Abstract
In the failing human heart, due to idiopathic dilated cardiomyopathy, it has been suggested that the β1-adrenergic receptor (β1AR) is a potential pathogenic autoantigen. The aim of the present study was to investigate whether immunization of rats with a synthetic peptide corresponding to the second extracellular loop of the β1AR (β1AR ECII) was able to induce the early stage of cardiomyopathy and also to investigate immunological and receptor functional parameters at a transcriptional level to permit insights into the autoimmune mechanism in cardiomyopathy. Eleven Whistar Fur rats were immunized with a β1AR ECII peptide (H26R) once a month during 12 months and seven control rats were injected with vehicle according to the same procedure used for the immunized group. Cardiac function, β1AR autoantibodies and their functional effects on cardiomyocytes were analysed. β1AR receptor signalling, immunological and cardiomyocyte stretch markers were determined on transcriptional level. In H26R immunized rats, β1AR autoantibodies were shown to be present and functionally active, cardiac functions in terms of fractional shortening were decreased and β1-adrenergic receptor kinase (GRK2) mRNA were increased compared with the control group. These data have shown that immunization of rats with a putative antigenic peptide was able to induce an early stage phenotype of cardiomyopathy in the form of cardiac dysfunction and up-regulation of GRK2 as the first step in the desensitization process of the β1AR, implying the pathological importance of the β1AR autoantibody.
Keywords: β1-adrenergic receptor, cardiac function, cardiomyopathy, immunization
Introduction
Idiopathic dilated cardiomyopathy (DCM) is one of the leading causes of severe heart failure and one of the most common reasons for heart transplantation. Mortality due to heart failure has decreased significantly during the last decade since ACE inhibitors, β-adrenergic receptor (β1AR) blockers and angiotensin II receptor blockers were introduced. Nevertheless, chronic heart failure remains one of the most important causes for morbidity and mortality and has a very high frequency of readmission to hospitalization because of aggravation of the heart failure, which accounts for a significantly higher health-care expenditure that is more than twice that of the cost for cancer. One of the most important reasons is that current heart failure management is aimed mainly at the restoration of neurohormonal balance, rather than targeting the primary causes of the disease.
What causes dilated cardiomyopathy remains unclear, and research has focused on three possible mechanisms of damage: genetic factors [1], viral persistence [2] and immunological abnormalities [3]. During the last 10 years there have been many investigations showing distinct autoantibodies or other immune factors in heterogeneous subsets of DCM [4,5] which have contributed supportive and confounding evidence to hypothesis that multiple autoimmune mechanisms are involved pathophysiologically in DCM. Studies have shown immune regulatory disturbances in: cytokine levels [6], autoantibodies against different cardiac proteins [7,8], T lymphocyte subset populations [9] and cell-mediated inflammation in DCM hearts [10]. These findings have also been supported in DCM animal models, where immunization with different identificated cardiac antigens [11–14] or transfer of peripheral blood lymphocytes from patients with DCM to severe combined immunodeficiency (SCID) mice [15] were able to induce cardiomyopathic changes. Emerging immune therapies in the treatment of dilated cardiomyopathy such as immunoadsorption show favourable effects on cardiac performance [16], adding further weight to the hypothesis that cardiomyopathy is possibly autoimmunity mediated.
According to Witebsky's criteria to define an autoimmune disease, immunization of animals with the antigen should result in production of the disease [17,18]. Autoantibodies against the second extracellular loop (ECII) of the β1AR has been shown in DCM patients to be the main autoimmune target [7,12,19] and monoclonal antibodies against the β1AR ECII have also been shown to induce a positive inotropic response [20] and apoptosis [21] in isolated cardiomyocytes. Recent studies have demonstrated that both immunization with a fusion protein of the β1AR ECII in rats [13] and β1AR DNA immunization in mice [22] have been shown to induce impaired cardiac function. The present study is a step further in this direction, aiming to demonstrate whether immunization of a peptide corresponding to the β1AR ECII could induce an early stage of DCM in rats and also investigate further the immunological and receptor functional parameters on a transcriptional level.
Materials and methods
Immunization
Immunization was performed in 11 male Whistar Fur rats, beginning at the age of 10 weeks. A synthetic peptide (H26R) corresponding to the human and rat β1AR ECII (residues 197–222: H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R), was produced by LSUHSC Core Laboratories (New Orleans, LA, USA). These rats were immunized by subcutaneous injection of the peptide (1 mg/ml), dissolved in 0·1 M Na2CO3/1%β-mercaptoethanol and emulsified in Freund's adjuvant once a month for 12 months. Another seven male Whistar Fur rats were used as control receiving vehicle in the same manner. At the end of the study, heart and sera were collected for analysis. The apex of the heart was used for mRNA analysis and frozen later in RNA; the rest of the heart was frozen immediately in optimal cutting temperature (OCT) for histology analysis or in liquid nitrogen for further analysis. All tissue and sera were then stored at −80°C.
Autoantibody detection
To detect β1AR autoantibodies in rat sera, the β1AR peptide H26R was used in an enzyme-linked immunosorbent assay (ELISA). To determine the specificity of β1AR peptide-induced immune response, another peptide belonging to the G-protein coupled receptor superfamily and sharing a high degree of homology in sequence, the β2-adrenergic receptor peptide (H-W-Y-R-A-T-H-Q-E-A-I-N-C-Y-A-N-E-T-C-C-D-F-F-T-N-Q) was also used. Nunc (Roskilde, Denmark) plates were coated with 10 µg/ml peptide dissolved in 0·1 M Na2CO3/1%β-mercaptoethanol for 1 h at room temperature. After saturation of the wells with PMT buffer [3% skimmed milk/0·1% Tween 20 in phosphate-buffered saline (PBS), pH = 7·4]. The rat sera was added to the plates and incubated in 37°C for 2 h. The antibodies were revealed by incubation with biotinylated donkey anti-rat immunoglobulin (IgG) antibodies (Jackson Immuno Research Laboratories Inc., San Diego, CA, USA) diluted 1 : 1000 in PMT and incubated at room temperature for 1 h following addition of streptavidin–peroxidase conjugate (Jackson Immuno Research) at 1 : 1000 dilution in the same buffer. Plates were then washed with PBS and 2,2-azino-di (3-ethylbenzothiazoline) sulphonic acid (ABTS)-H2O2 (Roche, Switzerland) substrate buffer was added and incubated for 30 min in the dark at room temperature. The absorbance was measured at 405 nm in an ELISA reader (Spectra Max Plus, Molecular Devices, Sunnyvale, CA, USA).
Transthoracic echocardiography
Transthoracic echocardiography was used for evaluation of left ventricular function and geometry using previously validated two-dimensional (2D), M-mode and Doppler techniques. The animals were anaesthetized briefly with isofluorane (Baxter, Deerfield, IL, USA) and echocardiography was performed as described previously [23].
Histochemistry
Cardiac structure and infiltration of immune cells were visualized with haematoxilin/eosin and collagen with Sirus red staining on cryosections from rat hearts, following analysis with routine light microscopy. Seven and six hearts, respectively, were used when calculating cardiomyocyte size to investigate dilatation from cells in the H26R immunized group compared to controls. Cells (15–30) were calculated per heart depending on how many cells were cross-sectioned and had the right conformation, with a round shape and the nucleus in the middle.
Isolation of RNA and real-time reverse transcription-polymerase chain reaction (RT-PCR)
We used real-time RT-PCR to assess the effect of immunization on mRNA expression in the heart tissue of components in the β1AR signalling cascade [β1AR and β1-adrenergic kinase(GRK2)], cardiomyocyte stretch biomarkers such as cardiotrophin-1 (CTF1) and brain natriuretic peptide (BNP); also, components of the immune system were analysed as the monocyte chemotactic protein-1 (MCP1) which recruits monocytes, complement factor 3 (C3) and inflammatory cytokine tumour necrosis factor (TNF)-α. Total RNA was isolated from myocardium using the SV total RNA isolation system (Promega, Madison, WI, USA), according to the manufacturer's recommendations. RT reaction using a TaqMan Gold RT-PCR Kit [Applied Biosystems (ABI), Foster City, CA, USA)] was performed for cDNA synthesis. Random hexamers were used as primers for the RT reaction. The cycling parameters are as follows: 10 min at 25°C, 30 min at 48°C and 5 min at 95°C. Real-time PCR analyses for C3, TNF-α, GUS, CTF1, BNP and GRK2 were performed with TaqMan assay-on-demand on the ABI 7700 Sequence Detection System, according to the manufacturer's recommendations. The sequences of primer/probe of β1AR were as follows (5′−3′): β1AR sense: TGCAGACGCTCACCAACCT; β1AR anti-sense: CAGCAGTCCCATGACCAGATC; β1AR FAM-MGB probe: TTCATCATGTCCCTGGCC.
The reactions were analysed in duplicate and the relative expression levels were calculated according to the standard curve method. The expression data were normalized to an endogenous control, β-glucuronidas (GUS). The logarithm of the RNA concentration was calculated from standard curves. The expression was determined as the ratio of RNAtarget/RNAGUS.
C3 in rat sera
Detection of C3 in rat sera was made with an ELISA. Nunc (Roskilde, Denmark) plates were coated with polyclonal goat IgG anti-rat C3 (ICN Pharmaceuticals Inc., Ohio, USA) at 1/2000 dilution in PBS and incubated in 37°C for 1 h. The wells were then saturated with PMT buffer for 1 h in room temperature, and samples and standard curves were then added to the plate. Pooled sera from healthy Whistar Fur rats (n = 3) was used in the standard curve, ranging from a dilution of 1/1000 to 1/128 000, and the samples were diluted 1/10 000 in PMT buffer. Both standards and samples were incubated at 37°C for 1 h 1/500 dilution of peroxidase-conjugated goat IgG to rat C3 (ICN Pharmaceuticals Inc.) was then added to the plates and incubated for 1 h in room temperature following the addition of substrate buffer, ABTS-H2O2 (Roche, Switzerland), and incubated for 30 min in the dark at room temperature. Absorbance was measured at 405 nm in an ELISA reader (Spectra Max Plus, Molecular Devices, CA, USA).
IgG purification and culture of neonatal beating cardiomyocytes
IgG from rat sera was purified by caprylic acid/ammonium sulphate purification, as described by McKinney and Parkinson [24].
The culture of neonatal cardiomyocytes was performed by removing hearts aseptically from 1–3-day-old Whistar rats and cultured as described previously [25]. The number of beats of a selected isolated myocardial cell or a cluster of synchronously contracting cells in each of 10 fields was counted for 15 s each time. Rat IgG, synthetic antigen peptide and the corresponding receptor agonists were added, respectively, and the cells were observed 60 min after each addition. This procedure was repeated twice in different cultures to yield results representing a total of 30 cells or cell clusters. The basal rate of beating was 136 ± 15 beats/min.
Membrane preparation and receptor binding assay
Apical heart segments from rats (n = 4) in each group were homogenized in ice-cold buffer I (50 m M Tris-HCl, 100 m M NaCl, 2 m M EDTA, pH 7·4) and centrifuged at 4°C for 15 min at 3000 g. All the following steps were performed at 4°C. The supernatant was filtered through a 100 µm nylon cell strainer (BD Biosciences, Bedford, MA, USA) and then centrifuged for 30 min at 80 000 g; the pellet was then washed in buffer II (50 m M Tris-HCl, 1 m M EDTA, pH 7·4) and centrifuged for 30 min at 80 000 g. Finally, the membrane was dissolved in the binding assay buffer (12 m M MgCl, 25 m M Tris, pH 7·2) and stored at −80°C.
β-Adrenergic receptor density (Bmax) was determined using [125I] cyanopindolol ([125I]-CYP; 2200 Ci/m M; Perkin Elmer Life and Analytical Sciences, Billerica, MA, USA). Membrane protein (25 µg) was incubated in the binding assay buffer with increasing concentrations of [125I]-CYP (12·5–400 pM) at 37°C for 30 min. The reaction was stopped by washing three times with ice-cold binding assay buffer and rapid filtration, using Wharman GF/C filters. Filter-bound radioactivity was measured by ã counting. The unspecific binding was defined as the bound radioactivity in the presence of unlabelled isoproterenol (4 µ M). Specific binding was determined by subtracting unspecific from total [125I]-CYP binding activity. Estimations of maximal bound (Bmax) and equilibrium dissociation constant (KD) were obtained by non-linear regression curve fitting with the one site binding model (hyperbola) using GraphPad Prism version 4·00 software (San Diego, CA, USA). Bmax was standardized against the protein concentration, determined with a bicinchoninic acid (BCA) protein assay regent kit (Pierce, Rockford, IL, USA).
Statistical analysis
Results are expressed as the mean ± s.e.m. Mann–Whitney's test was used for analysis of significance of differences between the groups. Values of P < 0·05 were considered to be statistically significant.
Results
Body weight, heart weight and heart/body weight ratio
All rats were alive throughout the study. There were no obvious signs of heart failure in the form of breathlessness and fluid retention. No differences in either body weight (452 ± 16 g versus 463 ± 20 g), heart weight (1·49 ± 0·16 mg versus 1·45 ± 0·2 mg) or heart/body weight ratio (3·3 ± 0·3 mg/g versus 3·2 ± 0·6 mg/g) between the H26R immunized and control group were observed.
β1AR antibody production
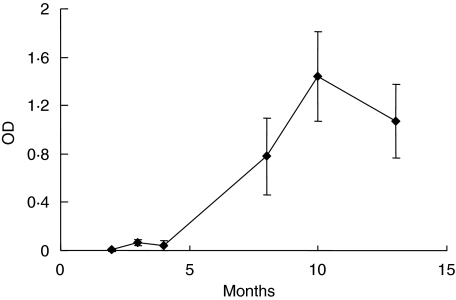
Rat sera were screened for β1AR autoantibodies with ELISA. It was shown that there was an increasing titre of anti-β1AR antibodies during the study period, with the highest concentrations at 10 months followed by a decrease of specific β1AR autoantibodies until the end of the study (Fig. 1). No autoantibodies were detectable in control rats. Rat sera were also screened for autoantibodies against another cardiac antigen, the β2-adrenergic receptor peptide, but no cross-reaction with this receptor peptide was shown.
Fig. 1.
Autoantibodies against the β1-adrenergic receptor extracellular loop 2 (β1AR ECII) during 13 months. β1AR autoantibodies in rat sera at 1/400 dilution were measured with peptide enzyme-linked immunosorbent assay (ELISA) at six time-points during the study period. The curve shows β1AR autoantibodies in the H26R immunized group. β1AR autoantibodies were undetectable in the control group.
Positive chronotropic effects of β1AR antibodies
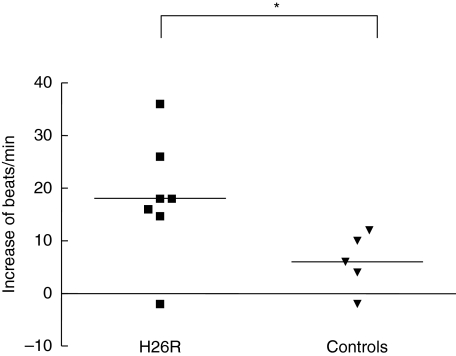
Functional properties of β1AR antibodies were analysed in vitro. IgG from H26R immunized animals showed positive chronotropic effects on spontaneous beating neonatal rat cardiomyocytes (Fig. 2).
Fig. 2.
Chronotropic action of rat immunoglobulin (IgG). Chronotropic response of IgG from H26R immunized (n = 7) and control rats (n = 5) on neonatal rat cardiomyocytes. The response is shown in increase of beats/min after addition of IgG compared to basal beating frequency and the horizontal lines corresponds to the mean value of each group. Statistical analyses between the groups were made with the Mann–Whitney test, *P < 0·05.
Heart function
At at 12 months following immunization echocardiography examinations showed a significant decrease in fractional shortening and circumferential shortening (fraction shortening/ejection time) in the H26R immunized group due to a tendency of dilatation of the left ventricular systolic dimensions in the H26R immunized group; all other echocardiographic parameters remained unchanged (Table 1). Examinations were also made at 9 months, but by that time no change in heart function was observed between the groups.
Table 1.
Echocardiographic parameters 12 months after immunization with H26R.
| H26R immunized (n = 7) | Controls (n = 7) | P-value | |
|---|---|---|---|
| HR (beats/min) | 342 ± 10 | 366 ± 9 | n.s. |
| CO (ml/min) | 165 ± 2 | 164 ± 2 | n.s. |
| LVD (mm) | 9·0 ± 0·3 | 9·1 ± 0·2 | n.s. |
| LVS (mm) | 6·4 ± 0·2 | 5·7 ± 0·3 | 0·08 |
| Posterior wall thickness in end-diastole (mm) | 1·53 ± 0·13 | 1·50 ± 0·07 | n.s. |
| IVSd | 1·3 ± 0·3 | 1·3 ± 0·4 | n.s. |
| FS (%) | 31·2 ± 1·1 | 37·4 ± 2·5 | 0·02* |
| Circumferential shortening (circ/s) | 3·92 ± 0·16 | 5·17 ± 0·42 | 0·012* |
Data are given as mean ± s.e.m.. Differences between groups were calculated with the Mann–Whitney test, * P < 0·05. HR = heart rate; CO = cardiac output (stroke volume · heart rate); LVD = left ventricle end-diastolic diameter; LVS = left ventricle end-systolic diameter; PWd = posterior wall diameter; IVSd = intraventricular septum thickness in end diastole; FS = fractional shortening (LVD-LVS/LVD × 100); circumferential fibre shortening (fraction shortening/ejection time).
Morphology, collagen and inflammation examination of heart tissue
When studying cardiomyocyte size, no significant differences between the H26R immunized group and the control group was observed. If anything, there was a small tendency towards greater size of the cardiomyocytes in the H26R immunized group compared to the controls (112·6 ± 11·9 versus 87·23 ± 11·1 µm2, P = 0·13). No differences in inflammation or collagen infiltration were observed between the groups.
β1AR antibody effect on β1AR signalling and immunological parameters at mRNA level
Using RT-PCR, mRNA encoding for GRK2 from heart tissues in the H26R immunized group was increased, whereas for β1AR, the cardiomyocyte stretch markers CTF1 and BNP, complement component C3, monocyte recruiter MCP1 and inflammatory cytokine TNF-α mRNA expression remained unchanged between groups (Table 2).
Table 2.
mRNA expressions in heart tissue.
| Gene | H26R immunizeda | Controlb | P-value |
|---|---|---|---|
| β 1AR | 1·58 ± 0·10 | 1·31 ± 0·14 | n.s. |
| β ARK | 2·11 ± 0·12 | 1·83 ± 0·07 | 0·03* |
| C3 | 1·80 ± 0·10 | 1·76 ± 0·17 | n.s. |
| TNF-α | 0·06 ± 0005 | 0·07 ± 0006 | n.s. |
| CTF1 | 1·05 ± 0·11 | 1·38 ± 0·12 | n.s. |
| BNP | 0·94 ± 0·10 | 1·14 ± 0·16 | n.s. |
| MCP1 | 0·46 ± 0·06 | 0·48 ± 0·07 | n.s. |
mRNA expressions were calculated via a standard curve and normalized against an endogen control.
Data are given as mean ± s.e.m. Statistical analysis between immunized and control animals was calculated with the Mann–Whitney test.
P < 0·05. β1AR: β1-adrenergic receptor; n.s.: not significant.
C3 in rat sera
ELISA analysis showed no signs of differences in C3 in rat sera between the groups: 109 ± 17% in the H26R immunized group compared to 83 ± 8% in the control group. The value of C3 is given as C3sample/C3standard in %.
β-adrenergic receptor density in rat hearts
Membrane prepared from rat hearts was analysed in a saturation binding assay with the non-selective 125I-labelled β-AR antagonist cyanopindolol. Saturation binding parameters were not significantly different, due probably to limited numbers of heart tissue (n = 4 in each group). The H26R-immunized animals showed a tendency towards down-regulation of β-adrenergic receptors: 170·3 ± 22·1 compared to 214·2 ± 20·9 fmol/mg in controls, P = 0·2. The Kd was not different in the H26R immunized group: 6·3 ± 0·7 versus 6·7 ± 0·6 p M in the control group.
Discussion
Growing evidence suggests a pathophysiological role of autoimmunity in cardiomyopathy and heart failure [7,8,14,16,26]. Our and others’ previous studies have shown that autoantibodies directed against the β1AR ECII seem to be involved in DCM [7,12,19], but questions regarding the exact role of these autoantibodies in the development of DCM remains to be clarified. In this study we focused on the effects of active immunization with a β1AR ECII peptide containing functional epitope(s) on cardiac structure, function and also the underlying mechanisms at transcriptional level.
Previous studies, using rabbits as host species for active immunization with β1AR peptide, have shown that immunization was able to induce cardiomyopathy-like changes [12,27,28]. However, the high susceptibility in rabbit species to induction of immune response raises the question of whether such active immunization is able to induce similar autoimmune damage in another animal species which is less susceptible to the immune response. This is of great importance, because in humans there is normally an effective immune defence system protecting the body from autoimmune diseases until this defence system has been broken down or manipulated. The rat is such a type of animal of choice.
Our results have shown high titres of antibodies against the β1AR in the sera from immunized rats, without cross-reactions with another closely related cardiac G-protein coupled receptor sharing a high degree of structural and biochemical similarities (β2-adrenergic receptor), indicating that an autoantigenic peptide was able to induce a specific and potent autoimmune response. We have also shown that these antibodies are functionally active, as they were able to induce positive chronotropic effects in cultivated neonatal cardiomyocytes.
A decrease in cardiac function was demonstrated without overt signs of heart failure in the β1AR peptide immunized rats. GRK2 expression seems to be a sensitive biomarker for heart failure, as it has been shown recently that the increase of GRK2 corresponds to disease severity in heart failure patients [29]. In our study we could show an increased expression of GRK2 on a transcriptional level, which often represents the early adaptation phase of the signal transduction pathway in myocytes and preceding desensitization of the β1AR, as shown in heart failure patients [30] and in a spontaneously hypertensive heart failure rat model [31]. The above changes indicate an early stage of cardiomyopathy, due probably to the limited response to the antigen, as the immune responses to a small antigen (< 5 KD) is more problematic to receive compared to an antigen of greater size. In our case, the molecular weight of the peptide is 3·3 KD and is in the limit zone of providing a good antigen response by itself, and this could explain the late peak of antibody production in the immunized animals.
Interaction of autoimmunity and cytokines, particularly in cardiomyopathy and heart failure, has received increased attention in recent years. However, in our present study we have not seen any changes in transcriptions of: a myocyte stretch marker (CTF1), a stress biochemical marker (BNP), a monocyte recruiter MCP1, a complement system component (C3) and an inflammatory cytokine (TNF-α). This might be due to the fact that our animal model is dealing with an early stage of cardiomyopathy. It will be interesting to enhance the immune response, e.g. by prolonging immunization duration or enhance the amount of immunized antigen, to see whether more pronounced cardiomyopathic change is associated with an increased expression of stretch biomarkers and augmented inflammation. On the other hand, this may strengthen our hypothesis that the early cardiomyopathic phenotype shown in this study is indeed caused exclusively by anti-β1-adrenergic receptor antibodies, and thereby the subsequent intracellular signalling cascade, without involvement of inflammatory components at this primary stage.
In summary, our data have shown that immunization of rats with a putative antigenic peptide was able to induce a phenotype of early stage of cardiomyopathy in the form of cardiac dysfunction and up-regulation of GRK2, as the first step in the desensitization process of the β1AR, implying the pathological importance of β1AR autoantibodies.
Acknowledgments
Many thanks to Azra Isic for her expert assistance. This study is supported by a grant from the Swedish Heart-Lung Foundation and Sahlgrenska Academy, University of Gothenburg, Sweden.
References
- 1.Michels VV, Moll PP, Miller FA, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with ‘idiopathic’ left ventricular dysfunction. Circulation. 2005;111:887–93. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 3.Gerli R, Rambotti P, Spinozzi F, et al. Immunologic studies of peripheral blood from patients with idiopathic dilated cardiomyopathy. Am Heart J. 1986;112:350–5. doi: 10.1016/0002-8703(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Carlquist JF, Hammond EH. Deficient natural killer cell activity in patients with idiopathic dilated cardiomyopathy. Lancet. 1982;2:1124–7. doi: 10.1016/s0140-6736(82)92786-6. [DOI] [PubMed] [Google Scholar]
- 5.Limas CJ. Autoimmunity in dilated cardiomyopathy and the major histocompatibility complex. Int J Cardiol. 1996;54:113–6. doi: 10.1016/0167-5273(96)02587-9. [DOI] [PubMed] [Google Scholar]
- 6.Marriott JB, Goldman JH, Keeling PJ, Baig MK, Dalgleish AG, McKenna WJ. Abnormal cytokine profiles in patients with idiopathic dilated cardiomyopathy and their asymptomatic relatives. Heart. 1996;75:287–90. doi: 10.1136/hrt.75.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–7. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 8.Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–8. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda T, Yokoyama T, Ohshima S, et al. T-lymphocyte subsets as noninvasive markers of cardiomyopathy. Clin Cardiol. 1990;13:617–22. doi: 10.1002/clc.4960130906. [DOI] [PubMed] [Google Scholar]
- 10.Noutsias M, Pauschinger M, Schultheiss H, U KH. Phenotypic characterization of infiltrates in dilated cardiomyopathy − diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med Sci Monit. 2002;8:CR478–87. [PubMed] [Google Scholar]
- 11.Fu ML, Schulze W, Wallukat G, Hjalmarson A, Hoebeke J. A synthetic peptide corresponding to the second extracellular loop of the human M2 acetylcholine receptor induces pharmacological and morphological changes in cardiomyocytes by active immunization after 6 months in rabbits. Clin Immunol Immunopathol. 1996;78:203–7. doi: 10.1006/clin.1996.0030. [DOI] [PubMed] [Google Scholar]
- 12.Matsui S, Fu ML, Katsuda S, et al. Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunized rabbits. J Mol Cell Cardiol. 1997;29:641–55. doi: 10.1006/jmcc.1996.0307. [DOI] [PubMed] [Google Scholar]
- 13.Jahns R, Boivin V, Hein L, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caforio AL, Grazzini M, Mann JM, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–42. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 15.Omerovic E, Bollano E, Andersson B, et al. Induction of cardiomyopathy in severe combined immunodeficiency mice by transfer of lymphocytes from patients with idiopathic dilated cardiomyopathy. Autoimmunity. 2000;32:271–80. doi: 10.3109/08916930008994101. [DOI] [PubMed] [Google Scholar]
- 16.Felix SB, Staudt A, Dorffel WV, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three-month results from a randomized study. J Am Coll Cardiol. 2000;35:1590–8. doi: 10.1016/s0735-1097(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 17.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. JAMA. 1957;164:1439–47. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 18.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited) Immunol Today. 1993;14:426–30. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 19.Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–54. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 20.Staudt A, Mobini R, Fu M, et al. beta (1)-Adrenoceptor antibodies induce positive inotropic response in isolated cardiomyocytes. Eur J Pharmacol. 2001;423:115–9. doi: 10.1016/s0014-2999(01)01113-x. [DOI] [PubMed] [Google Scholar]
- 21.Staudt Y, Mobini R, Fu M, Felix SB, Kuhn JP, Staudt A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/s0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 22.Gimenez LE, Hernandez CC, Mattos EC, et al. DNA immunizations with M2 muscarinic and beta1 adrenergic receptor coding plasmids impair cardiac function in mice. J Mol Cell Cardiol. 2005;38:703–14. doi: 10.1016/j.yjmcc.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Omerovic E, Bollano E, Basetti M, et al. Bioenergetic, functional and morphological consequences of postinfarct cardiac remodeling in the rat. J Mol Cell Cardiol. 1999;31:1685–95. doi: 10.1006/jmcc.1999.1004. [DOI] [PubMed] [Google Scholar]
- 24.McKinney MM, Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Meth. 1987;96:271–8. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 25.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–16. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 26.Gullestad L, Aass H, Fjeld JG, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–5. doi: 10.1161/01.cir.103.2.220. [DOI] [PubMed] [Google Scholar]
- 27.Matsui S, Fu ML, Hayase M, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Card Fail. 1999;5:246–54. doi: 10.1016/s1071-9164(99)90009-x. [DOI] [PubMed] [Google Scholar]
- 28.Iwata M, Yoshikawa T, Baba A, et al. Autoimmunity against the second extracellular loop of beta (1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;88:578–86. doi: 10.1161/01.res.88.6.578. [DOI] [PubMed] [Google Scholar]
- 29.Iaccarino G, Barbato E, Cipolletta E, et al. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–8. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 30.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–63. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KM, Eckhart AD, Willette RN, Koch WJ. The myocardial beta-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension. 1999;33:402–7. doi: 10.1161/01.hyp.33.1.402. [DOI] [PubMed] [Google Scholar]