Abstract
Dendritic cells (DC), as the most effective antigen presenting cells, are protagonists of the complex immune network involved in multiple sclerosis (MS) lesion formation. Glatiramer acetate (GA), a synthetic random copolymer, is thought to exert its therapeutical effect in MS by favouring both Th2 cell development and IL-10 production from peripheral lymphocytes as well as by systemically affecting the antigen presenting cells. In the present study we further analysed the mechanisms of action of GA by using an autologous DC-lymphocytes (Ly) coculture system from 11 MS patients and 12 matched healthy controls (HC). We found that, in MS patients, pretreatment with GA significantly decreases the in vitro proliferative effect of DC on lymphocytes as compared to HC and to unpulsed or myelin basic protein (MBP)-pulsed DC from MS patients (P < 0·05). In addition, GA-treated DC from both MS patients and HC significantly increase the lymphocyte production of IL-5 and IL-13 as compared to MBP-treated DC (P < 0·05). In conclusion our in vitro study may provide new therapeutical mechanisms of GA on lymphocytes, antiproliferative and Th2-favouring effects, which are mediated by monocyte-derived DC.
Keywords: glatiramer acetate, multiple sclerosis, dendritic cells, lymphocytes, cytokines
Introduction
According with present concepts on multiple sclerosis (MS) pathogenesis, infiltrating auto-reactive Th1 cells induce a complex pro-inflammatory cascade causing demyelination and axonal loss throughout the central nervous system [1].
Dendritic cells (DC) are the most effective antigen presenting cells (APC), able of priming naïve T cells toward either Th1 or Th2 subsets [2,3]. Depending on environmental instructions provided by both antigen type and cytokine milieu, monocyte-derived DC can be polarized into either Th1-driving DC1 by IL-12 and IFN-γ [4,5], or Th2-driving DC2 by prostaglandin E2 [6]. Thus, DC-modulated adaptive immune response could be determinant in MS phenomenology [7,8].
Glatiramer acetate (GA; also known as Copolymer-1; Copaxone) is an approved therapy for MS that reduces both clinical and radiological disease activities [9–11]. GA is a randomised mixture of synthetic peptides composed of four amino acids, selectively competing with activation and cytokine production of myelin basic protein (MBP)-specific auto-aggressive Th1 cells [12,13], and inducing MBP-specific Th2-regulatory cells [12]. Although bystander suppression [14–16] and increased IL-10 production [17,18] have been reported, mechanisms involved in GA-initiated Th2 responses are incompletely understood. According with the presence of both a systemic change of the APC system in GA-treated patients [19] and of an altered cytokine production from GA-treated DC [18], Farina and colleagues elegantly support the working idea that GA may positively act in MS also by changing the intrinsic properties of APC, including monocyte-derived DC [20].
To this purpose, we further analysed the effect of GA on DC-mediated lymphocyte activation with a novel autologous mixed lymphocyte reaction (MLR) obtained by coculturing antigen-pulsed or unpulsed irradiated DC with autologous lymphocytes from patients with MS and healthy controls.
Materials and methods
Patients and controls
Eleven patients with clinically definite MS (8 females), with more than three MS-like lesions on brain MRI and cerebrospinal fluid (CSF)-restricted oligoclonal IgG bands, were included in the study. Mean age was 24 ± 3 years and mean MS duration 5 ± 3 years. Patients were either untreated (3 patients) or treated with IFN-β-1a 30 µg/week (7 patients) or GA (1 patient). Immunological analyses were performed during clinical remission, defined as the absence of new neurological symptoms and signs during the 6 months preceding blood sampling. Twelve age- and sex-matched healthy controls (HC; 10 females) were also recruited. Ethical approval was previously obtained and all subjects gave an informed consent prior their inclusion to the study.
Culture media, recombinant human cytokines and monoclonal antibodies
RPMI 1640 (Gibco, Paisley, UK) was used as culture medium, supplemented with 2 mm l-glutamine, 1% nonessential amino acids, 10% foetal calf serum, 50 U/ml penicillin and 50 µg/ml streptomycin (all from Gibco). To DC cultures, the following factors were added: recombinant human (rh) granulocyte-macrophage-stimulating factor (GM-CSF, 800 U/ml; Leucomax; Novartis, Basel, Switzerland), IL-4 (500 U/ml; R & D System, Minneapolis, MN, USA), GA (20 µg/ml, Teva, Petah Tiqva, Israel), and human MBP, 50 µg/ml. To lymphocyte cultures, the following were added: IL-2 (200 µl/m; Amersham Biosciences, Buckinghamshire, UK) and phytohaemagglutinin (PHA, 10 µg/ml; Roche, Mannheim, Germany).
For DC phenotyping by flow cytometry, the following monoclonal antibodies (mAbs) were used: phycoerythrin (PE)-conjugated anti-CD123 (IL-3Rα), CD80 and CD11c, and fluorescein isothiocyanate (FITC)-conjugated anti-CD86, CD83 and CD1a (Pharmingen, San Diego, CA, USA); a lineage cocktail comprising of FITC-conjugated mAbs against CD3, CD14, CD16, CD19, CD20 and CD56 along with PerCP-conjugated HLA-DR (Becton Dickinson, San Jose, CA, USA). Isotype control mAbs were: FITC-, PE- or PerCP-conjugated mAbs against IgG1 or IgG2a (Becton Dickinson). For lymphocytes, the following mAbs were used: FITC-conjugated mAbs against IgA2, CD95, CD69 and CD45RO; FITC-conjugated mAbs against CD25 and HLA-DR; and PerCP-conjugated mAbs against CD3 and CD4 (Becton Dickinson).
Generation and analysis of DC
Peripheral blood mononuclear cells (MNC) were isolated from heparinized blood by centrifugation on a discontinuous density gradient (Lymphoprep; 1·077 g/ml; Nycomed, Oslo, Norway). MNC were subsequently centrifuged on a Percoll gradient (Pharmacia, Uppsala, Sweden) consisting of three density layers (1·076, 1·059 and 1·045 g/ml). The light density fraction, containing predominantly monocytes, was allowed to adhere to Nunclon culture flasks (Nunc, Roskilde, Denmark) in RPMI 1640 medium. Adherent cells were plated into six-well plates (Costar, Cambridge, MA, USA) with complete medium containing rhGM-CSF (800 U/ml) and rhIL-4 (500 U/ml) and cultured for 6 days. At day 3, the media including the supplements were refreshed. DC were identified on day 6 of culture by flow cytometry as positive for CD11c, HLA-DR, CD1a, and costimulatory molecules (CD80 and CD86), but lacking the lineage markers for CD3 (T cells), CD19 and CD20 (B cells), CD16 and CD56 (NK cells), CD14 (monocytes) and CD123 (plasmocytoid DC). The DC were then irradiated and used as feeder layer for lymphocyte stimulation in cocultures.
After 6 days of culture, DC were collected and washed with phosphate-buffered saline. Purity of DC was > 80%, and cell viability > 70%. Cell-surface molecules on DC were examined by flow cytometry using three-colour staining: lineage-negative CD11c+ and lineage-negative CD123+ cells represent myeloid and lymphoid DC subpopulations, respectively. DC maturation and activation were evaluated by expression levels of CD83 and HLA-DR, and of the costimulatory molecules CD80 and CD86.
Lymphocyte cultures and analysis
The heavy density fraction of the Percoll, which contained predominantly lymphocytes, was cultured in 6-well plastic plates (Multiwell 6 well; Becton Dickinson). PHA (10 µg/ml) and IL-2 (200 µl/ml) were used to keep long-term lymphocyte cultures. Lymphocyte purity was assessed by flow cytometry for CD3+CD4+ and CD3+CD4- subpopulations. On day 7, aliquots of each lymphocyte culture were analysed with flow cytometry for purity (> 80% CD3+ cells). An aliquot of each lymphocyte culture was seeded together with mature, irradiated DC in cocultures. The remaining lymphocyte culture was exposed to either MBP (50 µg/ml; MBP-Ly) or GA (20 µg/ml; GA-Ly) or left unexposed to antigens as control lymphocytes (Ly) to be used for MLR; 96-well round-bottom plates (Nunc) with 2 × 105 lymphocytes for each well were used.
Autologous mixed lymphocyte reaction
On day 6, DC cultures were pulsed either with MBP (50 µg/ml) or GA (20 µg/ml) as antigen to be presented to lymphocytes. After two hours of incubation, DC were washed extensively. DC were then irradiated (15 Gy from a 137Cs source) and used to stimulate lymphocytes; 1 × 104 irradiated DC from MS patients or HC were plated in 96-well round-bottomed plates (Nunc) in triplicate and overlaid with 2 × 105 autologous lymphocytes as responder cells. Co-culture wells with irradiated but unpulsed (blank) DC were included for reference. Accordingly, a single 96-well plate for an individual donor (1 MS patient and 1 HC) included the following items:
blank DC, MBP-DC, GA-DC;
unpulsed control lymphocytes (Ly);
blank DC + Ly, MBP-DC + Ly, GA-DC + Ly.
After 72 h, all the items, including the cocultures of DC + Ly, were incubated with BrDU. After an additional 24 h in culture, proliferation assays were performed following instructions from the manufacturer (Amersham Biosciences). According to the manufacturer, an OD of 1 corresponds to a 24 h proliferation of 500 cells/well of the tumoral cell line L929.
Measurement of cytokines by ELISA
On day 9 of culture, supernatants from MLR were harvested. All ELISA tests (Euroclone, West York, UK) for cytokine measurement were performed in duplicate. The following cytokines were tested: TNF-α, IL-12p40 and IL-2 from DC cultures; IL-4, IL-5, IL-10, IL-13, TNF-α and IFN-γ from MLR and lymphocytes cultures. Optical densities were also measured using an ELISA reader. Cytokine concentrations were calculated using standard curves in order to minimize interassay variability.
Statistics
The statistical analysis of the study results was performed by using mean and standard deviation (SD). The ‘horizontal’ comparison within each group (either patient or control groups) under different experimental conditions has been performed by using the paired two-tailed T-test. Also, the unpaired two-tailed T-test was used for ‘vertical’ comparison between patients and controls. Significance level (P) was conventionally set at < 0·05.
Results
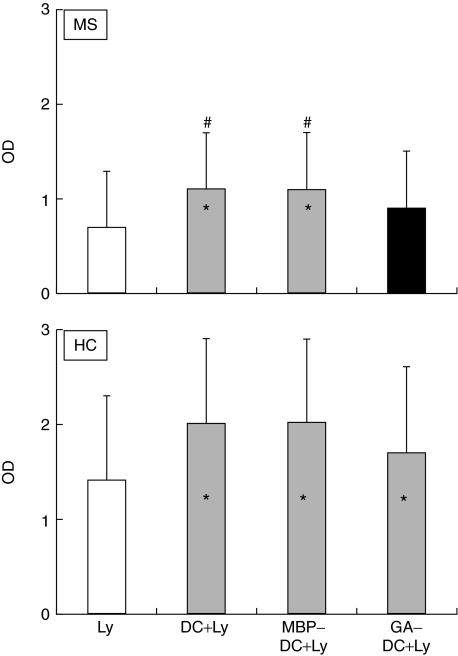
MBP- but not GA-pulsed DC enhance lymphocyte proliferation in autologous MLR from MS patients
To clarify the interaction between DC and lymphocytes upon distinct antigenic stimulations, we analysed the effect of control DC relative to GA- and MBP-pulsed DC from MS patients and HC on Ly proliferation in autologous MLR. DC were cultured in the presence of GM-CSF and IL-4 for six days. Thereafter, DC were exposed to either GA or MBP, or kept without antigen for 48 h (control DC). After washing, DC were irradiated and cocultured with autologous Ly for 72 h. As control, autologous lymphocytes were cultured with IL-2 and PHA only (Ly).
In MS patients, MBP-DC and control DC significantly enhanced autologous Ly proliferation (P < 0·05 compared to control Ly; Fig. 1). On the contrary, GA-DC showed no significant effect on lymphocyte proliferation.
Fig. 1.
Blank column shows the proliferation of control lymphocytes (Ly) from MS patients and healthy controls (HC). When proliferation of MS patients’ Ly was measured upon coculture with autologous irradiated DC exposed to MBP (MBP-DC + Ly) or GA (GA-DC + Ly) or no antigen (DC + Ly), MBP-DC as well as control DC significantly enhanced autologous lymphocyte proliferation (shaded columns; P < 0·05 for both comparisons). This was not observed for GA-pulsed DC (black bar). In HC, coculture with DC resulted in elevated lymphocyte proliferation levels irrespective if the DC had been exposed or not to antigens (shaded columns; P < 0·05 for all 3 comparisons); *P < 0·05 for comparison between Ly and MLR. There is a significant difference in proliferation between autologous MLR with GA-DC pretreatment and MLR with MBP or without any DC pretreatment; #P < 0·05 for comparison between MLR with DC without pretreatment and MLR with GA-pretreatment. According to manufacturer (Amersham Biosciences), an OD of 1 corresponds to the proliferation of 500 cells/well of the tumoral cells L924 in 24 h.
In HC, MBP-DC, GA-DC and control DC significantly, and to a similar extent, enhanced autologous lymphocyte proliferation (P < 0·05 for all comparisons; Fig. 1).
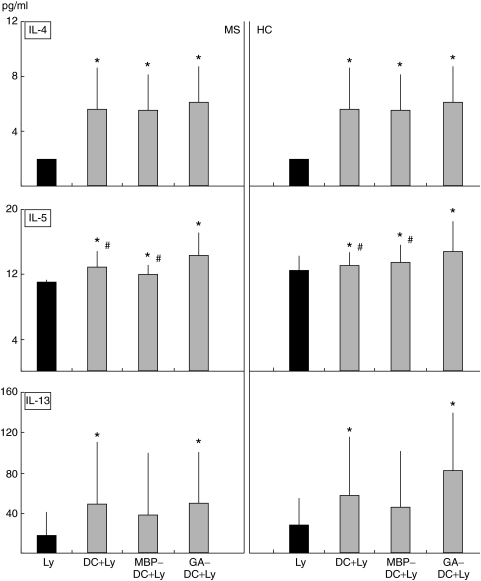
DC enhance IL-4 production in autologous MLR
To evaluate the interaction between DC and lymphocytes at the functional level, we examined the effect of DC on cytokine production by Ly. Concentrations of IL-4, IL-5, IL-10, IL-13, TNF-α and IFN-γ were analysed in supernatants of MLR and Ly cultures. TNF-α and IFN-γ production were not enhanced in DC + Ly MLR supernatants of both MS and HC. On the contrary, an elevated IL-4 production was detectable in the culture supernatant of control DC, MBP-DC and GA-DC MLRs (P < 0·05 for all comparisons; Fig. 2) as compared to supernatant of Ly culture alone.
Fig. 2.
Black bars show the concentration of IL-4, IL-5 and IL-13 in supernatants of control Ly from MS patients and HC. Shaded columns show the concentration of IL-4, IL-5 and IL-13 in supernatants of MLR cultures from MS patients and HC. DC from MS and HC were exposed to MBP or GA or no antigen, and cocultured with autologous lymphocytes. As compared to control Ly, IL-4, IL-5 and IL-13 production were augmented upon DC stimulation (both with and without antigen) both in MS and HC. Cytokines level is given as pg/ml; *P < 0·05 for comparison between Ly and MLR. A significantly higher production of IL-5 in GA-DC MLR as compared to MBP- or control DC MLR both in MS patients and HC. Significant differences are indicated by #P < 0·05.
GA-pulsed DC enhance IL-5 production in autologous MLR
Control DC, MBP-DC and GA-DC significantly enhanced IL-5 production in MLR from both MS and HC (P < 0·05 for all comparisons; Fig. 2), the effect being stronger in GA- as compared to MBP- or control-DC MLRs.
GA- but not MBP-pulsed DC enhance IL-13 production in autologous MLR
Control-DC and GA-DC significantly enhanced IL-13 production in MLRs from both MS and HC (P < 0·05 for both comparisons; Fig. 2). Differently, MBP-DC showed no significant effects on IL-13 production in MLR neither in MS nor in HC. In addition and in MS patients only, we found that while the MBP-DC-induced lymphocyte proliferation was high and IL-13 production low, the GA-DC-induced proliferation was low and IL-13 production high (P < 0·05).
Discussion
In this study we have used a MLR technique involving T cells and APC, the latter represented by autologous peripheral monocyte-derived irradiated DC, immature or matured by antigenic exposure to either MBP or GA, as previously described [21]. The main outcome measures of the study were the measurable effects of GA-pulsed irradiated DC on proliferation and cytokine production of lymphocytes obtained by MS patients and HC. The salient findings of the study are worthy of comment.
MBP-DC, control DC and GA-DC from HC significantly enhanced, to a comparable extent, the autologous lymphocyte proliferation. Differently, MBP-DC and control DC from MS patients significantly enhanced autologous lymphocyte proliferation while GA-DC exerted no significant effects. Thus, pretreatment of DC with GA appears to down-modulate their ability to initiate an intense lymphocyte proliferation only in MS patients.
How GA could act at this level is still unknown, but it could represent an important therapeutic effect of GA in MS. It is generally accepted that peripherally activated auto-reactive T cells pass through the blood–brain barrier, thereafter being re-activated in loco by local or secondarily recruited, blood-derived APC. Recently, DC have been detected in the CSF from MS patients [8] though their origin, whether brain- or blood-borne, remains an open question. Once reactivated in the CNS, T cells could proliferate and initiate a network of complex interactions, including the production of pro-inflammatory cytokines, leading to the final mechanisms of demyelination and axonal damage. Inhibiting lymphocyte proliferation by GA-exposed DC could result in a reduction of activated lymphocytes along with the known modulation in favour of Th2 cytokines with anti-inflammatory activities.
As a second step of the study, we have evaluated a panel of cytokines in three different experimental conditions: control MLR (DC + Ly), MBP-DC + Ly and GA-DC + Ly. The reason for choosing these two antigens is obvious: MBP is a putative auto-antigen in MS whereas GA represents an antigen specifically counterbalancing MBP-driven T cell activation, thus having the capacity of inducing beneficial therapeutic effects in MS. Differently to what described in other studies [14–18], we found no evidence of enhanced IL-10 production. We show that irradiated monocyte-derived DC predominantly induced IL-4, IL-5 and IL-13, but not the pro-inflammatory cytokines TNF-α and IFN-γ. Interestingly, the genes coding for IL-5 and IL-13 are located in the same chromosome and are regulated in a coordinated manner [22] which may lead us to hypothesize their clustered expression.
In our study, MLRs performed in both MS and HC by using untreated DC, GA-DC as well as MBP-DC resulted in an elevated IL-4 production as compared to control lymphocyte cultures. Based on these findings, we may hypothesize that IL-4 secretion in MLR is dependent on DC stimulation but independent on antigen-specific DC maturation.
In all MLR conditions IL-5 production was also influenced. However, GA-DC MLR resulted in the highest IL-5 production relative to unpulsed and MBP-pulsed DC MLR. These results seem to indicate, contrarily to IL-4, that IL-5 production could depend on antigenic stimulation of DC and that the Th2-inducer GA may exert a rather special signal for IL-5.
Finally, control DC and GA-treated DC significantly enhanced IL-13 production in both MS and HC (Fig. 2). Differently, MBP-DC induced no significant IL-13 increase as compared to control lymphocytes, neither in MS nor in HC. Moreover, IL-13 production is lower in MBP- as compared to GA- or control-DC MRL, though, perhaps due to the high standard deviation, not statistically significant. These results indicate that similarly to IL-5, IL-13 production is dependent on antigenic stimulation of DC. We may suggest that one of the therapeutic effects of GA in MS could be also dependent on IL-13 production.
As recently reviewed [20] previous studies on therapeutic mechanisms of GA were mainly focused on the direct effect of GA on T cells. Recent works have shown that the drug also notably affects the properties of APC, such as monocytes and DC which might offer an alternative explanation for the previously observed Th2 shift [18–20]. In the light of this considerations, our study provides confirmatory evidence of the GA-mediated Th2 shift based on the interaction between DC and T-cells. In addition, we add a novel aspect of the therapeutic effect of GA in MS, here represented by its selective antiproliferative effect on lymphocytes through DC modulation.
In conclusion, our results implicate additional mechanisms of action of GA in MS: one as an antiproliferative effect of monocyte-derived DC on lymphocytes and one by promoting an anti-inflammatory lymphocyte response mediated by DC.
Acknowledgments
The financial supports of RAS (Regione Autonoma Sardegna) and Fondazione Italiana Sclerosi Multipla (FISM), Genoa; grant number 2002/R/51, are gratefully acknowledged.
References
- 1.Olsson T. Humanized antibodies against an adhesion molecule block the CNS inflammation in multiple sclerosis. Lakartidningen. 2003;100:1705. [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 4.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12- inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–6. [PubMed] [Google Scholar]
- 5.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–12. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- 6.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv Exp Med Biol. 1997;417:363–7. doi: 10.1007/978-1-4757-9966-8_59. [DOI] [PubMed] [Google Scholar]
- 7.Pashenkov M, Soderstrom M, Huang Y-M, Link H. Cerebrospinal fluid affects phenotype and functions of myeloid dendritic cells. Clin Exp Immunol. 2002;128:379–87. doi: 10.1046/j.1365-2249.2002.01850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KP, Brooks BR, Ford CC, et al. Glatiramer acetate (Copaxone): comparison of continuous versus delayed therapy in a six-year organized multiple sclerosis trial. Mult Scler. 2003;9:585–91. doi: 10.1191/1352458503ms961oa. [DOI] [PubMed] [Google Scholar]
- 10.Comi G, Moviola L. Glatiramer acetate. Neurologia. 2002;17:244–58. [PubMed] [Google Scholar]
- 11.Paty D, Arnason B, Li D, et al. Interferons in relapsing remitting multiple sclerosis. Lancet. 2003;361:1822–4. doi: 10.1016/S0140-6736(03)13418-6. [DOI] [PubMed] [Google Scholar]
- 12.Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci USA. 2003;100:14157–62. doi: 10.1073/pnas.2336171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuhaus O, Archelos JJ, Hartung HP. Immunomodulation in multiple sclerosis: from immunosuppression to neuroprotection. Trends Pharmacol Sci. 2003;2:131–8. doi: 10.1016/S0165-6147(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 14.Dhib-Jalbut S, Chen M, Said A, Zhan M, Johnson KP, Martin R. Glatiramer acetate-reactive peripheral blood mononuclear cells respond to multiple myelin antigens with a Th2-biased phenotype. J Neuroimmunol. 2003;140:163–71. doi: 10.1016/s0165-5728(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 15.Putheti P, Soderstrom M, Link H, Huang Y-M. Effect of glatiramer acetate (Copaxone) on CD4+CD25high T regulatory cells and their IL-100 production in multiple sclerosis. J Neuroimmunol. 2003;144:125–31. doi: 10.1016/j.jneuroim.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Rieks M, Hoffmann V, Aktas O, et al. Induction of apoptosis of CD4+ T cells by immunomodulatory therapy of multiple sclerosis with glatiramer acetate. Eur Neurol. 2003;50:200–6. doi: 10.1159/000073860. [DOI] [PubMed] [Google Scholar]
- 17.Hussien Y, Sanna A, Soderstrom M, Link H, Huang Y-M. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–10. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- 18.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 19.Weber MS, Starck M, Wagenpfeil S, Meinl E, Hohlfeld R, Farina C. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain. 2004;127:1370–8. doi: 10.1093/brain/awh163. [DOI] [PubMed] [Google Scholar]
- 20.Farina C, Weber MS, Meinl E, Wekerle H, Hohlfeld R. Glatiramer acetate in multiple sclerosis. update on potential mechanisms of action. Lancet Neurol. 2005;4:567–75. doi: 10.1016/S1474-4422(05)70167-8. [DOI] [PubMed] [Google Scholar]
- 21.Wiesemann E, Klatt J, Sonmez D, Blasczyk R, Heidenreich F, Windhagen A. Glatiramer acetate (GA) induces IL-13/IL-5 secretion in naive T cells. J Neuroimmunol. 2001;119:137–44. doi: 10.1016/s0165-5728(01)00379-4. [DOI] [PubMed] [Google Scholar]
- 22.Kelly BL, Locksley RM. Coordinate regulation of the IL-4, IL-13, and IL-5 cytokine cluster in Th2 clones revealed by allelic expression patterns. J Immunol. 2000;165:2982–6. doi: 10.4049/jimmunol.165.6.2982. [DOI] [PubMed] [Google Scholar]