Makinodan T. Relationship between aging and immune system. Prog Allergy. 1981;29:134–81. [PubMed] [Google Scholar]
- 2.LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–26. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 3.Schneider EL. Infectious diseases in the elderly. Ann Intern Med. 1983;98:395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–70. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 6.Doria G, D’Agostaro G, Poretti A. Age-dependent variations of antibody avidity. Immunology. 1978;35:601–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Goidl EA, Innes JB, Weksler ME. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976;144:1037–48. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–5. [PubMed] [Google Scholar]
- 9.Manoussakis MN, Tzioufas AG, Silis MP, Pange PJ, Goudevenos J, Moutsopoulos HM. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin Exp Immunol. 1987;69:557–65. [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi Y, Kurashima C, Utsuyama M, Hirokawa K. An animal model of autoimmune sialadenitis in aged mice. Pathol Immunopathol Res. 1989;8:118–24. doi: 10.1159/000157143. [DOI] [PubMed] [Google Scholar]
- 11.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–39. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 12.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–91. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 13.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–4. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 14.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–11. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley SB, Cooke MP, Fulcher DA, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–35. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 18.Kirman I, Zhao K, Wang Y, Szabo P, Telford W, Weksler ME. Increased apoptosis of bone marrow pre-B cells in old mice associated with their low number. Int Immunol. 1998;10:1385–92. doi: 10.1093/intimm/10.9.1385. [DOI] [PubMed] [Google Scholar]
- 19.Souvannavong V, Lemaire C, Andreau K, Brown S, Adam A. Age-associated modulation of apoptosis and activation in murine B lymphocytes. Mech Ageing Dev. 1998;103:285–99. doi: 10.1016/s0047-6374(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 20.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Craxton A, Otipoby KL, Jiang A, Clark EA. Signal transduction pathways that regulate the fate of B lymphocytes. Adv Immunol. 1999;73:79–152. doi: 10.1016/s0065-2776(08)60786-5. [DOI] [PubMed] [Google Scholar]
- 23.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404–13. [PubMed] [Google Scholar]
- 24.Watanabe N, Nomura T, Takai T, Chiba T, Honjo T, Tsubata T. Antigen receptor cross-linking by anti-immunoglobulin antibodies coupled to cell surface membrane induces rapid apoptosis of normal spleen B cells. Scand J Immunol. 1998;47:541–7. doi: 10.1046/j.1365-3083.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 26.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–700s. [PubMed] [Google Scholar]
- 27.Lin EY, Orlofsky A, Berger MS, Prystowsky MB. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993;151:1979–88. [PubMed] [Google Scholar]
- 28.Rothstein TL, Wang JK, Panka DJ, et al. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–5. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein TL, Zhong X, Schram BR, et al. Receptor-specific regulation of B-cell susceptibility to Fas-mediated apoptosis and a novel Fas apoptosis inhibitory molecule. Immunol Rev. 2000;176:116–33. doi: 10.1034/j.1600-065x.2000.00616.x. [DOI] [PubMed] [Google Scholar]
- 30.Hahne M, Renno T, Schroeter M, et al. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–4. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 31.Zuniga E, Motran CC, Montes CL, Yagita H, Gruppi A. Trypanosoma cruzi infection selectively renders parasite-specific IgG+ B lymphocytes susceptible to Fas/Fas ligand-mediated fratricide. J Immunol. 2002;168:3965–73. doi: 10.4049/jimmunol.168.8.3965. [DOI] [PubMed] [Google Scholar]
- 32.Tian MT, Chou CH, DeFranco AL. Apoptosis induced by the antigen receptor and Fas in a variant of the immature B cell line WEHI-231 and in splenic immature B cells. Int Immunol. 2001;13:581–92. doi: 10.1093/intimm/13.4.581. [DOI] [PubMed] [Google Scholar]
- 33.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–10. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 34.Cossarizza A, Ortolani C, Monti D, Franceschi C. Cytometric analysis of immunosenescence. Cytometry. 1997;27:297–313. doi: 10.1002/(sici)1097-0320(19970401)27:4<297::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Ginaldi L, De Martinis M, D’Ostilio A, et al. The immune system in the elderly. I. Specific humoral immunity. Immunol Res. 1999;20:101–8. doi: 10.1007/BF02786466. [DOI] [PubMed] [Google Scholar]
- 36.Wen R, Chen Y, Xue L, et al. Phospholipase Cgamma2 provides survival signals via Bcl2 and A1 in different subpopulations of B cells. J Biol Chem. 2003;278:43654–62. doi: 10.1074/jbc.M307318200. [DOI] [PubMed] [Google Scholar]
- 37.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–41. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu HC, Shi J, Yang P, et al. Activated CD8 (+) T cells from aged mice exhibit decreased activation-induced cell death. Mech Ageing Dev. 2001;122:1663–84. doi: 10.1016/s0047-6374(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 39.Lens SM, den Drijver BF, Potgens AJ, Tesselaar K, van Oers MH, van Lier RA. Dissection of pathways leading to antigen receptor-induced and Fas/CD95-induced apoptosis in human B cells. J Immunol. 1998;160:6083–92. [PubMed] [Google Scholar]
- 40.Rathmell JC, Goodnow CC. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J Immunol. 1994;153:2831–42. [PubMed] [Google Scholar]
- 41.Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J Immunol. 1996;157:65–71. [PubMed] [Google Scholar]
- 42.Yoshida T, Higuchi T, Hagiyama H, Strasser A, Nishioka K, Tsubata T. Rapid B cell apoptosis induced by antigen receptor ligation does not require Fas (CD95/APO-1), the adaptor protein FADD/MORT1 or CrmA-sensitive caspases but is defective in both MRL-+/+ and MRL-lpr/lpr mice. Int Immunol. 2000;12:517–26. doi: 10.1093/intimm/12.4.517. [DOI] [PubMed] [Google Scholar]
- 43.Kozono Y, Kotzin BL, Holers VM. Resting B cells from New Zealand Black mice demonstrate a defect in apoptosis induction following surface IgM ligation. J Immunol. 1996;156:4498–503. [PubMed] [Google Scholar]