Abstract
Oral infection by Anisakis simplex third stage larvae (L3) frequently gives rise to an allergic response. To comprehend the allergic and immune responses induced by L3, we investigated the kinetics of specific antibody isotype expression and the time course of biological and immunochemical allergy states using sera prepared from rats orally infected with L3 twice, with an interval of 9 weeks between infections. Biological and immunochemical allergy states were analysed by RBL-2H3 exocytosis and by indirect ELISA for IgE, respectively. The peak IgM at reinfection (RI) was comparable or similar to that at primary infection (PI) both in levels analysed by indirect ELISA and in antigen recognition analysed by Western blot. IgG1 and IgG2a levels were higher and showed accelerated kinetics after RI vs. after PI. However, the level of IgG2b was substantially lower than that of IgG2a. Peak immunochemical and biological allergy states for RI were higher and were reached faster than those for PI. The peak biological allergy state was observed at 1 week postreinfection and this occurred sooner than that for the peak immunochemical allergy state found at 2 weeks postreinfection. Our analysis of the relationship between specific IgE avidity and biological allergy state did not show any meaningful correlation. These results suggest that the allergic response induced by L3 oral infection is predominantly caused by reinfection and that this is accompanied by an elevated IgM level, which further suggests that the biological allergy state might not be related to specific IgE avidity.
Keywords: Anisakis simplex, basophils/mast cells, hypersensitivity, rats
Introduction
Anisakis simplex belongs to the Anisakinae family and normally parasitizes marine mammals. The second intermediate hosts of Anisakis simplex are marine fishes and cephalopods in which third stage larvae (L3) grow and develop. Live L3 carried by raw intermediate hosts infect humans orally [1]. In contrast to the reduced allergic response reported in hosts with a Schistosoma haematobium infection [2], L3 frequently causes allergies [3–5].
Several research groups have investigated the kinetics of specific antibody production in mice [6–9]. However, these results in mice could not be associated with an allergic response, as the presence of specific IgE was not verified. Amano et al. [10] first established the time course of L3-dependent allergies by peritoneal inoculation in a rat model. However, the immune response depends on the infection route and the human L3 allergy is caused by oral infection and not by peritoneal infection; thus, it is necessary to investigate the time course using rats infected with L3 per os [10].
Daschner et al. [11] and Alonso-Gomez et al. [12] showed that the L3 allergy induced by re-exposure to L3 was accompanied by an increase in specific IgM. They suggested that the elevation of specific IgM in the secondary immune reaction was caused by a new antigen or epitope that was not recognized in the primary immune response. As specific IgM production is a characteristic feature of the primary immune reaction, specific IgM produced in the secondary immune reaction in human L3 allergies should be compared with that produced in the primary immune reaction.
Several methods exist to characterize an allergy state. Immunochemical methods that analyse allergy state involve identifying a specific IgE by indirect ELISA or characterizing the IgE antigen by Western blot. Biological methods for studying allergy state involve either passive cutaneous anaphylaxis (PCA) or the measurement of exocytosis in rat basophilic leukaemia (RBL)-2H3 cells [13,14]. The results of RBL-2H3 exocytosis are highly correlated with those of PCA [15,16]. The relationship between biological allergy state and specific IgE avidity was described as a reverse relationship in a study using artificially prepared antibodies [17,18]. This relationship needs to be re-examined with a naturally induced allergy by L3.
This investigation used both biological and immunochemical methods to examine the time course of allergy states and the kinetics of specific antibody production in rats infected orally with L3. Our results showed that allergies caused by L3 oral infection were mainly induced by reinfection and occurred in combination with the elevation of specific IgM. Moreover, the biological allergy state was not found to be related to the avidity of specific IgE in the allergic response induced by L3.
Materials and methods
Materials
Sprague-Dawley rats purchased from Samtako (Osan shi, South Korea) were kept in an animal room, according to the guidelines of the Experimental Animal Committee of the Korea University College of Medicine. Avidin-conjugated alkaline phosphatase, alkaline phosphatase substrate (Sigma 104), and p-nitrophenyl N-acetyl-β-D-glucosaminide were purchased from Sigma (St. Louis, MO, USA). RBL-2H3 cells were maintained in MEM-Earles (Sigma) supplemented with 15% fetal bovine serum, l-glutamine, sodium pyruvate, and penicillin/streptomycin according to the protocol supplied by ATCC [19].
Anisakis simplex fourth stage larva (L4) excretory-secretory products (ESP and L4ESP) preparation
L3 harvested from mackerel body cavities were soaked in 0·1 M glycine solution (pH 2·0) twice for 30 min each time and then cultured in RPMI 1640 supplemented with the antibiotics gentamicin, kanamycin, streptomycin and penicillin in a humid, 37° C, 5% CO2 incubator [19]. The supernatant harvested on culture day 5 was referred to as L4ESP. This supernatant was lyophilized, resuspended in distilled water, and filtered.
Parasite infection and blood collection
The Sprague-Dawley rats were orally infected with L3 twice with an interval of 9 weeks between infections by placing 5 or 20 larvae on the pharynx. Rat sera were prepared by careful bleeding once per week from the ophthalmic plexus vein using a heparin-coated capillary tube [19].
Indirect ELISA
Indirect ELISA was performed as described by Kim et al. [19]. Briefly, ELISA plates coated with antigen (100 µl/well at 4 µg/ml) were incubated at 4° C for 16 h with each serum diluted 1 : 200. To detect the presence of each immunoglobulin isotype, plates were incubated for 2 h at 37° C with peroxidase-conjugated goat anti-rat IgM antibodies (ICN/Cappel, Westchester, PA, USA) or peroxidase-conjugated goat anti-rat IgG1, IgG2a, or IgG2b antibodies (Zymed, San Francisco, CA, USA) diluted 1 : 1000. o-Phenylene diamine (1 mg/ml, Sigma) was used as the substrate for peroxidase and the optical density (OD) read at 450/650 nm using a Microplate reader (Molecular Devices, Menlo Park, CA, USA). The ELISA reader showed optical density in the range from 0 to 4. For specific IgE, plates were incubated with purified B5 (1 µg/ml) [20] and then incubated for 2 h at 37 °C with goat anti-mouse IgG conjugated to alkaline phosphatase (Sigma) diluted 1 : 1000. Sigma 104 (1 mg/ml, Sigma) was used as the substrate and optical density read at 405/650 nm.
Rat IgE capture assays
Rat IgE capture assays were performed as described by Kim et al. [21] to measure the level of total rat IgE. Briefly, ELISA plates (Costar, Cambridge, MA, USA) coated with purified B5 (4 µg/ml) at 100 µl/well were incubated with rat serum samples for 2 h in a 37 °C incubator diluted 1 : 200. The plates were then incubated for 2 h at 37 °C with biotinylated mare-1 (mouse IgG anti-rat IgE, Zymed) diluted 1 : 1000 and finally incubated for 2 h at 37 °C with alkaline phosphatase-conjugated Extravidin diluted 1 : 1000. Sigma 104 (1 mg/ml) was used as the substrate. The concentration of rat IgE was determined by comparison with a standard curve generated using rat IgE of IR162 (Zymed). A standard curve was calibrated using the Softmax program (Molecular Devices, Menlo Park, CA, USA).
RBL-2H3 cell activation and the measurement of β-hexosaminidase release
The activation of RBL-2H3 was performed as described by Kim et al. [21]. Briefly, ∼5 × 104 RBL-2H3 cells per well were incubated for 2 h with rat serum diluted 1 : 40 at 100 µl per well in a humid 37 °C, 5% CO2 incubator. Exocytosis was induced by incubation with L4ESP (200µg/ml, 100µl/well) for 2 h. As an indicator of RBL-2H3 exocytosis, β-hexosaminidase release was measured using p-nitrophenyl-N-acetyl-β-D-glucosaminide as a substrate [19]. OD was read at 405/650 nm. Exocytosis results were analysed by averaging the data obtained from triplicate wells and expressed as release percentages. Total release was achieved by lysing cells with 1% Triton X-100.
SDS-PAGE and Western blot
SDS-PAGE was performed with a discontinuous gel consisting of a 10% resolving gel and 4% stacking gel using a Mini-Format Vertical Electrophoresis System (Amersham Biosciences, Uppsala, Sweden) as described by Kim et al. [19]. The proteins were blotted onto nitrocellulose membranes (Schleicher & Schuell, Germany) and specific antigens were visualized by Ig isotype, i.e. IgM, IgG1 and IgE.
Specific IgE avidity
The L4ESP-specific IgE thiocyanate avidity assay used was a modified form of an L4ESP-specific IgE ELISA, as previously described by Mitchell et al. [18]. Sera were assayed at dilutions determined to give a final absorbance of approximately 0·1. After incubation of the plate with diluted serum and washing, 100 µl of sodium isothiocyanate (0·75, 1·5, 3 and 6 M) in 0·2 M phosphate buffer was added (in duplicate) and the plate incubated for 15 min at RT. After the elution with isothiocyanate, the plate was washed three timed with PBS-T and the assay was completed as described above for the indirect ELISA of IgE against L4ESP. IC50% was defined as the concentration of isothiocyanate required to reduce the absorbance of a sample to 50% of the noninhibited value. This was determined by interpolation using inhibition curves and assuming linearity at the 50% point.
Statistical analysis
Data are presented as mean ± SE. Statistical analyses were performed using Excel (Microsoft). Standard error bars (SE) are shown in the figures.
Measurements of endotoxin activity
The endotoxin activities of each ESP preparation were determined using a Limulus amebocyte lysate (LAL) assay kit (QCL-1000) from Cambrex (Walkersville, MD, USA) according to the protocol supplied by manufacturer. Four different preparations of L4ESP were tested.
Results
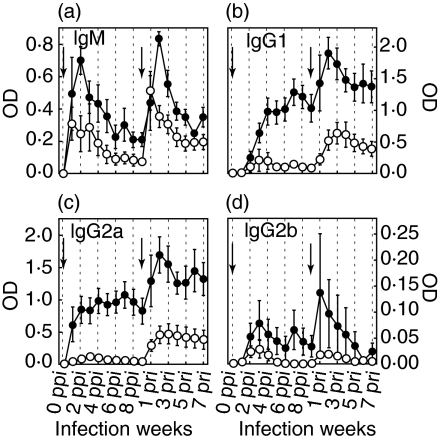
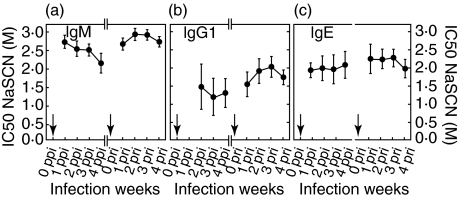
The rat sera collected from each group infected with 5-L3 or 20-L3 were examined by indirect ELISA to measure the levels of specific antibodies (IgM, IgG1, IgG2a, and IgG2b) against L4ESP (Fig. 1). Analyses of serum dilutions showed that the OD values from the ELISAs were in a linear relationship with specific antibody level. Each experimental group contained 8 rats. The ELISAs showed that the OD levels of 20-L3 were much higher than those of 5-L3, and that the OD levels at RI were higher that those at PI. The OD levels of IgM at peak RI were similar or higher than those at PI (Fig. 1a). Compared to the kinetics of IgG1 and IgG2a, the change in IgM OD level was more rapid. IgG1 and IgG2a showed a similar pattern to IgM in that OD levels at RI increased faster than those at PI and stayed at a higher level for a longer period (Figs 1b,c). In contrast to the OD level and kinetics of IgG2a, the production of IgG2b was low and irregular.
Fig. 1.
The time course of production of specific antibody isotypes: IgM, IgG1, IgG2a, and IgG2b. The presence of each specific antibody isotype was analysed by indirect ELISA. (a) IgM, (b) IgG1, (c) IgG2a, and (d) IgG2b. • 20-L3 group; ○ the 5-L3 group. The arrows indicate times of infection. Each rat was infected with either 20- or 5-L3. SEs are shown as bars.
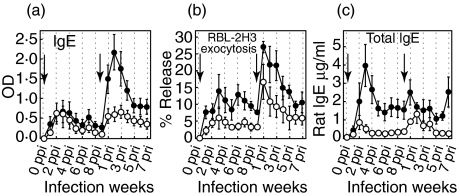
Both RBL-2H3 exocytosis and indirect ELISA for IgE revealed that higher levels of IgE were present at RI than at PI, and the kinetics of IgE showed a pattern of rapid change, similar to the IgM kinetics. The kinetics of specific IgE at RI peaked at 3 weeks post reinfection (pri) for 5-L3 and at 2 weeks pri for 20-L3. However, the kinetics of release percentage in the RBL-2H3 exocytosis assay peaked at 1 week pri for both 5-L3 and 20-L3 and then decreased gradually (Figs 2a,b). The kinetics of total IgE showed rapidly changing patterns for both PI and RI (Fig. 2b).
Fig. 2.
The kinetics of specific IgE, total IgE, and RBL-2H3 exocytosis. Allergic state was analysed immunochemically by indirect ELISA for specific IgE (a), by rat IgE capture assay for total IgE level (b) and biologically by RBL-2H3 exocytosis (c). • 20-L3 group; ○ 5-L3 group. The arrows indicate the times of infection. SEs are shown as bars.
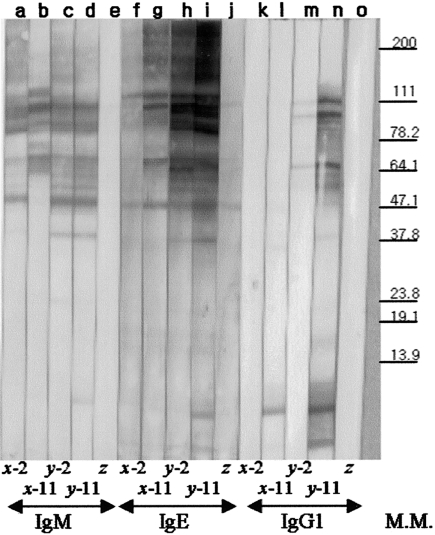
Antigens recognized by a specific antibody isotype were analysed by Western blot using rat sera from 2 rats, one with 5-L3 infection (x) and the other with 20-L3 infection (y) at 2 weeks post primary infection (ppi) and at 2 weeks pri (Fig. 3). Antigen recognition by specific IgM showed that the antigens identified were similar, regardless of L3 number or number of infections (Fig. 3, lanes a, b, c, d).
Fig. 3.
Immunoblot analysis with specific antibody isotypes of IgM, IgG1 and IgE. Antigens recognized by the specific antibody isotypes IgM (lanes a–e), IgE (lanes f–j) or IgG1 (lanes k–o) were analysed by Western blot. Sera were from two individual rats infected either with 5-L3 (x) or 20-L3 (y) or from negative control rat (z) either at 2 week ppi (lanes a, c, f, h, k, m) or at 2 week pri (11 week ppi, lanes b, d, g, i, l, n). The molecular marker is shown on the right.
The avidities of specific antibody isotypes IgM, IgG1 and IgE were analysed using sera from 1 to 4 weeks ppi and from 1 to 4 weeks pri. The avidity at RI tended to exceed that at PI for IgM, IgG1, and IgE (Figs 4a–c). In the case of specific IgE avidity, no noticeable differences were found 1, 2, or 3 weeks pri (Fig. 4c). This similarity in specific IgE avidities was also observed using another rat sera preparation (data not shown).
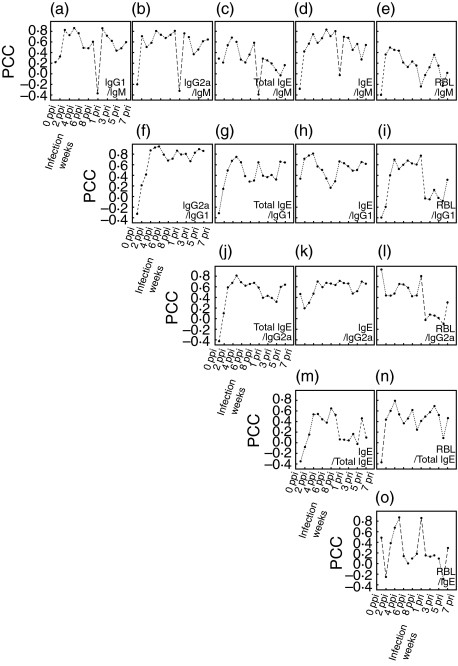
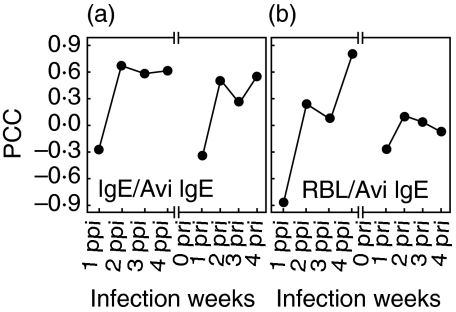
The correlation coefficients (CC) were calculated between IgM and each of the following parameters of the time course: IgG1, IgG2a, IgE, total IgE or RBL-2H3 exocytosis. The analysis showed that the CCs for each comparison with specific IgM decreased abruptly at 1 week pri (Fig. 5a–e). The CC between IgE avidity and specific IgE was particularly low at 1 week ppi and at 1 week pri (Fig. 6a). And, the CC between IgE avidity and RBL-2H3 exocytosis showed low values both at 1 week ppi and at 1 week pri (Fig. 6b).
Fig. 5.
Correlation between parameters of specific antibody, total IgE and RBL-2H3 exocytosis. Pearson's correlation coefficients were calculated between IgM and each of the following parameters: IgG1, IgG2a, IgE, total IgE and RBL-2H3 exocytosis. The x-axis indicates weeks after infection.
Fig. 6.
Correlation between IgE avidity and allergic state. Pearson's correlation coefficients were calculated (a) between specific IgE avidity and specific IgE, and (b) between specific IgE avidity and RBL-2H3 exocytosis. RBL indicates RBL-2H3 exocytosis. The x-axis indicates weeks after infection.
Discussion
Amano et al. [10] investigated the time course of immune reactions against ESP prepared by incubating L3 for 5 days, which we refer to as L4ESP. Since the kinetics of the specific IgG for L4ESP showed a pattern of increase, Amano et al. concluded that the infecting L3 developed into L4 in the rat peritoneum. In this investigation, because the sera from rats orally infected with L3 contained a high level of antibodies to L4ESP, most of the orally introduced L3 had developed into L4 within the rat intestine. Ishikura reported that orally introduced L3 disappeared from the rat intestine within a day [22]. In the present study, no larvae were found within rat bodies in the autopsies at either 1 week ppi or at 1 week pri in 4 rats of 5-L3 or 4 rats of 20-L3 (data not shown). Thus, most of the orally introduced L3 are believed to have been expelled during the early infection period. Due to the fast discharge of orally introduced L3, oral infection was expected to produce an insufficient and irregular immune reaction [9,10]. However, our results showed that the orally introduced L3 induced a constant and strong immune reaction (Fig. 1). The elevated IgM production in L3 oral infection decreased faster than that in L3 peritoneal inoculation (Fig. 1a) [10], a phenomenon which we attributed to less L4ESP absorption from L4 within the intestine than within the peritoneum.
Although specific IgM production upon primary infection was observed even in cases of Toxocara canis infection, specific IgM production upon reinfection has not been reported in humans, an abnormal host [23]. In addition, it is generally believed that the production of IgM at PI is usually much higher than that at RI, as observed for bacterial or viral infections. However, IgM elevation for L3 RI was comparable to that for L3 PI and was considered a marker of live L3 infection. The kinetics of specific IgM differed from that of other isotypes as IgM increased rapidly regardless of the number of infections.
Mast cells were known to participate in primary defence against bacterial infection and activated by lipopolysaccharide (LPS) of bacterial product [24,25]. However, mast cell exocytosis was not induced by LPS. In this investigation, a clear larva culture supernatant was applied and the prepared L4ESP was regarded to carry a low level bacteria (LAL test: 25–200 EU/ml). The contaminated L4ESP was characterized by turbid larva culture supernatant, in spite of daily change of culture media (LAL test: 1700 EU/ml). In addition, even the exocytosis induction of RBL-2H3 sensitized by anti L4 rat sera by contaminated L4ESP was not over that by clear and low-contaminated L4ESP (data not shown). RBL-2H3 was rat mucosal mast cell line. This indicates that the induction of RBL-2H3 exocytosis in this investigation was not affected by any bacterial products.
Although the severity of the allergy state for PI did not change with respect to L3 number (i.e. 5-L3 or 20-L3), the allergy state for RI showed a clear dependence upon L3 number in both immunochemical and biological assays (Figs 2a,b). Unlike the results of L3 peritoneal inoculation, the allergy state induced by L3 oral infection appeared to be positively associated with L3 number. However, the peak of the biological allergy state at 1 week after L3 oral infection was similar to that after L3 peritoneal inoculation.
Of the immunoglobulin isotypes measured, the production of the specific antibodies IgE, IgG1 and IgG2a was apparent. This observation indicates that the synthesis of Th2 cytokines was prevalent [26,27], a conclusion compatible with mRNA analyses (data not published). Although some rat sera produced IgG2b, the production of which is controlled by Th1 cytokines, the low OD levels and high SEs showed that Th1 cytokine synthesis was irregular and weak.
Though Daschner et al. [11] have suggested that the IgM elevation seen in gastro-allergic anisakiasis may be due to the appearance of a new antigen, Western blots in this study showed that the elevation of IgM by L3 RI was not induced by a novel antigen (Fig. 3, line a-d). Rather, increased IgM avidity appeared to be associated with the memory of IgM production (Fig. 4a). This hypothesis of IgM memory needs to be further explored. As discussed, the biological allergy state for RI was stronger by a factor of 2 and occurred 2 weeks sooner than that for PI, regardless of the infecting L3 number. The difference in severity in allergy states in the time course study suggests that the L3 allergy is induced by re-exposure to the allergen, regardless of IgM elevation. This description of L3 allergy is compatible with the general description of an allergy. Although not strongly recognized by specific IgM, an antigen at approximately 7 kD was clearly recognized by both specific IgE (for y-11) and by specific IgG (for x-11 and y-11). This antigen needs further investigation as a marker for L3 reinfection (Fig. 3).
Fig. 4.
The time course of specific antibody avidity for L4ESP. The avidity of specific antibody for L4ESP was analysed for a) IgM, (b) IgG1 and (c) IgE. The arrows indicate the times of infection.
In the analysis of specific antibody avidity for L4ESP antigen, IgM and IgG1 avidity at RI was higher than at PI, indicating an increased avidity at RI (Figs 4a,b). Although there was a tendency for IgE to have a higher avidity at RI also, the difference in IgE avidity between PI and RI was not as great as for IgM and IgG1. In addition, no meaningful change was observed from week 1 to week 3 pri for IgE avidity (Fig. 4c). Even the CC between IgE avidity and RBL-2H3 exocytosis did not show a reverse relationship (Fig. 6b). However, since there were abrupt decreases in the CC between IgM and other isotypes at 1 week pri (Fig. 5a–d) and between IgE avidity and specific IgE at 1 week pri (Fig. 6a), sera at 1 week pri appear to be different in immunological response. These results indicate that, although the biological allergy state was not related to specific IgE avidity in L3 allergy, there was an abrupt change in the sera at 1 pri. To ensure its usefulness as an effective naturally induced allergy model, the L3 allergy requires further investigation to clarify the induction of the biological allergy state.
In this investigation, the time course of immune reactions and the biological and immunochemical allergy states were analysed using rats orally infected with L3. The results indicate that the L3 allergy occurs upon re-exposure to L3, and that this is accompanied by IgM elevation. Furthermore, our immunochemical results suggest that the biological allergy state is not related to IgE avidity.
Acknowledgments
This research was supported by a grant from the Medical Research Center for Environmental Toxicogenomics and Proteomics funded by the Medical Research Center Project of the Korean Science and Engineering Foundations. We also take this opportunity to thank Joo Hee Shin, our coworker, for her dedicated efforts during the course of this work, but who tragically, recently passed away.
References
- 1.Sudduth RH. Anisakidosis. In: Strickland GT, editor. Hunter's Tropical Medicine and Emerging Infectious Diseases. 8. Philadelphia: W.B. Saunders Co; 2000. pp. 799–801. [Google Scholar]
- 2.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2001;356:1723–7. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 3.Kasuya S, Hamano H, Izumi S. Mackerel-induced urticaria and Anisakis. Lancet. 1990;335:665. doi: 10.1016/0140-6736(90)90455-e. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Ancillo A, Caballero MT, Cabanas R, Contreras J, Martin-Barroso JA, Barranco P, Lopez-Serrano MC. Allergic reactions to Anisakis simplex parasitizing seafood. Ann Allergy Asthma Immunol. 1997;79:246–50. doi: 10.1016/S1081-1206(10)63009-8. [DOI] [PubMed] [Google Scholar]
- 5.del Pozo MD, Audicana M, Diez JM, et al. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy. 1997;52:576–9. doi: 10.1111/j.1398-9995.1997.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias R, Leiro J, Ubeira FM, Santamarina MT, Sanmartin ML. Anisakis simplex: stage-specific antigens recognized by mice. J Helminthol. 1995;69:319–24. doi: 10.1017/s0022149x00014899. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias R, Leiro J, Ubeira FM, Santamarina MT, Sanmartin ML. Anisakis simplex: antigen recognition and antibody production in experimentally infected mice. Parasite Immunol. 1993;15:243–50. doi: 10.1111/j.1365-3024.1993.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones RE, Deardorff TL, Kayes SG. Anisakis simplex: histopathological changes in experimentally infected CBA/J mice. Exp Parasitol. 1990;70:305–13. doi: 10.1016/0014-4894(90)90112-p. [DOI] [PubMed] [Google Scholar]
- 9.Perteguer MJ, Cuellar C. Isotype-specific immune responses in murine experimental anisakiasis. Zentralbl Veterinarmed B. 1998;45:603–10. doi: 10.1111/j.1439-0450.1998.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 10.Amano T, Nakazawa M, Sugiyama H, Secor WE, Oshima T. Specific antibody patterns of Wistar rats inoculated with third stage larvae of Anisakis simplex. J Parasitol. 1995;81:536–42. [PubMed] [Google Scholar]
- 11.Daschner A, Cuellar C, Sanchez-Pastor S, Pascual CY, Martin-Esteban M. Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol. 2002;24:243–51. doi: 10.1046/j.1365-3024.2002.00458.x. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Gomez A, Moreno-Ancillo A, Lopez-Serrano MC, Suarez-De-Parga JM, Daschner A, Caballero MT, Barranco P, Cabanas R. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol Res. 2004;93:378–84. doi: 10.1007/s00436-004-1085-9. [DOI] [PubMed] [Google Scholar]
- 13.Hogarth-Scott RS. Rabbit reagin-like antibodies. Int Arch Allergy Appl Immunol. 1967;32:201–7. doi: 10.1159/000229928. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann A, Vieths S, Haustein D. Biologic allergen assay for in vivo test allergens with an in vitro model of the murine type I reaction. J Allergy Clin Immunol. 1997;99:227–32. doi: 10.1016/s0091-6749(97)70101-5. [DOI] [PubMed] [Google Scholar]
- 15.Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981;11:317–23. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 16.Kaul S, Hoffmann A. Mediator release assay of rat basophil leukemia cells as alternative for passive cutaneous anaphylaxis testing (PCA) in laboratory animals. ALTEX. 2001;18:55–8. [PubMed] [Google Scholar]
- 17.Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science. 1998;281:568–72. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AJ, Moss ND, Collins AM. The biological activity of serum IgE changes over the course of a primary response. Scand J Immunol. 2002;55:33–43. doi: 10.1046/j.1365-3083.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Kim KH, Cho S, Park HY, Cho SW, Kim YT, Joo KH, Lee JS. Immunochemical and biological analysis of allergenicity with excretory-secretory products of Anisakis simplex third stage larva. Int Arch Allergy Immunol. 2005;136:320–8. doi: 10.1159/000084225. [DOI] [PubMed] [Google Scholar]
- 20.Conrad DH, Studer E, Gervasoni J, Mohanakumar T. Properties of two monoclonal antibodies directed against the Fc and Fab′ regions of rat IgE. Int Arch Allergy Appl Immunol. 1983;70:352–60. doi: 10.1159/000233347. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Cho S, Joo KH, Lee JS, Conrad DH, Cho SW. Identification of functionally different rat IgE in RBL-2H3 exocytosis. Immune Network. 2002;2:195–201. [Google Scholar]
- 22.Ishikura H. Epidemiological aspects of intestinal anisakiasis and its pathogenesis. In: Ishikura H, Kikuchi K, editors. Intestinal Anisakiasis in Japan. Tokyo: Springer-Verlag; 1990. pp. 3–21. [Google Scholar]
- 23.Lillywhite JE, Bundy DA, Didier JM, Cooper ES, Bianco AE. Humoral immune responses in human infection with the whipworm Trichuris trichiura. Parasite Immunol. 1991;13:491–507. doi: 10.1111/j.1365-3024.1991.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–41. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 25.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001;167:2250–6. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 26.Cetre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713–9. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binder J, Graser E, Hancock WW, Wasowska B, Sayegh MH, Volk HD, Kupiec-Weglinski JW. Downregulation of intragraft IFN-γ expression correlates with increased IgG1 alloantibody response following intrathymic immunomodulation of sensitized rat recipients. Transplantation. 1995;60:1516–24. doi: 10.1097/00007890-199560120-00025. [DOI] [PubMed] [Google Scholar]