Abstract
4-1BB ligand (4-1BBL) expressed on antigen-presenting cells interacts with 4-1BB on activated T cells (especially CD8+ cells) and co-stimulates the latter to secrete cytokines and to proliferate. The role of 4-1BB–4-1BBL interaction was studied here in a model of colitis based on naive CD4+ T cell transfer to SCID mice, a disease model in which CD8 cells do not take part. We found that CD4+ T cells from 4-1BB-deficient mice, after transfer in SCID mice, proliferated more rapidly compared to wild-type CD4+ T cells. Mice reconstituted with naive CD4+ T cells from 4-1BB-deficient mice developed colitis, however, with a mixed Th1/Th2 response, in contrast to the Th1-type response in mice reconstituted with wild-type naive CD4+ T cells. Importantly, this altered cytokine response did not temper colitis severity. Although it has been reported previously that 4-1BB co-stimulation may contribute to regulatory T cell functioning, we found that CD4+CD25+ regulatory T cells from 4-1BB-deficient mice were perfectly able to prevent naive CD4+ T cell-induced colitis. In conclusion, our data provide evidence that 4-1BB–4-1BBL interaction modulates the effector CD4+ T cell-driven immune response and cytokine production in experimental colitis without affecting regulatory T cell function.
Keywords: 4-1BB, CD137, experimental colitis, interferon-gamma, interleukin-4
Introduction
4-1BB (CD137) is a 30 kDa glycoprotein of the tumour necrosis factor (TNF) receptor superfamily [1,2]. It is expressed on activated CD4+ and CD8+ T cells, activated B cells and natural killer cells, on resting monocytes and dendritic cells [3–6]. Its natural ligand, the 4-1BB ligand (4-1BBL), is a type II transmembrane glycoprotein of the TNF superfamily expressed on monocytes, macrophages, dendritic cells, B cells and on activated T cells [2,7]. 4-1BB engagement enhances expansion of T cells of both murine and human CD4+ and CD8+ T cells and activates them to secrete cytokines [interleukin (IL)-2, IL-4, interferon (IFN)-γ, tumour necrosis factor (TNF-α)] [8–10] independent of, but in synergy with, CD28 triggering [1,9]. 4-1BB is involved in T cell-dependent antibody production by B cells [11] and it promotes T cell survival [9,12]. 4-1BB triggering on dendritic cells stimulates them to produce IL-6 and IL-12 and induces IL-8 and TNF-α production in monocytes [4,6]. In vivo, studies with 4-1BBL-deficient mice and with agonistic anti-4-1BB monoclonal antibodies (mAb) demonstrated a role for 4-1BB–4-1BBL interactions in the induction and maintenance of optimal CD8+ T cell-mediated immune responses against viral infections [13,14] and tumours [15,16]. The effects of 4-1BB co-stimulation on CD4+ T cells in vivo are more controversial. Agonistic anti-4-1BB mAb optimizes the CD4+ T cell response against tumours [17,18] and reduces the suppressive function of regulatory T cells [19]. Defective CD4+ T cell priming associated with ageing can be rescued by signalling through 4-1BB [20], and transgenic non-obese diabetic (NOD) mice over-expressing a single-chain anti-4-1BB Fv on pancreatic β cells develop more severe diabetes than non-transgenic littermates [21]. Amelioration of autoimmune diseases with agonistic anti-4-1BB has been reported, presumably through anergy induction of CD4+ T cells during priming at the dendritic cell interface in lupus-prone NZB × NZW F1 mice [22] or through activation-induced cell death of autoreactive lymphocytes in experimental autoimmune encephalomyelitis and in spontaneous lupus disease in Fas-deficient MRL/lpr mice [23,24]. CD4+CD25+ regulatory T cells are important to maintain peripheral tolerance. 4-1BB is expressed constitutively on these regulatory T cells [25] and Choi et al. reported 4-1BB-dependent inhibition of activated CD4+CD25+ regulatory T cell functions in a graft-versus-host model [19]. The role of 4-1BB in vivo thus appears to be complex, and varies according to the experimental model studied.
The aim of the present study was to analyse the effects of 4-1BB–4-1BBL interaction on the induction and regulation of colitis in the T cell transfer model. In this model, SCID mice lacking mature T and B lymphocytes are reconstituted with syngeneic naive CD4+CD45RBhi T cells and consequently develop colitis which, however, can be prevented by concomitant transfer of CD4+CD45RBlo T cells, due to the presence of regulatory T cells in this latter fraction [26,27]. This model enabled us to study the effects of the 4-1BB–4-1BBL interaction specifically on CD4+ T cell-dependent inflammation in the absence of CD8 and B cells. Because we have reported previously functional expression of 4-1BB in inflamed gut tissue in Crohn's disease, an idiopathic chronic inflammatory bowel disease in humans [28], the present study on 4-1BB–4-1BBL interactions was also intended to clarify its potential role in the pathogenesis of Crohn's disease.
Materials and methods
Reagents and mice
The mAb used in this study include anti-mouse CD3ɛ mAb [clone 500A2, hamster immunoglobulin (Ig) G], phycoerythrin (PE)-conjugated anti-mouse CD4 mAb (clone GK1·5, rat IgG2b), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD25 mAb (clone 7D4, rat IgM, κ) and FITC-conjugated anti-mouse CD45RB mAb (clone 16 A, rat IgG2a) (PharMingen, San Diego, CA, USA). Purified rat anti-mouse-4-1BB mAb (clone 3H3, rat IgG2a) to perform immunohistochemistry and for in vivo use was a kind gift from Robert Mittler, Department of Surgery and Emory Vaccine Center, Emory University School of Medicine, Atlanta, USA. Peroxidase-labelled rabbit anti-rat Ig and swine anti-rabbit Ig were purchased from Dako (Glostrup, Denmark). Rat IgG2a mAb as an isotype control was purified from cultures supernatants of the hybridoma GL117 secreting anti-α-galactosidase of Escherichia coli (a gift from H. Heremans, Rega Instituut, Leuven, Belgium).
Specific pathogen-free female Balb/c mice were obtained from Harlan (Horst, the Netherlands). Specific pathogen-free female 4-1BB–/– Balb/c mice were generated as reported previously [29]. Specific pathogen-free female C.B-17 SCID mice were obtained from the breeding facility of the REGA Institute (University of Leuven, Leuven, Belgium). All mice were maintained in the animal care facility of the faculty of medicine, Catholic University of Leuven in micro-isolator cages with filtered air and free access to autoclaved food and water. SCID mice were 6 weeks of age when experiments were initiated. All studies were approved by the local ethical committee of the Catholic University of Leuven.
SCID colitis model
Spleen cells from wild-type or 4-1BB–/– Balb/c mice were used as a source of CD4+ cells and separated further into a CD45RBhi and CD45RBlo fraction under sterile conditions by two-colour sorting on a FACS Vantage (Becton Dickinson, CA, USA), as described previously [30]. For the experiments with Treg cells, CD4+ T cells were stained with FITC-conjugated anti-CD25 mAb and PE-conjugated anti-CD45RB mAb and fractionated on a FACSVantage into CD4+CD45RBhighCD25– and CD25+CD4+ T (with low to intermediate CD45RB expression) (Treg) cells. All populations were > 98% pure on re-analysis. To induce colitis, SCID mice were injected intraperitoneally (i.p.) with sorted syngeneic wild-type or 4-1BB–/– CD45RBhiCD4+ T cells (4 × 105 cells/mouse). Some mice were injected i.p. with wild-type CD45RBhi (4 × 105 cells/mouse) plus wild-type or 4-1BB–/– CD45RBlo (2 × 105) CD4+ T cells. Some mice were injected i.p. with total CD4+ T cells (5 × 105 cells/mouse) from wild-type or 4-1BB–/– mice. Disease activity was monitored weekly on the basis of body weight and soft stool or diarrhoea and experiments were abrogated 8 weeks after T cell transfer.
Histological examination
Colon tissue samples were collected at the end of the experiments and fixed in 6% formalin. Paraffin-embedded sections (5 µm) were cut and stained with haematoxylin and eosin. The sections were analysed without prior knowledge of the type of T cell reconstitution or treatment. Gut inflammation was scored, using a previously described score system [30].
Immunohistochemistry
Colon tissue and mesenteric lymph nodes (MLN) were harvested from SCID mice 8 weeks after reconstitution with CD4+ T cells as indicated. Cryostat sections (5 µm) were cut and stained immunohistochemically for the presence of 4-1BB. All procedures were conducted at room temperature. Briefly, cryostat sections were air-dried overnight, fixed in acetone for 10 min and rinsed in phosphate-buffered saline (PBS). Sections were then incubated in dual endogenous enzyme-blocking reagent (DakoCytomation, Glostrup, Denmark; code S2003) for 10 min, washed in PBS and incubated for 45 min with rat anti-mouse 4-1BB mAb (25 µg/ml). Sections were rinsed with PBS and incubated for 30 min with peroxidase-labelled rabbit anti-rat Ig (1/75 dilution). After washing with PBS the sections were incubated with peroxidase-labelled swine anti-rabbit Ig (1/100 dilution) for 30 min. Sections were rinsed in PBS. The reaction product was developed during 10 min using a solution of 50 mg 3-amino-9-ethylcarbazole and 5 ml dimethylformamide in 100 ml acetate buffer (0·05 M, pH 4·9) to which 30% H2O2 was added. After colour development, sections were rinsed in acetate buffer for 5 min and were counterstained with Mayer's haematoxylin for 1 min. After wash with distilled H2O, sections were mounted in glycerol medium (BDH, Dorset, UK). As a negative control, the same procedure was performed with rat IgG2a as a primary antibody. The number of positively staining cells was counted with a calibrated graticule in the eyepiece of an Axiolab Zeiss microscope (G. De Hertogh and K. Geboes). Four high-power fields (HPF) (total magnification × 400) focused on lamina propria in the four quadrants of a transversal section of the colon were selected for counting of 4-1BB-expressing cells among mononuclear cells. One HPF consists for about 50% of lamina propria, the rest being crypt epithelium. In MLN, 5 HPF of the four quadrants and the centre of one biopsy section were selected. Only mononuclear cells were counted, with the exclusion of epithelial cells.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Colonic tissue samples (from diseased areas in case of colitis or randomly in the absence of macroscopic colitis) were obtained from SCID mice at the end of the experiments as indicated. All samples were frozen immediately in liquid nitrogen after dissection and stored at −80°C until use. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Leusden, the Netherlands) according to the manufacturer's instructions. A constant amount of 2 µg target RNA was used for cDNA synthesis (ready-to-use kit; Pharmacia, Uppsala, Sweden). After 90 min at 37°C, the reverse transcriptase was inactivated by incubating the cDNA samples for 5 min at 95°C. The cDNA samples were then subjected to real-time quantitative RT-PCR, performed in the ABI prism 7700 sequence detector (Applied Biosystems, Foster City, CA, USA) as described previously [31]. The sequences of the primers and probes for murine IFN-γ, TNF-α, IL-10 and β-actin have been reported previously [31]. All primers and probes were designed with the assistance of the computer program Primer Express (AB) and purchased from Eurogentec (Seraing, Belgium) or Applied Biosystems. The 5′-nuclease activity of the Taq polymerase was used to cleave a non-extendable dual-labelled fluorogenic probe. Fluorescent emission was measured continuously during the PCR reaction. PCR amplifications were performed in a total volume of 25 µl containing 5 µl cDNA, 12·5 µl universal PCR Master Mix without AmpErase® UNG (AB), 100–300 nM concentrations of each primer and 200 nM concentrations of the corresponding detection probe. Each PCR amplification was performed in triplicate wells using the following conditions: 94°C for 10 min, followed by 40 or 45 cycles at 94°C for 15 s and 60°C for 1 min cDNA plasmid standards, consisting of purified plasmid DNA specific for each individual target, were used to quantify the target gene in the unknown samples, as described [31]. All results were normalized to β-actin to compensate for differences in the amount of cDNA in all samples.
Isolation and culture of lamina propria mononuclear cells (LPMC)
LPMC were harvested from the colon of T cell-reconstituted SCID mice, as reported previously [30]. The isolated LPMC were diluted into culture medium at a final concentration of 5 × 105 viable cells in 1 ml (24-well plates) and incubated at 37°C in 5% CO2 humidified air. The culture medium was RPMI-1640 solution (Bio-Whittaker, Verviers, Belgium) supplemented with 0·3 mg/ml l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Bio-Whittaker, Verviers, Belgium) and 10% iron-supplemented fetal calf serum (HyClone, Logan, UT, USA). Cells were stimulated with soluble anti-mouse CD3ɛ (5 µg/ml) and mouse CD80-transfected mitomycin C-treated (50 µg/ml) P815 cells (5 × 105 in 1 ml). Supernatants were collected after 48 h for cytokine measurement.
Cytokine assay
Measurements of IFN-γ and IL-4 production levels in the supernatants of LPMC cultures were performed by sandwich ELISA, using matched mAb pairs according to the manufacturer's instructions (BioSource International, Fleurus, Belgium).
Carboxyfluorescein succinimidyl ester (CFSE) labelling of T cells
T cell proliferation in vivo was assessed by flow cytofluorometry of CFSE (Molecular Probes, Eugene, OR, USA) labelled cells. CD4+ T cells were isolated from spleens of wild-type and 4-1BB–/– mice and resuspended in RPMI-1640 at 50 × 106 cells/ml; 110 µl of 50 µM CFSE solution was added to one ml of cells and mixed rapidly. After 5 min at room temperature, cells were washed three times with PBS containing 5% fetal calf serum (FCS), resuspended in culture medium and 5 × 106 wild-type or 4-1BB–/– CFSE-labelled CD4+ T cells were injected i.p. into 6-week-old SCID mice. As negative and positive controls, unstained and stained CD4+ T cells were kept in medium for 24 h to analyse CFSE uptake. SCID mice were killed 5 days after reconstitution and cells were harvested from the peritoneal cavity and stained with PE-conjugated anti-CD4. CFSE intensity in a gate for CD4+ T cells was assessed with flow cytofluorometry.
Statistics
Statistical analysis was performed with GraphPad Prism, version 3 (San Diego, CA, USA). The non-parametric Mann–Whitney U-test was used. Statistical significance was established at P < 0·05.
Results
Proliferation of 4-1BB–/–CD4+ T cells in the peritoneal cavity
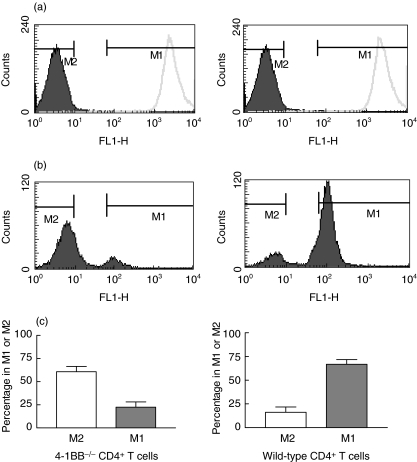
In lymphopenic mice, T cells undergo vigorous expansion as a compensatory response to lymphopenia [32]. We explored whether 4-1BB affects CD4+ T cell expansion in vivo after transfer to SCID mice. Wild-type and 4-1BB–/– CD4+ T cells were labelled with the cytoplasmic dye CFSE and transferred into SCID mice. At day 5 after transfer, no CD4+ T cells could be harvested from the spleen, suggesting that at that time-point, cells had not yet migrated in abundance to the periphery. Among T cells recovered from the peritoneal cavity at day 5, 66·8 ± 4·9% wild-type CD4+ T cells had retained high CFSE expression, suggesting that these had undergone no or only few cell divisions (M1) (Fig. 1). In contrast, 60·8 ± 5·6% of 4-1BB–/–CD4+ T cells recovered from the peritoneal cavity were CFSE negative and thus had undergone repeated cell divisions (M2) (Fig. 1). The differences were highly significant (P = 0·0079), and show that in the absence of 4-1BB, CD4+ T cells proliferate more rapidly in a lymphopenic host.
Fig. 1.
In vivo homeostatic CD4+ T cell proliferation in the peritoneal cavity after transfer to SCID mice. CD4+ T cells were isolated from spleen cells of wild-type and 4-1BB–/– mice and stained with the cytoplasmic dye carboxyfluorescein succinimidyl ester (CFSE). (a) Unstained and CFSE-stained CD4+ T cells from 4-1BB–/– mice (left) and wild-type mice (right) as a negative and positive control, respectively. CFSE-stained cells were then injected intraperitoneally (i.p.) into SCID mice. At day 5, T cells were recovered from the peritoneal cavity and CFSE expression was analysed by flow cytofluorometry. (b) Representative FACS plots are shown; (c) summary bar graph representing the mean percentage plus s.e.m. of cells in the M1 and M2 area are shown (five mice per group). M1: cells which have undergone no or only few cell divisions; M2: cells which have undergo repeated cell divisions.
4-1BB is expressed in colonic tissue and mesenteric lymph nodes (MLN) in T cell-reconstituted SCID mice
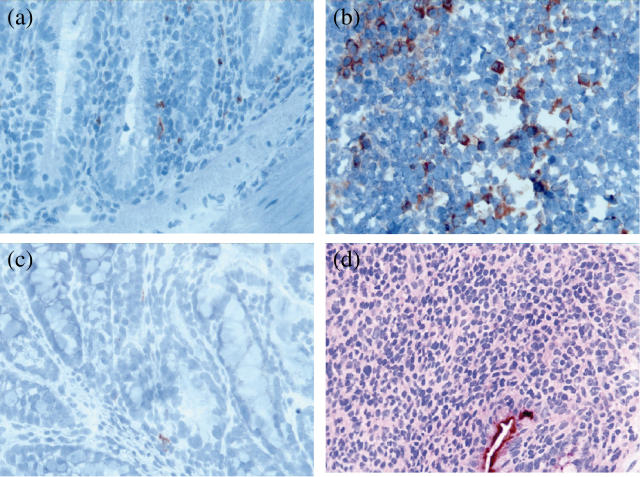
The in situ expression of 4-1BB in colonic tissue and MLN from wild-type and 4-1BB-deficient CD45RBhiCD4+ T cell-reconstituted mice was studied. 4-1BB positive mononuclear cells were seeded in the lamina propria (4·6 ± 1·1 4-1BB positive cells/HPF) in colon tissue from wild-type CD45RBhiCD4+ T cell-reconstituted mice (n = 13) (Fig. 2a). MLN from wild-type CD45RBhiCD4+ T cell-reconstituted mice were enlarged compared to those from CD45RBhi+loCD4+ T cell-reconstituted mice. As shown in Fig. 2b, 4-1BB positive mononuclear cells were present in the MLN from wild-type CD45RBhiCD4+ T cell-reconstituted SCID mice (7·2 ± 1·5 4-1BB positive cells/HPF, n = 11), and they had migrated preferentially to the cortex where they were organized as focal agglomerates. 4-1BB-positive mononuclear cells were rare (1·2 ± 0·4 4-1BB positive cells/HPF, n = 5) in colon tissue from 4-1BB–/– CD45RBhiCD4+ T cell-reconstituted mice (Fig. 2c). The 4-1BB positive cells might be of host origin, as other than CD4+ T cells can also express 4-1BB [3–6]. In the negative control staining, no positive cells were found (Fig. 2d).
Fig. 2.
4-1BB expression in colon and mesenteric lymph nodes of wild-type CD45RBhiCD4+ T cell-reconstituted SCID mice. Microphotographs of cryostat sections of colons (a) and mesenteric lymph nodes (b) from wild-type CD45RBhiCD4+ T cell-reconstituted mice were stained with anti-mouse 4-1BB monoclonal antibodies (mAb) and peroxidase-labelled rabbit anti-rat and swine anti-rabbit Ig. (c) Microphotograph of a cryostat section of colon of a 4-1BB-deficient CD45RBhiCD4+ T cell-reconstituted SCID mice and stained as in (a) and (b). (d) Microphotograph of a cryostat section of colon from wild-type CD45RBhiCD4+ T cell-reconstituted SCID mice stained in the presence of a control primary antibody (original magnification × 400).
4-1BB–/–CD45RBhiCD4+ T cell-reconstituted SCID mice develop severe colitis
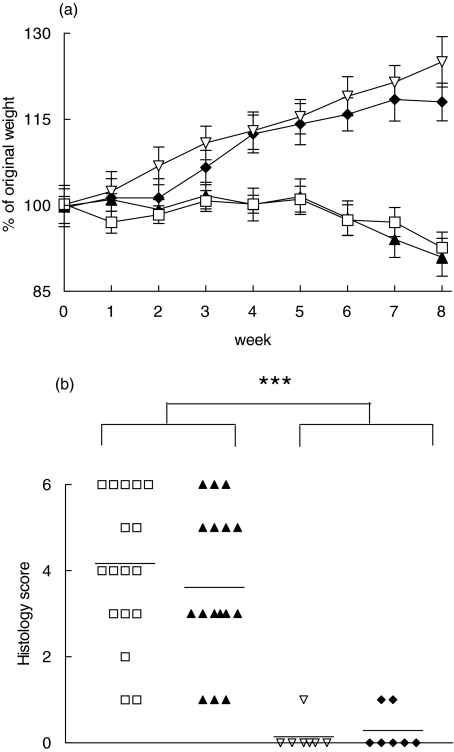
To study further the functional consequences of 4-1BB–4-1BBL co-stimulation in experimental colitis, SCID mice reconstituted with CD45RBhiCD4+ T cells from either wild-type or 4-1BB-deficient mice were monitored weekly up to 8 weeks. Data from two independent experiments were pooled. SCID mice reconstituted with wild-type or 4-1BB–/–CD45RBhiCD4+ T cells both developed progressive weight loss starting around 5 week after reconstitution, as shown in Fig. 3a. Macroscopically, the colon was enlarged and had a greatly thickened wall in both wild-type and 4-1BB–/– CD45RBhiCD4+ T cell-reconstituted mice. On microscopy, the cellular inflammatory infiltrate was composed of large numbers of lymphocytes. Neutrophils were common in ulcerative areas. Epithelial lesions included ulceration, occasionally with a mountain-peak appearance, and less severe lesions such as mucin depletion, loss of goblet cells and crypt abscesses. Architectural changes included crypt hyperplasia, crypt elongation and villous transformation of the surface. Features and severity of inflammation were not different in wild-type and 4-1BB–/– CD45RBhiCD4+ T cell-reconstituted SCID mice (mean score 3·61 ± 0·40 and 4·17 ± 0·41, respectively) (Fig. 3b).
Fig. 3.
Comparison of disease activity after transfer of CD45RBhi and CD45RBlo CD4+ T cells from wild-type or 4-1BB–/– mice to SCID recipients. SCID mice were reconstituted with either wild-type or 4-1BB–/– CD45RBhiCD4+ T cells, or with wild-type CD45RBhiCD4+ T cells plus wild-type or 4-1BB–/– CD45RBloCD4+ T cells as indicated. Data from two independent experiments were pooled. (a) The change of weight over the observation time is expressed as a percentage of the original weight at the start of the experiment. Data represent the mean ± s.e.m. (b) Histological scores of colonic sections. Data represent the individual score for each mouse. ***P < 0·001. (a) ♦, CD45RBhi + 4-1BB+/+ CD45RBlo T cells (n = 7); ▿, CD45RBhi + 4-1BB-/- CD45RBlo T cells (n = 7); ▴, 4-1BB+/+ CD45RBhi T cells (n = 17); □, 4-1BB-/- CD45RBhi T cells (n = 18). (b) □, 4-1BB-/- CD45RBhi T cells (n = 18); ▴, 4-1BB+/+ CD45RBhi T cells (n = 17); ▿, CD45RBhi + 4-1BB-/- CD45RBlo T cells (n = 7); ♦, CD45RBhi+ + 4-1BB+/+ CD45RBlo T cells (n = 7).
4-1BB co-stimulation influences the CD4+ T cell cytokine response in the inflamed lamina propria
To study the cytokine response in mice with colitis, colon tissue was harvested from SCID mice 8 weeks after reconstitution with either wild-type or 4-1BB–/– CD45RBhiCD4+ T cells. Levels of cytokine mRNA in colonic tissue were determined by real-time quantitative RT-PCR. Data from two independent experiments were pooled. As shown in Table 1, mRNA levels of IFN-γ were elevated in wild-type CD45RBhiCD4+ T cell-reconstituted SCID mice, compared to wild-type CD45RBhi+loCD4+ T cell-reconstituted SCID mice which do not develop colitis (see below). In contrast, mRNA levels of IL-10 were significantly lower in wild-type CD45RBhiCD4+ T cell-reconstituted mice, compared to wild-type CD45RBhi+loCD4+ T cell-reconstituted mice (P < 0·0029). The latter is in accordance with the role ascribed to IL-10 in the prevention of colitis induction [33]. IFN-γ mRNA levels were significantly lower and IL-10 mRNA levels significantly higher in 4-1BB–/–CD45RBhiCD4+ T cell-reconstituted mice compared to wild-type CD45RBhiCD4+ T cell-reconstituted mice (P < 0·05). In fact, IL-10 mRNA levels in these mice were comparable to those of wild-type CD45RBhi+loCD4+ T cell-reconstituted mice. To explore further these differences in cytokine production, we isolated LPMC from wild-type and 4-1BB–/– CD45RBhiCD4+ T cell-reconstituted mice and analysed the Th1/Th2 cytokine production (IFN-γ and IL-4) in LPMC cultures. As shown in Table 2, the secretion of the Th2 type cytokine IL-4 was higher in the culture supernatants of 4-1BB–/– compared to wild-type CD45RBhiCD4+ T cell-reconstituted mice (P < 0·01). The IFN-γ : IL-4 ratio in the supernatants from LPMC cultures was significantly lower in 4-1BB–/–CD45RBhiCD4+ T cell-reconstituted mice compared to wild-type CD45RBhiCD4+ T cell-reconstituted mice (36 ± 14 versus 142 ± 52, P = 0·026). Together these data show that CD45RBhiCD4+ T cells from 4-1BB–/– mice, after transfer into SCID mice, produce a mixed Th1/Th2 cytokine pattern in the colon lamina propria to which they are recruited, while transferred wild-type CD45RBhiCD4+ T cells produce a selective Th1 cytokine pattern.
Table 1.
Cytokine mRNA level (cytokine : β actin ratio) in the colon of SCID mice reconstituted with CD4+ T cell subsets1.
| Cytokine mRNA | |||
|---|---|---|---|
| Type of CD4+ T cell reconstitution | IFN-γ | TNF-α | IL-10 |
| 4-1BB–/–CD45RBhi (n = 16) | 241 ± 41†** | 490 ± 104 | 43 ± 14† |
| 4-1BB+/+CD45RBhi (n = 16) | 598 ± 151** | 326 ± 59 | 11 ± 4** |
| CD45RBhi+ CD45RBlo (n = 6) | 17 ± 13 | 225 ± 52 | 50 ± 9 |
mRNA levels of cytokines in colonic samples from all groups were analysed using quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR). The numbers represent the ratio of cDNA copy number for the cytokine divided by the cDNA copy number for β-actin and multiplied by 106 (mean ± s.e.m.). Data from two independent experiments are pooled.
P < 0·05 versus 4-1BB+/+CD45RBhiCD4+ T cell-reconstituted mice;
P < 0·01 versus CD45RBhi+ CD45RBloCD4+ T cell-reconstituted mice.
Table 2.
Cytokine secretion by lamina propria mononuclear cell cultures1.
| Type of CD4+ T cell reconstitution | IFN-γ pg/ml | IL-4 pg/ml | IFN-γ : IL-4 ratio |
|---|---|---|---|
| 4-1BB–/–CD45RBhi (n = 18) | 5017 ± 855 | 420 ± 80** | 36 ± 14* |
| 4-1BB+/+CD45RBhi (n = 17) | 6461 ± 1314 | 113 ± 20 | 142 ± 52 |
Lamina propria mononuclear cells (LPMC) were isolated from colonic tissues from SCID mice reconstituted with CD4+ T cell subsets as indicated. LPMC (5 × 105 in 1 ml) were stimulated with anti-CD3ɛ (5 µg/ml) and mouse CD80 transfectants (5 × 105/ml). Supernatants were harvested after 48 h of culture and assayed for interferon (IFN)-γ and interleukin (IL)-4 by enzyme-linked immunosorbent assay (ELISA). Data are pooled from two experiments and expressed as the mean ± s.e.m.
P < 0·05;
P < 0·01 versus 4-1BB+/+CD45RBhiCD4+ T cell-reconstituted mice.
4-1BB–4-1BBL interaction is not involved in CD4+CD25+ regulatory T cell function in the T cell transfer model
Gut inflammation induced by CD45RBhiCD4+ T cells can be prevented by concomitant reconstitution with CD45RBloCD4+ T cells, due to the activity of a CD4+CD25+ regulatory T cell population in this latter subset [26,27]. Because recent data suggest a role for 4-1BB in the function of CD4+CD25+ regulatory T cells [19,34], we studied whether 4-1BB is important for the function of regulatory T cells in this colitis model. First, we compared the ability of wild-type and 4-1BB–/– CD45RBloCD4+ T cells to prevent colitis in wild-type CD45RBhiCD4+ T cell-reconstituted SCID mice. As shown in Fig. 3a, SCID mice reconstituted with either 4-1BB–/– or wild-type CD45RBloCD4+ T cells plus wild-type CD45RBhiCD4+ T cells appeared healthy, with a gradual increase of body weight and absence of diarrhoea during the period of observation. Macroscopically the colon appeared normal at the time of dissection and microscopically only few lymphocytes were observed in the lamina propria, without any difference in the inflammatory score (histology score: 0·29 ± 0·18 versus 0·14 ± 0·14, respectively, for wild-type and 4-1BB–/–CD45RBloCD4+ T cells, n = 7) (Fig. 3b). Additionally, SCID mice reconstituted with total CD4+ T cells from 4-1BB-deficient or from wild-type mice also showed gradual increases of body weight, and no detectable pathological changes in the intestine were seen (data not shown).
Discussion
Using a chronic colitis model based on naive CD4+ T cell transfer to SCID mice, we report here that 4-1BB–4-1BBL interaction modulates CD4+ T cell effector functions. We were able to show that 4-1BB was expressed on mononuclear cells in inflamed colon tissue and MLN from reconstituted SCID mice, despite the absence of CD8 cells. To gain further insight into the role of 4-1BB on CD4+ T cells, we reconstituted SCID mice with either wild-type or 4-1BB–/– CD45RBhiCD4+ T cells. All mice developed a wasting disease with diarrhoea due to colitis. This indicates that the induction of the effector response of CD45RBhiCD4+ T cells and their homing to the lamina propria is 4-1BB independent. Wild-type CD45RBhiCD4+ T cell-reconstituted mice developed colitis with a Th1 profile, as evidenced by high levels of IFN-γ mRNA in colon tissue and secretion of high amounts of IFN-γ in LPMC cultures. Interestingly, 4-1BB–/–CD45RBhiCD4+ T cell-reconstituted mice had significantly lower levels of IFN-γ mRNA in colon tissue compared to wild-type CD45RBhi CD4+ T cell-reconstituted mice. Moreover, LPMC harvested from 4-1BB–/–CD45RBhiCD4+ T cell-reconstituted mice produced more IL-4 when stimulated in vitro compared to wild-type CD45RBhiCD4+ T cell-reconstituted mice, thus resulting in a significantly different IFN-γ : IL-4 ratio. These data suggest that 4-1BB–4-1BBL interaction is important to balance Th1/Th2 cytokine production in CD4+ T cell-driven inflammation. More importantly, however, these data indicate that the cytokine profile is not important for the outcome of the disease severity. Indeed, weakening a Th1 response by tilting the balance towards a Th2 response did not attenuate colitis, as disease severity was not different in wild-type and 4-1BB-deficient CD45RBhiCD4+ T cell-reconstituted SCID mice. This observation is in accordance with recent data showing that Th1 as well Th2 differentiated CD4+ T cells are able to induce colitis in the SCID model [35,36].
IL-10 is required for prevention of colitis by regulatory T cells in this model [33]. However, IL-10 is apparently not sufficient to prevent colitis. Indeed, 4-1BB–/–CD45RBhiCD4+ T cell-reconstituted mice had significantly higher IL-10 mRNA levels in colon tissue compared to wild-type CD45RBhiCD4+ T cell-reconstituted mice. Interestingly, IL-10 mRNA levels in tissue were even similar to those observed in wild-type CD45RBhi+loCD4+ T cell-reconstituted mice. The fact that high levels of IL-10 mRNA are not sufficient to prevent colitis may perhaps be explained by the observation that, besides IL-10, TGF-β is also needed to prevent colitis in SCID mice [37,38]. CD4+CD25+ regulatory T cells express 4-1BB constitutively [25] and 4-1BB co-stimulation augments the proliferation of CD4+CD25+ regulatory T cells [34]. The SCID colitis model is eminently suitable to study regulatory T cells, as mentioned above. We studied the suppressive capacity of regulatory T cells harvested from 4-1BB-deficient mice. Similar to CD45RBhi plus 4-1BB+/+CD45RBloCD4+ T cell-reconstituted mice, SCID mice reconstituted with CD45RBhi plus 4-1BB–/–CD45RBloCD4+ T cells increased in weight over time and had no histological features of colon inflammation. Thus, 4-1BB–4-1BBL interaction is not essential for regulatory T cell function in this model. This contrasts to observations in a graft-versus-host model [19] and points to the complexity and heterogeneity of regulatory T cell control mechanisms.
Our data in the colitis model might be relevant for elucidating the potential role of 4-1BB in the pathogenesis of Crohn's disease. We have reported previously functional expression of 4-1BB in Crohn's disease [28]. Interestingly, expression in ulcerative colitis was much lower compared to Crohn's disease. Instead, other members of the TNF/TNFR superfamily such as CD40/CD154 and OX40/OX40L are all expressed equally in both types of inflammatory bowel disease [39]. Crohn's disease is characterized by a Th1 type of inflammation [40]. In contrast, ulcerative colitis is characterized by high IL-10 production [41]. This phenomenon could be influenced at least in part by 4-1BB, as shown by our data in this experimental model of colitis. Differences in 4-1BB expression in Crohn's disease versus ulcerative colitis may be important in the different cytokine expression pattern and clinical presentation of both diseases.
In conclusion, our data support recent observations that 4-1BB–4-1BBL interaction is involved in CD4+ T cell-mediated immune responses [17,22,24]. The picture, however, remains complex. In an in vivo model, 4-1BB–/– CD4+ T cells display enhanced cell division and clonal expansion during a primary response resulting in a more enhanced effector CD4+ T cell response to ovalbumin (OVA) [42]. The authors suggested that 4-1BB triggering might thus have a down-regulatory role on CD4+ T cells, by limiting their clonal expansion. Our results are in accordance with these findings, as we also showed some enhanced activities of CD45RBhiCD4+ T cells from 4-1BB-deficient mice after transfer to SCID mice. This was manifested by enhanced homeostatic-type proliferation and Th2-type cytokine production, with higher IL-4 and IL-10. Down-regulation of CD4+ T cells by 4-1BB triggering might also help to explain the beneficial effects of agonistic anti-4-1BB mAb in several autoimmune disease models [22–24]. A further exploration of these potential down-regulatory effects of 4-1BB triggering on CD4+ T cell function seems warranted.
Acknowledgments
We thank M. Adé and E. Dilissen for expert technical assistance in performing ELISA and RT-PCR. We thank W. Scheers and V. Van Duppen for their help with cell sorting and Christel Vandenbroeck for performing IHC. We thank Dr R. Mittler for providing us with anti-4-1BB mAb and Dr H. Heremans for providing us with rat IgG2a mAb. This work was supported by a grant from the Foundation for Scientific Research Flanders (G.0080·02) by a grant from the Research Council of the Catholic University of Leuven (OT 02/43) and a Schering-Plough IBD fellowship to Philippe Maerten. B. S. Kwon was supported by the SRC Fund to the IRC University of Ulsan from KOSEF and the Korean Ministry of Sciences and technology, and NIH grants RO1EY, 13325 and P30EY002377.
References
- 1.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 2.Alderson MR, Smith CA, Tough TW, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–27. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85:1043–52. [PubMed] [Google Scholar]
- 4.Kienzle G, von Kempis J. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int Immunol. 2000;12:73–82. doi: 10.1093/intimm/12.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–7. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin RG, Din WS, Davis-Smith T, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB. a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–41. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Kim SH, Mantel P, Kwon BS. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses. Eur J Immunol. 1998;28:881–90. doi: 10.1002/(SICI)1521-4141(199803)28:03<881::AID-IMMU881>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–24. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 10.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 11.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–40. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–9. [PubMed] [Google Scholar]
- 13.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–68. [PubMed] [Google Scholar]
- 14.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–85. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 15.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 16.Taraban VY, Rowley TF, O'Brien L, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–27. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174–83. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 18.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 19.Choi BK, Bae JS, Choi EM, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–91. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 20.Bansal-Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137) J Immunol. 2002;169:5005–9. doi: 10.4049/jimmunol.169.9.5005. [DOI] [PubMed] [Google Scholar]
- 21.Sytwu HK, Lin WD, Roffler SR, et al. Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun. 2003;21:247–54. doi: 10.1016/s0896-8411(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 22.Foell J, Strahotin S, O'Neil SP, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. J Clin Invest. 2003;111:1505–18. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Chen HM, Subudhi SK, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002;8:1405–13. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Lin X, Chen HM, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:1457–65. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 25.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+) CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 26.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 SCID mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 27.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+) CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maerten P, Geboes K, Hertogh GD, et al. Functional expression of 4-1BB (CD137) in the inflammatory tissue in Crohn's disease. Clin Immunol. 2004;112:239–46. doi: 10.1016/j.clim.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–90. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Geboes K, Colpaert S, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40–CD154 interactions. J Immunol. 2000;164:6005–14. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- 31.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 32.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+) CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 33.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:2428–34. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 35.Xu D, Liu H, Komai-Koma M, et al. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 36.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Kitani A, Fuss I, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 39.Maerten P, Liu Z, Ceuppens JL. Targeting of costimulatory molecules as a therapeutic approach in inflammatory bowel disease. Biodrugs. 2003;17:395–411. doi: 10.2165/00063030-200317060-00003. [DOI] [PubMed] [Google Scholar]
- 40.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 41.Melgar S, Yeung MM, Bas A, et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–37. doi: 10.1046/j.1365-2249.2003.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Vella AT, Kwon BS, Croft M. Enhanced CD4 T Cell responsiveness in the absence of 4-1BB. J Immunol. 2005;174:6803–8. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]