Abstract
The family of Toll-like receptors (TLRs) initiates innate immune responses, and Toll-like receptor 4 (TLR4) was considered to be an important player in the initiation and progression of atherosclerotic disease. The aim of the study was to investigate the expression of TLR4 on peripheral-blood mononuclear cells (PBMCs) in patients with coronary arteriosclerosis disease (CAD). We have examined the expression of TLR4 protein and mRNA by flow cytometry (FCM) and real-time quantitative reverse transcription polymerase chain reaction (RT-PCR). In addition, the levels of plasma lipids were determined by automatic biochemistry analyzer. The results showed that the positive rates and the mean mRNA copy number of TLR4 in CAD group were significantly higher than that in controls. But no significant difference was found in the positive rate and the mean mRNA copy number of TLR4 between CAD group with normal level of plasma lipids and the CAD group with abnormal level of plasma lipids. We suggest that expression level of TLR4 on peripheral-blood mononuclear cells is increased in atherosclerotic, but the differential expression of TLR4 has no correlation with the level of plasma lipids.
Keywords: toll-like receptor 4, atherosclerosis, lipid, inflammation
Introduction
Atherosclerosis is a multifactorial process and its outcome, coronary heart disease, is considered to be responsible for the greatest number of deaths worldwide. Immune responses including innate and adaptive immune system play important roles in initiation and progression of atherosclerosis [1]. The family of Toll-like receptors is a transmembrane signal receptor that initiates an innate immune response after recognition of pathogen-associated molecular patterns (PAMPs). TLRs are important in linking the innate to the adaptive immune response [2]. Toll was first discovered as a Drosophila gene. In 1997 a human Toll homologue was identified, now known as TLR4 [3]. Evidence is accumulating that TLRs, and particularly TLR4, are important players in the initiation and progression of atherosclerotic disease [4].
There is increasing evidence supporting the role of TLR4 in atherosclerotic lesion development. Kiechl et al. [5] showed that humans with the Asp299Gly TLR4 polymorphism had a lower risk of carotid atherosclerosis and less intima media thickness in the common carotid artery. Toll-like receptor 4 activation vascular cells up-regulate the expression of monocyte chemoattractant protein-1(MCP-1) and other chemokines involved in the recruitment of monocytes and initiating the inflammatory response [6]. Recently, evidence emerged that vascular and systemic inflammation induced by oxidized low density lipoprotein (oxLDL), infection and heat-shock proteins, three key players in human atherogenesis, are mediated in part via the TLR4/nuclear factor-kappaB(NF-κB) pathway [7]. It suggests that TLR4 may provide a potential pathophysiological link between lipids and infection/inflammation and atherosclerosis.
Toll-like receptor 4 is expressed by different cell types in the atherosclerotic vessel wall: endothelial cells [8], macrophages [8,9], adventitional fibroblasts [6] and dendritic cells [10] and it is prominently expressed and activated in lipid-rich macrophage-infiltrated human atherosclerotic plaques [9]. We hypothesized that the expression of TLR4 on peripheral-blood mononuclear cells may be altered in patients with atherosclerosis diseases and the alteration may be correlated with the level of plasma lipids. In order to explore this hypothesis we assessed the expression of TLR4 at both the protein and mRNA level in 50 patients with coronary arteriosclerosis disease (CAD) and 40 healthy controls by flow cytometry and real-time quantitative RT-PCR. Besides, the levels of plasma lipids were determined by automatic biochemistry.
Materials and methods
Subjects
The subjects were selected from inpatients with angiographically verified coronary artery disease from August to December 2004 in Shanghai Changzheng Hospital. The study group consisted of 50 patients (32 men and 18 women; mean (± SD) age 65 ± 9 years); the control group consisted of 40 volunteers (21 men and 17 women; mean (± SD) age 61 ± 7 years) who have no CAD history, no other diseases and have normal levels of plasma lipids concentrations. All patients gave their informed consent for the study. Fasting blood samples were collected into anticoagulation (with EDTA-K2) tubes from 90 participants.
Analysis of expression of surface TLR4
TLR4 flow cytometry was performed using phycoerythrin (PE) antihuman TLR4 antibody (eBioscience, San Diego, CA, USA) by the manufacturer's instruction. Briefly, 20 µl PE-conjugated mouse antihuman TLR4 antibody was incubated with 100 µl whole blood for 30 min at room temperature in the dark. After incubated with the RBC lysis buffer, the mixture was washed in stain buffer containing bovine serum albumin and sodium azide. Cells were resuspended in 500 µl stain buffer and analyzed by flow cytometer (BeckmanCoult, Fullerton, CA, USA). Negative control was prepared by incubating with an isotype-matched control antibody (IgG2a).
PBMCs preparation and RNA extraction
Peripheral-blood mononuclear cells were isolated from whole blood of the subjects by Ficoll-Hypaque density gradient centrifugation. Total cellular RNA was extracted using the Trizol RNA extraction kit in accordance with the manufacturer's instructions. RNA yield and purity were determined spectrophotometrically at 260/280 nm.
Standard plasmid construction
For real-time quantitative RT-PCR, primers and Taqman probes were designed by Beacon Designer 2.1 software and the sequences were showed in Table 1. cDNA was synthesized by RT-PCR reactions using SuperScript™ III Platinum® two-step quantitative RT-PCR kit (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. PCR products were purified through gel extraction. Ligation of the pGEM-T plasmid vector and purified PCR fragment was performed with T4 DNA ligase (Promega, Madison, WI, USA). Plasmids were purified on columns with a Qiagen kit and quantified by A260 measurements. To control the validity of our primers, the amplified products were sequenced. Recombinant plasmid pGEM-T-TLR4 was as positive control and pGEM-T-β-actin was as endogenous control.
Table 1.
Primer and probe sequences of TLR4 and β-actin.
| Sequence | Amplicon size (bp) | |
|---|---|---|
| TLR4 | ||
| ″Sense-primer | 5′-TGGAAGTTGAACGAATGGAATGTG-3′ | 147 |
| ″Antisense-primer | 5′-ACCAGAACTGCTACAACAGATACT-3′ | |
| ″Probe | 5′-FAM-AGCACACTGAGGACCGACACACCAA-TAMRA-3′ | |
| β-actin | ||
| ″Sense-primer | 5′-AGATCAAGATCATTGCTCCTCCTG-3′ | 145 |
| ″Antisense-primer | 5′-CATTTGCGGTGGACGATGGA-3′ | |
| ″Probe | 5′-FAM-CGGACTCGTCATACTCCTGCTTGCTG-TAMRA-3′ | |
Real-time quantitative RT-PCR
To generate standard curve, the recombinant plasmids were gradient diluted with sterilized water to 1 × 109, 108, 107, 106 105, 104 and 103copies/µl. We synthesized cDNA from the extracted total RNA using SuperScript™ III Platinum® two-step quantitative RT-PCR kit. Real-time quantitative PCR reactions were performed using an ABI-Prism 5700 sequence detector. Each PCR mixture (50 µl total volume) contained the following: 4 µl cDNA template, 25 µl Platinum® Quantitative PCR SuperMix-UDG, 1 µl ROX, primers (1 µl each), 1 µl Taqman probe and 17 µl sterilized water. Cycling parameters were as follows: 50 °C for 2 min, 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 minute. We analyzed results using Sequence Detection Software (Applied Biosystems, Foster City, CA, USA).
Plasma lipids detection
The level of plasma lipids was determined by automatic biochemistry analyzer (Beckman CX4), including total cholesterol, triglyeride, low density lipoprotein cholesterol, high density lipoprotein cholesterol and lipoprotein(a). Any one of the diadynamic criteria beyond the normal range was considered plasma lipids abnormal.
Statistical analysis
All real-time quantitative RT-PCR determinations were made in duplicate. Measurement data was presented as x¯±s. Statistical differences in the amounts of TLR4 mRNA or the positive rates of TLR4 between groups were tested by 2-way t-test. A P-value < 0.05 was considered significant.
Results
Expression of TLR4 protein in the atherosclerotic peripheral blood cells
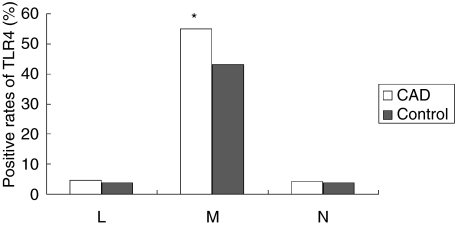
TLR4 protein expression in atherosclerotic peripheral blood cells was detected using FCM. The positive rates of TLR4 in lymphocytes and neutrophilic granulocytes were less than 5%, and there was no significant difference between CAD group and control group. TLR4 is mainly expressed in human monocytes and the positive rates of TLR4 in monocytes in CAD group was significantly higher than that in controls (55.11 ± 16.3%versus 42.91 ± 15.7%, P < 0.01) (Fig. 1).
Fig. 1.
The positive rates of TLR4 in peripheral blood cells. L = lymphocytes; M = monocytes; N = neutrophilic granulocytes. *P < 0.01.
Construction of standard plasmids
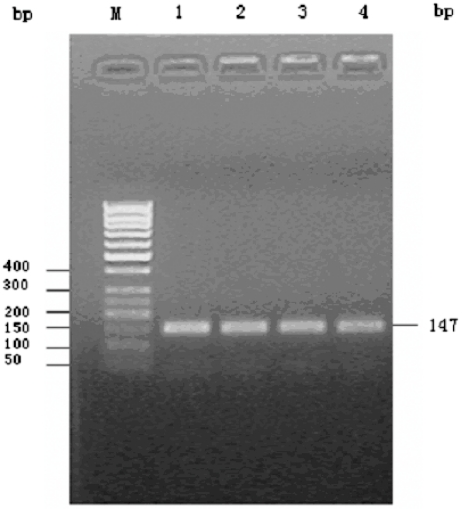
cDNA was synthesized by RT-PCR using total RNA extracted from PBMCs as template. Amplified products were visualized on 1.5% agarose gels stained with ethidium bromide and photographed under UV light (Fig. 2). The constructed plasmids were confirmed by polymerase chain reaction. The target fragments proper sequence and orientation were confirmed by sequencing, as indicated that plasmid had been successfully constructed.
Fig. 2.
Agarose gel electrophoresis of TLR4 RT-PCR product m: 50 bp DNA Ladder marker; 1–4: TLR4 RT-PCR product.
Creation of standard curve
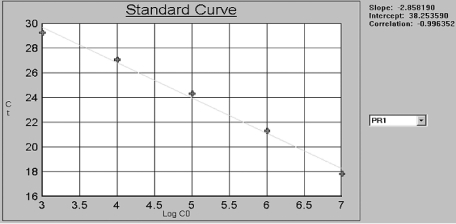
Plasmids were purified and quantified by A260 measurements, and then converted to the number of copies using the molecular weight of the DNA. To create standard curve with the plasmids DNA template, we diluted the constructed plasmids to 1 × 109, 108, 107, 106 105, 104 and 103copies/µl. By including a serial dilution such a standard in each PCR run, with known amounts of input copy number, theTLR4 gene can be quantified in the unknown samples. For each dilution, ΔRn was measured and plotted against cycle number. By plotting Ct values against the known input copy number, a standard curve is generated [11]. (Fig. 3)
Fig. 3.
Standard curve of TLR4.
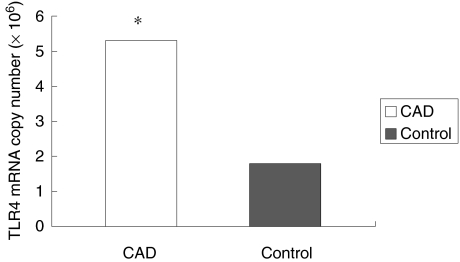
Toll-like receptor 4 mRNA was quantified by 5’nuclease assay using fluorescent-labelled TaqMan probe and analyzed using real-time quantitative RT-PCR. Correction for inefficiencies in RNA input or reverse transcriptase was performed by normalization to the housekeeping gene –β-actin. All real-time quantitative RT-PCR determinations were made in duplicate. The results showed that TLR4 mRNA copy number ranged from 7.7 × 105 ∼ 2.3 × 107 copy/µg RNA in 50 CAD patients and 9.8 × 104 ∼ 5.5 × 106 copy/µg RNA in 40 healthy controls. The mean TLR4 mRNA copy number in CAD group was significantly higher than that in controls (5.3 ± 3.8 × 106versus 1.8 ± 1.4 × 106; P < 0.01]. (Fig. 4)
Fig. 4.
The mean TLR4 mRNA copy number in CAD group was significantly higher than that in controls. *P< 0.01.
Relationship between TLR4 expression and the level of plasma lipids
There were 21 CAD patients with normal level of plasma lipids and 29 beyond the normal range. All subjects of control group had normal level of plasma lipids. It was found that the positive rates of TLR4 and the mean mRNA copy number of TLR4 in CAD group with normal level of plasma lipids were also significantly higher than that in controls(P < 0.01). But no significant difference was found in the positive rate and the mean mRNA copy number of TLR4 between CAD group with normal level of plasma lipids and the CAD group with abnormal level of plasma lipids (P > 0.05). The results were summarized in Table 2.
Table 2.
Relationship between the expression of TLR4 and plasma lipids.
| Groups | n | TLR4 positive rate | mRNA (copies/µgRNA) |
|---|---|---|---|
| CAD with normal lipids | 21 | 54.16 ± 14.6%** | (5.1 ± 4.7) × 106** |
| CAD with abnormal lipids | 29 | 56.41 ± 18.3%** | (5.4 ± 3.0) × 106** |
| Controls | 40 | 42.91 ± 15.7% | (1.8 ± 1.4) × 106 |
P < 0.01 versus control group.
Discussion
Although other studies have looked at human leucocyte expression of TLR4, this is the first study to examine how this expression may be altered in patients with coronary arteriosclerosis disease and whether the alteration may be correlated with the level of plasma lipids. The main finding of this study was that we detected significantly elevated levels of both protein and mRNA expression of TLR4 on PBMCs in CAD group. However, no significant difference was found in the positive rate and the mean mRNA copy number of TLR4 between CAD group with normal level of plasma lipids and the CAD group with abnormal level of plasma lipids. Together, these findings raise the possibility that enhanced TLR4 expression in peripheral blood may play a role in inflammation in atherosclerosis, supporting the emerging paradigm.
Although the precise pathogenesis of atherosclerosis has not been clarified now, experimental work over the past decade has linked inflammation to atherogenesis and plaque disruption [12]. Immune mechanisms, including innate immunity and acquired immunity, play important roles in the process of atherosclerosis. The family of Toll-like receptors initiates an innate immune response [13], and TLR4 is considered to be an important player in the initiation and progression of atherosclerotic disease. By now, the precise trigger for the inflammatory response is not known but may include modified lipoproteins and local or distant infections [14]. TLR4 recognize PAMPs [15,16] and activate the inflammatory cell via the NF-κB pathway [17,18]. Activation of monocytes/macrophages is an important initial step in the cascades of events leading to many inflammatory diseases, including atherosclerosis. Recent data showed that TLR4 was expressed in lipid-rich, macrophage-infiltrated atherosclerotic lesions of mice and humans [9]. These findings showed that TLR4 might be the important linker among lipids, infection/inflammation, and atherosclerosis.
TLR4 is well known as the receptor for lipopolysaccharide(LPS), a product of the outer membrane of Gram-negative bacteria [19,20]. We did not measure the LPS level, but all subjects with CAD and controls were confirmed to have normal hemogram through blood routine examination suggesting that the increased TLR4 expression possibly have no relationship with LPS. A high concentration of serum low density lipoprotein (LDL) is a major risk factor for CAD [21]. Oxidative modification of LDL makes it more atherogenic than its native form. Minimally modified LDL (mmLDL) and its oxidized phospholipids have been found to bind to CD14 or activate TLR4 on macrophages [22]. Human epidemiological studies support the involvement of CD14 and TLR4 in cardiovascular diseases. Our study showed that the expression level of TLR4 had no correlation with the level of plasmid lipids, this possibly because that we detected the native LDL, HDL and other plasma lipids which could not respond the concentration of mmLDL and oxLDL in vivo.
In summary, we observed that the expression levels of TLR4 mRNA and protein on PBMCs in patients with CAD were increased compared to healthy controls. However, we found that the positive rate and the mean mRNA copy number of TLR4 had no correlation with the level of native plasma lipids. TLR4 may be the new target of antiatherogenic therapy in the future even though we still have some problems to be improved understanding, such as the molecular mechanisms driving TLR4 overexpression and the central role of TLR4 in atherosclerosis initiation and progression.
Acknowledgments
This study was supported by grant from the China National Natural Science Foundation Council (30471616).
References
- 1.Hansson GK, Libby P, Schonbeck U, Zhong QY. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–91. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;8:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Pasterkamp G, Keulen JK, Kleijn DPV. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–34. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 6.Vink A, Schoneveld AH, van der Meer JJ, et al. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–90. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 7.Kiechl S, Wiedermann CJ, Willeit J. Toll-like receptor 4 and atherogenesis. Ann Med. 2003;35:164–71. doi: 10.1080/07853890310008215. [DOI] [PubMed] [Google Scholar]
- 8.Edfeldt K, Swedenborg J, Hansson GK, Zhong QY. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- 9.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–31. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 11.Giulietti1 A, Overbergh1 L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR. applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immumol. 2004;173:5901–7. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK. Plaque disruption and thrombosis: potential role of inflammation and infection. Cardiol Clin. 1999;17:271–81. doi: 10.1016/s0733-8651(05)70074-6. [DOI] [PubMed] [Google Scholar]
- 15.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 17.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-êB through toll like receptor 4 in cultured human dermal endothelial cells: differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–63. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 18.Dirks SH, van Deventer SJH, Peppelenbosch MP. Lipopolysaccharide recognition, internalisation, signaling and other cellular effects. J Endotoxin Res. 2002;7:335–48. [PubMed] [Google Scholar]
- 19.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in the tlr4 gene. J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4) -deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 21.George J, Afek A, Gilburd B, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis: lessons from experimental models. Lupus. 2000;9:223–7. doi: 10.1191/096120300678828190. [DOI] [PubMed] [Google Scholar]
- 22.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–8. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]