Abstract
Human visceral leishmaniasis (VL), also known as kala azar (KA) in India, is a systemic progressive disease caused by Leishmania donovani. In VL, Th1 responses correlate with recovery from and resistance to disease and resolution of infection results in lifelong immunity against the disease. However, recent data suggest an important role for interleukin (IL)-10 in maintaining the resistant state. We evaluated whole cell extract (WE) and 11 antigenic fractions [F1–F11, molecular weight (MW) range of 139–24·2 kDa] from L. donovani (2001 strain, a fresh field isolate from Bihar), for their ability to induce in vitro T cell proliferation and production of interferon (IFN)-γ, interleukin (IL)-12, IL-10 and IL-4 by peripheral blood mononuclear cells (PBMCs) of exposed immune individuals (14 patients with history of VL, 10 household endemic contacts) and 20 non-endemic healthy controls. Twenty-one of 24 exposed individuals and no healthy controls showed proliferative response to WE. Whole-extract activated IFN-γ, IL-12, IL-10 levels were higher in the exposed group than in controls; IL-4 was not detectable in any of the samples. Among 21 responders to WE, frequent proliferative responses were seen to fractions F1–F4 (MW > 64·2 kDa) and none to fractions F5–F11; fractions F1–F11 stimulated comparable levels of IFN-γ and IL-12 while IL-10 levels were higher in response to F5–F11 compared to F1–F4. These data demonstrate the presence of immunostimulatory antigens in the high MW fractions of whole L. donovani antigen. However, these fractions do not stimulate a Th1 response and produce variable amounts of IFN-γ and the regulatory cytokine, IL-10. Hence, these high MW immunostimulatory fractions need to be evaluated in greater depth for their possible role as protective antigens.
Keywords: cytokines, immune response, T cell proliferation, vaccination, visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL) is endemic in the Indian subcontinent and is a cause of significant morbidity and mortality [1]. Although limited treatment options are available, there is much interest in the development of an effective immunoprophylactic agent for VL [2].
The control of leishmanial infection is mediated by Th1 type immune response [3], and experimental studies in murine models of cutaneous leishmaniasis (CL) have established a clear-cut dichotomy between Th1-mediated protection and Th2-mediated disease susceptibility [4]. As in CL, Th1 responses contribute to resistance and protection in canine visceral disease [5,6]; however, the Th1/Th2 dichotomy is less well defined in murine models of VL [7,8]. The majority of human leishmanial infections are subclinical or self-limited and once infected, individuals develop long-term immunity and are protected against reinfection [9]. The Th1/Th2 dichotomy is also observed in human CL; however, immune response defining disease versus protection is not so well established in human VL [10]. Interferon (IFN)-γ and interleukin (IL)-12, the signature cytokines for Th1 responses, are decreased during acute VL [11]. These responses persist at high levels after successful treatment and are accompanied by high IL-10 levels. Recently, IL-10 has been suggested to play a role in counterbalancing the exacerbated polarized response that may develop following cure [12].
Based on the current understanding of immune responses in this infection, leishmanial antigens that predominantly stimulate Th1 responses are considered as ‘potential protective antigens’ and antigens that predominantly stimulate a Th2 response to be associated with pathology. However, it has been observed that several leishmanial antigens that induce Th1 response during infection do not provide protection in vaccination studies [13–18]. Hence, it may not be appropriate to use only stimulation of Th1 responses as readouts for antigen selection in vaccine development against leishmaniasis. Studies have evaluated systematically biochemically purified proteins and whole parasite lysates for potential immunostimulatory activity [19–24]. However, selections of these molecules had an element of bias, because their identification was based either on reactivity with immune sera or were from the soluble protein fraction. The present study was undertaken to evaluate the nature of the immune response of treated cured patients and contacts of a fresh field isolate of Leishmania donovani to the total parasite antigen and its 11 antigenic fractions separated on the basis of molecular weight (MW).
Materials and methods
Culture of parasites
L. donovani strain 2001, isolated from an Indian kala azar patient, was maintained in golden hamsters as described previously [25]. Amastigotes from spleen of infected animals were cultured in L-15 medium (Gibco-Invitrogen, Grand Island, NJ, USA) at 25°C, with 10% heat-inactivated fetal calf serum (FCS) (Gibco-Invitrogen), 0·3% tryptose phosphate broth (Himedia, Mumbai, India) and gentamycin (40 mg/l; Sigma, St. Louis, USA). Promastigotes were grown to stationary phase, harvested by centrifugation and washed in ice-cold phosphate-buffered saline (PBS) before use.
Study groups
Twenty-four people from a kala azar hyperendemic region in Bihar were enrolled for the study. Fourteen individuals [seven males; median age 16 (range 5–30 years)] were diagnosed, treated and cured patients of VL, confirmed by demonstration of parasites in splenic aspirates. All patients had received a full course of either amphotericin B, miltefosine or parmomycin. At the time of blood collection, these patients had no medical complaints and spleen was not palpable on physical examination. Samples were collected 6 months after completion of treatment in nine patients (amphotericin = 5, miltefosine = 2, parmomycin = 2) and 3 months in the other five patients (amphotericin = 2, miltefosine = 1, parmomycin = 2). Ten individuals [five males; median age 29 (range 6–33 years)] were asymptomatic household contacts of diagnosed cases. They showed no clinical symptoms nor received any anti-kala azar treatment. Twenty healthy adult donors [10 males; median age 26 (range 15–30 years)] from a non-endemic region served as controls. Blood was drawn after informed consent was obtained from each subject for peripheral blood mononuclear cells (PBMC) and serum separation. Serum was stored at −80°C in small aliquots for cytokine determination.
Preparation of antigens from L. donovani promastigotes
Whole cell extract (WE)
Stationary-phase promastigotes were harvested by centrifugation and the pellet was dissolved in sodium dodecyl sulphate (SDS) extraction buffer, vortexed and centrifuged at 1500 g for 5 min. An equal volume of cold acetone was then added drop by drop to precipitate the protein. The mixture was kept on ice for 15 min and centrifuged at 2000 g for 15 min at 4°C. Protein concentration was determined and aliquots of antigen were stored at −80°C.
Antigenic fractions
Antigenic fractions were prepared from metacyclic parasites, pelleted by centrifugation at 600 g for 15 min at 4°C. The pellet, comprised of approximately 6 × 108 parasites (equivalent to approximately 3 mg of protein), was suspended in 1× gel loading buffer and proteins were subjected to electrophoresis on 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto nitrocellulose membrane, which was dried and cut into 11 1-cm horizontal strips. These strips were processed to precipitate the antigen-bearing particles as described previously [26]. The fractions were tested for toxicity against PBMCs by MTT assay and were found to be non-cytotoxic (more than 90% viability at all doses). The 11 antigenic fractions with their corresponding MW are shown in Table 1. Additional strips of blank nitrocellulose (blotted from a blank portion of the gel) were also processed for use as appropriate antigen controls.
Table 1.
Antigenic fractions (F1–F11) prepared from whole parasite of Leishmaniasis donovani (2001 isolate) promastigote separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and their corresponding molecular weights.
| Fraction no. | MW (kDa)a | Fraction no. | MW (kDa)a |
|---|---|---|---|
| F1 | 139–120 | F7 | 48·2–42·0 |
| F2 | 120–101 | F8 | 42·0–36·5 |
| F3 | 101–82·0 | F9 | 36·5–31·5 |
| F4 | 82·0–64·2 | F10 | 31·5–27·3 |
| F5 | 64·2–56·0 | F11 | 27·3–24·0 |
| F6 | 56·0–48·2 |
Molecular weight (MW) represented in kilodaltons (kDa)
T cell proliferation assay
Mononuclear cells were separated from venous blood by density gradient centrifugation as described previously [27]. PBMCs were suspended at a concentration of 5 × 104 cells per well in RPMI-1640 medium (Gibco-Invitrogen) supplemented with HEPES, l-glutamine, sodium bicarbonate, sodium pyruvate, antibiotic–antimycotic mix (Sigma) and 10% heat-inactivated FCS. Cultures were set up in triplicate in 96-well round-bottomed plates (Tarsons, Kolkata, India) with WE at 10 µg/ml, 5 µg/ml and 2·5 µg/ml and 11 antigenic fractions at 1 : 5 and 1 : 50 dilutions. Cultures with phytohaemagglutinin (PHA) (Gibco-Invitrogen) at a dose of 1 : 100 served as positive control; wells without any stimulants served as negative controls. At the end of 24 h, 120 µl of supernatant was harvested carefully from each well and stored at −80°C for cytokine determination. The wells were replenished with 120 µl of cRPMI and incubated further at 37°C in 5% CO2 atmosphere for 2 days for PHA-stimulated cultures and 4 days for cultures stimulated with WE and antigenic fractions. Because responses to the 1 : 5 dilution of F1–F11 were consistently higher than to the 1 : 50 dilution (data not shown), only data for 1 : 5 dilutions were analysed.
Proliferation was assessed by thymidine (0·5 µCi/well, BARC, Mumbai, India) incorporation. The stimulation index (SI) was obtained by dividing counts per minute (cpm) of stimulated cultures by cpm of unstimulated cultures. An SI of 2·5 and above was taken as a positive response. Individuals having a positive proliferative response of ≥ 2·5 to one of the three doses of WE were termed responders. In the case of a positive response to more than one dose, the highest value of lymphocyte transformation test (LTT) response was taken for analysis and the values of the corresponding supernatants for cytokine levels.
Cytokine measurements
IFN-γ, IL-12p40, IL-10 and IL-4 levels in the 24-h antigen-stimulated supernatants and serum were determined with an OptEIA set enzyme-linked immunosorbent assay (ELISA) kit (Pharmingen, San Jose, CA, USA). The results were expressed as picograms (pg) of cytokine/ml, based on the standards provided in the kit. The lower detection limits for various cytokines were as follows: 4·7 pg/ml for IFN-γ, 31·3 pg/ml for IL-12p40 and 7·8 pg/ml for IL-4 and IL-10.
Statistical analysis
Statistical analyses were performed using SPSS version 10 software (SPSS, Inc., Chicago, IL, USA). The Mann–Whitney U-test was used for intergroup comparison of quantitative variables. Correlations between variables were determined by Spearman's rank correlation analysis. P-values less than 0·05 were considered to denote statistical significance.
Results
Responses to WE
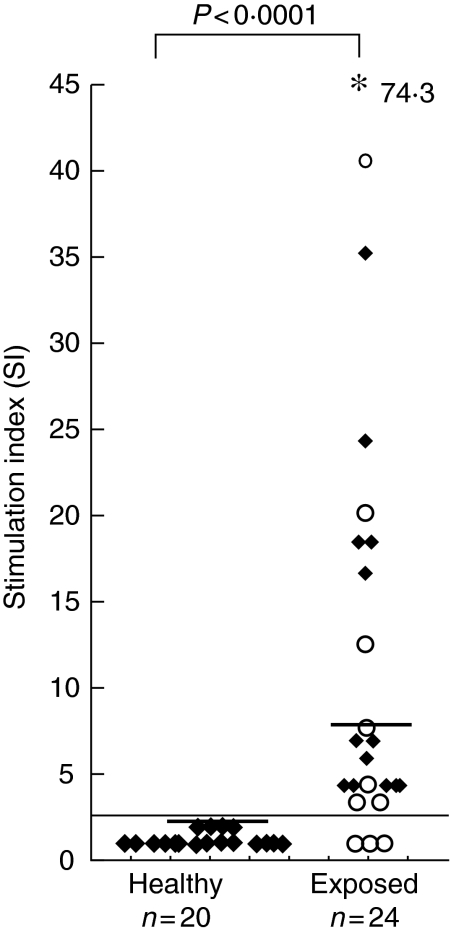
A positive lymphoproliferative response (SI ≥ 2·5) to WE was observed in 21 of the 24 exposed individuals; that is, all 14 cured patients and seven of the 10 endemic contacts. The frequency of positive responses among the exposed group [median 7·1 (range 1–74·3)] was significantly higher than among healthy controls [median 1·2 (range 0·5–1·6) P < 0·0001], none of whom showed a positive response (Fig. 1). The magnitude of responses in the treated cured group [median 8·1 (range 5–74·3)] and endemic contacts [median 6·4 (range 1–41)] was comparable.
Fig. 1.
Proliferative responses of peripheral blood mononuclear cells (PBMCs) from healthy controls and exposed individuals [treated cured visceral leishmaniasis patients (♦) and endemic contacts (○)] stimulated in vitro with whole cell extract (WE) of Leishmania donovani promastigotes. Each data point represents one individual; median is indicated by line. Stimulation index (SI) of ≥ 2·5 was taken to be a positive response by the patient and is indicated by a horizontal line. *Represents SI value above the scale. P indicates level of significance. Statistical analysis was performed by Mann–Whitney U-test; n = number of individuals.
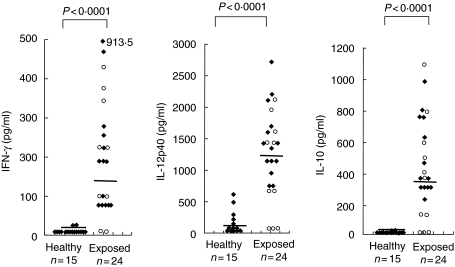
Levels of IFN-γ in the WE-stimulated supernatants were significantly higher in the exposed group [median 188 pg/ml (range 1–913)] compared to healthy controls [median 3·6 pg/ml (range 1–28) P < 0·0001]. The levels of IL-12 and IL-10 in the exposed group [median 1355 pg/ml (range 59–2718), median 364 pg/ml (range 1–1100), respectively]) were also significantly higher than in controls [IL-12: median 93·2 pg/ml (range 2·1–623·8), IL-10: median 3·6 pg/ml (range 2·1–28·1); P < 0·0001; Fig. 2]. The levels of these cytokines were comparable between treated cured patients and endemic contacts. IL-4 was not detectable in any of the control or study group samples.
Fig. 2.
Interferon (IFN)-γ, interleukin (IL)-12p40 and IL-10 levels in supernatants of peripheral blood mononuclear cells (PBMCs) from healthy controls and exposed individuals [treated cured visceral leishmaniasis patients (♦) and endemic contacts (○)] stimulated in vitro with whole cell extract (WE) of Leishmania donovani promastigotes. Each data point represents one individual; median is indicated by line. Values are given as concentration in pg/ml; *IFN-γ levels above the scale. P indicates level of significance. Statistical analysis was performed by Mann–Whitney U-test; n = number of individuals. IL-4 levels were not detectable.
There was a significant positive correlation in the magnitude of lymphoproliferative responses to WE and the levels of IFN-γ, IL-12 and IL-10 in the WE-stimulated supernatants (r = 0·8388, r = 0·7805, r = 0·8104, respectively, P < 0·01). A significant positive correlation was also seen between the levels of WE-stimulated IFN-γ and IL-12, IFN-γ and IL-10, IL-12 and IL-10 levels (r = 0·9231, r = 0·9359, r = 0·9344, respectively, P < 0·01) in the exposed group. PHA responses were present in all samples.
Cytokine levels in serum
IL-10 levels were significantly higher in the serum of the exposed individuals in comparison to the healthy controls (P < 0·001). The median serum IL-10 level in 14 treated cured patients was significantly higher [798 pg/ml (range 267–1364)] than in endemic contacts [486 pg/ml (range 21–1359); P < 0·05]. Among the endemic contacts, the median serum level was 710 pg/ml (range 400–1359) for the seven responders and 121 pg/ml (range 21–122) for the three non-responders. However, IFN-γ and IL-12 levels were comparable in the two groups; IL-4 levels were not detectable (Table 2).
Table 2.
Levels of interferon (IFN)-γ, interleukin (IL)-12p40, IL-10 and IL-4 in serum of healthy controls and exposed immune individuals.
| Cytokinea | Controls n = 20 | Exposed n = 24 | P-valueb |
|---|---|---|---|
| IFN-γ | 26·7 (1–144) | 35·5 (1–167) | n.s. |
| IL-12p40 | 13 (1–26) | 14 (1–30) | n.s. |
| IL-10 | 11 (1–24) | 726·2 (1–1363) | *< 0·001 |
| IL-4 | n.d. | n.d. |
P = level of significance.
Values are given as median (range) concentration in pg/ml.
Statistical analysis was performed by Mann–Whitney U-test. n.d. = not detectable; n.s. = not significant.
Responses to fractionated L. donovani antigens
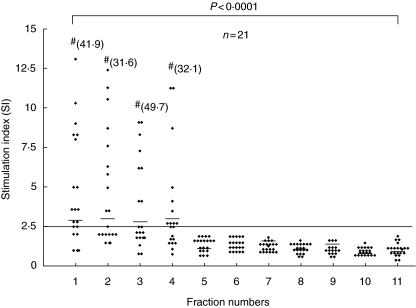
Proliferative responses to WE were taken as a marker for prior exposure to the disease resulting in a measurable immune response. Subsequent cytokine and T cell activation studies with the 11 antigenic fractions were conducted for the 21 exposed individuals showing positive responses to the WE. Of the 21 responders, 20 showed frequent proliferative responses to one or more of fractions F1–F4 (MW range of 139–64·2 kDa); 15 showed responses to F1 [median (range), 3·2 (1–41·9)], 13 to F2 [median (range), 3·2 (1–31·6)], 11 to F3 [median (range), 2·4 (1–49·7)] and 13 to F4 [median (range), 2·5 (1–32·1)]. None of these individuals showed any response to fractions F5–F11 (MW < 64·2 kDa). One treated cured case, who showed a proliferative response to WE (SI = 5), did not show a response to any of the fractions. The magnitude of response to each of the fractions F1, F2, F3 and F4 was equivalent, while the responses to fractions F1, F2, F3 and F4 were significantly (P < 0·0001) higher than to fractions F5–F11 (Fig. 3). LTT responses to PHA were present in all samples.
Fig. 3.
Proliferative responses of peripheral blood mononuclear cells (PBMCs) from exposed individuals (n = 21) stimulated in vitro with F1–F11 antigenic fractions of Leishmania donovani promastigotes. Each data point represents one individual; median is indicated by line. Stimulation index (SI) of ≥ 2·5 was taken as positive response and is indicated by a horizontal line. #SI value above the scale. P indicates level of significance between F1–F4 and F5–F11. Statistical analysis was performed by Mann–Whitney U-test.
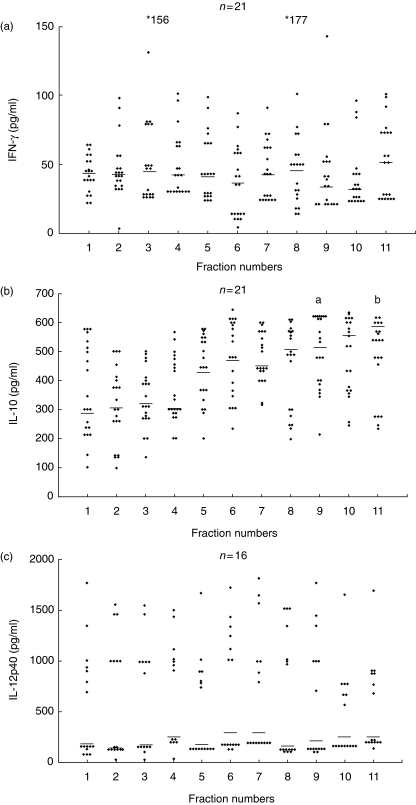
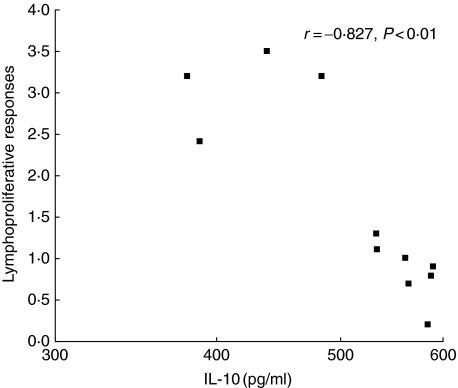
Fractions F1–F11 stimulated endemic variable levels of IFN-γ, IL-10 and IL-12 in both patients and contacts (Fig. 4a–c). There was no measurable IL-4 response to any of the fractions. The IFN-γ and IL-12 levels in F1–F11-stimulated supernatants were comparable and there was no correlation with the proliferative response to the same fractions. The IL-10 levels had higher values in supernatants of cultures stimulated with F5–F11 compared to F1–F4. However, intergroup comparisons showed significantly higher IL-10 levels in only F11 compared to F1, and F9 compared to F3 (marked as ‘a’ and ‘b’ in Fig. 4b, P < 0·05). There was a significant negative correlation between the median proliferative responses to each of the 11 fractions and the median IL-10 levels to the corresponding fractions (r = –0·827, P < 0·01, Fig. 5). A significant negative correlation was also observed between the ratios of IL-10 to IFN-γ levels and proliferative responses (r = –6013, P < 0·01). PHA responses to IFN-γ, IL-12, IL-10 and IL-4 were present in all samples.
Fig. 4.
Interferon (IFN)-γ (a), interleukin (IL)-10 (b) and IL-12p40 (c) levels in supernatants of peripheral blood mononuclear cells (PBMCs) from exposed individuals stimulated in vitro with F1–F11 antigenic fractions of Leishmania donovani promastigotes. Each data point represents one individual; median is indicated by line. Values are given as concentration in pg/ml. *(a) IFN-γ levels above the scale. Statistical analysis was performed by Mann–Whitney U-test; n = number of individuals. (b) aF9 versus F3, P < 0. 05; bF11 versus F1, P < 0.05).
Fig. 5.
Correlation between proliferative responses and interleukin (IL)-10 levels in response to stimulation with F1–F11 antigenic fractions of Leishmaniasis donovani promastigotes of exposed group. Each data point represents the median value for the lymphoproliferative responses and the IL-10 levels to each of 11 fractions. P indicates level of significance. Correlation was determined by Spearman's rank correlation; r = regression constant for correlation.
Discussion
Successful recovery from VL due to L. donovani is associated with the development of lifelong immunity and resistance to reinfection [28]. The parameters of protective immunity are less well defined in human VL and immunostimulatory antigens of L. donovani have not been well studied. In this paper, we have described the immune status of treated, cured and exposed individuals living in an area endemic for L. donovani. Further, we have evaluated the immune response of these individuals to antigen fractions separated from the whole promastigote antigens of a fresh isolate of L. donovani from the same region. We report here that immune individuals living in a kala azar endemic region secrete high levels of IL-10 together with IL-12 and IFN-γ. For the first time, we report that antigens in the MW range > 64·2 kDa induced proliferative responses and low IL-10 levels while antigens in the < 64·2 kDa region did not induce proliferative responses and induced high IL-10 levels.
L. donovani WE stimulated significant proliferative responses in the majority (21 of 24) of exposed individuals. Thus, individuals who control parasitaemia successfully either following treatment in the case of patients or due to adequate immunity, as in endemic contacts, exhibit good T cell reactivity to the Leishmania antigen. In fact, the proliferative responses of these two groups were comparable in our study. The presence of a positive immune response in seven of 10 of our endemic contacts suggests that the frequency of subclinical infection in an endemic area such as Bihar is high, as has been reported in other areas of the world [29,30]. The majority of people infected with L. chagasi or L. donovani have been shown to clear their infection spontaneously and develop protective immunity, characterized by lymphocyte proliferation and secretion of IFN-γ in response to antigen in vitro.
In this study, WE stimulated production of high levels of IFN-γ, IL-12 and IL-10 by PBMCs of exposed individuals; IL-4 levels were undetectable. The cytokine levels in the treated cured patients and endemic contacts were comparable, suggesting that endemic contacts develop a strong protective immunity. A similar observation has been reported for L. major infection from Sudan [31]. The high IFN-γ and IL-12 levels, which correlated with the proliferative responses, is in keeping with the Th1 profile that accompanies a state of protective immunity in this infection [32,33]. IL-12 has been shown to restore IFN-γ production both in vitro by cells of patients who have a Th2 skewed response and in vivo in animal models of CL and VL [34–37]. The role of IL-4 as the driving Th2 cytokine responsible for disease progression has been shown only for murine CL [38]. Its role in human disease is far less clear. Our inability to detect IL-4 in the WE-stimulated supernatants and serum suggests that this cytokine is down-regulated rapidly following cure.
WE-stimulated culture supernatants of immune individuals had levels of IL-10 that were comparable to levels of IFN-γ and IL-12. As well as Th2 cells, human macrophages, mast cells and B cells produce IL-10. CD4+ CD25+ T cells, the Tregs, secrete IFN-γ, IL-10 and transforming growth factor (TGF)-β simultaneously and play an important role in down-regulation of the immune response [12,39]. High IL-10 levels are found during active VL and IL-10 is believed to be the major macrophage deactivating cytokine, favouring parasite persistence [40]. The persistence of high IL-10 levels in serum and high levels of IL-10 produced by WE-stimulated cells of both treated cured cases and endemic contacts in our study indicates that this cytokine is important for maintaining the protective state. A similar observation has been made in Sudan, where a higher number of cells producing both IFN-γ and IL-10 were seen in cured VL cases than in controls [41]. Simultaneously, high IFN-γ and IL-10 has also been reported in cured CL cases from Tunisia [12]. In a murine model for L. major, IL-10 was shown to be essential for parasite persistence and it has been suggested that the chronic state is maintained by infected dendritic cells (DCs) and macrophages that remain responsive to both cytokines [42]. That IL-10 may play a similar role in humans also is suggested by the observation that high plasma IL-10 levels and expression by keratinocytes was predictive of post-kala azar dermal leishmaniasis (PKDL) development [43]. Thus, treatment may not result in sterile cure and these individuals may need IL-10 for maintaining a fine balance in the host that prevents sterile immunity and reinfection.
When fractionated antigens were used to stimulate the cells of the immune contacts (21 of 24 individuals with a positive LTT response), only fractions containing protein in the MW range 139–64·2 kDa were found to stimulate T cell proliferation, with the majority (20 of 21) showing a response to one or more of F1–F4 fractions, and not a single response was seen to any of the fractions having protein of < 64·2 kDa. This is in contrast to previous reports that have evaluated the full parasite antigen profile [19–24]. This difference may be attributed to antigenic differences among Leishmania species, differences in the level of intensity of infection or genetic background of the host and differential combinations in each fraction. It should be pointed out that as particulate (nitrocellulose-bound) antigens were used for stimulation, the magnitude of the response between various fractions could not be compared. However, there were relatively few protein bands of low intensity in the upper MW range (MW > 80 kDa) compared to the numerous and dense bands in the middle and lower MW ranges. Hence, the proliferation by the high MW regions is especially significant.
IFN-γ and IL-12 levels in F1–F11-stimulated culture supernatants were comparable. Thus, IFN-γ levels did not show correlation with protective immunity, as has been reported in earlier studies. IL-10 levels in response to F1–F4 stimulation was lower than to F5–F11 and showed a negative correlation with proliferative responses. Several leishmanial antigens such as Ldp23, which have been shown to induce Th1 response during infection, are not necessarily protective in vivo. In contrast, strong Th2 response-inducing antigens such as LACK and CPB2·8, have been shown to induce substantial protection in mice if administered in conjunction with adjuvants that stimulate Th1 responses. LmSTI1, which stimulated mixed Th1/Th2 responses, gave excellent protection in mice and monkeys if used with IL-12 or monophosphoryl lipid A plus squalene as adjuvant [13,16,44,45].
PapLe22, a 22 kDa antigen of L. infantum, has been shown to stimulate preferentially IL-10 and not IFN-γ in cured VL cases [46]. Our low MW fractions may contain similar molecules and although it is unclear as to which cell type is stimulated, a possible role for an expanded regulatory T cell subset cannot be excluded. However, these cells may be too few to be reflected in the proliferative responses. The CD4+ CD25+ regulatory subset of cells has been shown to produce high levels of IL-10 associated with intermediate levels of IFN-γ and has been characterized both in mouse and humans [41,47,48]. We have also observed high levels of IL-10 accompanied by low/intermediate levels of IFN-γ to the low MW fractions. Thus, these data suggest that protection against leishmaniasis requires more than the activation of Leishmania-specific IFN-γ-producing T cells.
Our results have highlighted the complex nature of the immune response to different antigens in VL. In view of the role of IL-10 in maintaining a chronic state, which may be crucial for continued protection against reinfection, the differential stimulation of proliferation, IL-10 and IFN-γ raises issues that need to be resolved before appropriate molecules are selected to be included in an effective vaccine.
Acknowledgments
We acknowledge the financial support of the Department of Biotechnology, Government of India, New Delhi for this work. We are thankful to Mr Dinesh Chandra for helping us to obtain samples. Parul Tripathi is a PhD student supported by SGPGI, Lucknow.
References
- 1.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–8. [PubMed] [Google Scholar]
- 2.Modabber F. Development of vaccines against leishmaniasis. Scand J Infect Dis. 1990;76:72–8. [PubMed] [Google Scholar]
- 3.Liewn FY, O'Donnell CA. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–9. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 4.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 5.Lemesre JL, Holzmuller P, Cavaleyra M, Gonçalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23:2825–40. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Gabriela M, Gomes S, Capela MJR, Ramada J, Campino L. Experimental canine leishmaniasis: evolution of infection following re-challenge with Leishmania infantum. Acta Tropica. 2003;87:235–44. doi: 10.1016/s0001-706x(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 7.de Souza EP, Bernardo RR, Palatnik M, de Sousa CBP. Vaccination of Balb/c mice against experimental visceral leishmaniasis with the GP36 glycoprotein antigen of Leishmania donovani. Vaccine. 2001;19:3104–15. doi: 10.1016/s0264-410x(01)00031-7. [DOI] [PubMed] [Google Scholar]
- 8.Tewary P, Mehta J, Sukumaran B, Madhubala R. Vaccination with Leishmania soluble antigen and immunostimulatory oligodeoxynucleotides induces specific immunity and protection against Leishmania donovani infection. FEMS Immunol Med Microbiol. 2004;42:241–8. doi: 10.1016/j.femsim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Kharazmi A, Kemp K, Ismail A, et al. T-cell response in human leishmaniasis. Immunol Lett. 1999;65:105–8. doi: 10.1016/s0165-2478(98)00132-1. [DOI] [PubMed] [Google Scholar]
- 10.Gaafar A, Kharazmi A, Ismail A, et al. Dichotomy of T cell response to leishmania antigens in patients suffering from cutaneous leishmaniasis: absence or scarcity of Th1 activity is associated with severe infections. Clin Exp Immunol. 1995;100:239–45. doi: 10.1111/j.1365-2249.1995.tb03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirmez C, Yamamura M, Uyemura K, et al. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–5. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinchieri G. Regulatory role of T cells producing both interferon-γ and interleukin-10 in persistent infection. J Exp Med. 2001;194:F53–F57. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock KG, McNeil KS, Mottram JC, et al. The Leishmania mexicana cysteine protease, CPB2.8, induces potent Th2 responses. J Immunol. 2003;170:1746–53. doi: 10.4049/jimmunol.170.4.1746. [DOI] [PubMed] [Google Scholar]
- 14.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2004;172:3793–7. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 15.Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective leishmania antigen. Science. 1995;268:563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 16.Campos-Neto A, Porrozzi R, Greeson K, et al. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–8. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–3. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 18.Webb JR, Kaufmann D, Campos-Neto A, Reed SG. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J Immunol. 1996;157:5034–41. [PubMed] [Google Scholar]
- 19.White AC, Jr, Castes M, Garcia L, Rtujillo D, Zambrano L. Leishmania chagasi antigens recognized in cured visceral leishmaniasis and asymptomatic infection. Am J Trop Med Hyg. 1992;462:123–31. doi: 10.4269/ajtmh.1992.46.123. [DOI] [PubMed] [Google Scholar]
- 20.Laskay T, Mariam H, Berhane TY, Fahniger TE, Kiessling R. Immune reactivity to fractionated Leishmania aethiopica antigens during active human infection. J Clin Microbiol. 1991;29:757–63. doi: 10.1128/jcm.29.4.757-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melby PC, Neva FA, Sacks DL. Profile of human T cell response to leishmanial antigens. Analysis by immunoblotting. J Clin Invest. 1989;83:1868–75. doi: 10.1172/JCI114093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst P, Stromberg E, Ghalib HW, et al. Identification and characterization of a T cell stimulating antigens from Leishmania by CD4 T cell expression cloning. J Immunol. 2001;166:498–05. doi: 10.4049/jimmunol.166.1.498. [DOI] [PubMed] [Google Scholar]
- 23.Jeronimo SMB, Higgs E, Vedvick T, et al. Identification of Leishmania chagasi antigens recognized by human lymphocytes. J Infect Dis. 1995;172:1055–60. doi: 10.1093/infdis/172.4.1055. [DOI] [PubMed] [Google Scholar]
- 24.Reed SG, Carvalho EM, Sherbert CH, et al. In vitro responses to leishmania antigens by lymphocytes from patients with leishmaniasis or Chagas’ disease. J Clin Invest. 1990;85:690–6. doi: 10.1172/JCI114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg R, Gupta SK, Tripathi P, Naik S, Sundar S, Dube A. Immunostimulatory cellular responses of cured leishmania infected patients and hamsters against integral membrane proteins and non-membranous soluble proteins of a recent clinical isolate of Leishmania donovani. Clin Exp Immunol. 2005;140:149–56. doi: 10.1111/j.1365-2249.2005.02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abou-Zeid C, Filley E, Steele J, Rook GAW. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen bearing particles. J Immunol Meth. 1987;98:5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi P, Saxena S, Yadav VS, Naik S, Singh VK. Human S-antigen: peptide determinant recognition in uveitis patients. Exp Mol Pathol. 2004;76:122–8. doi: 10.1016/j.yexmp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Locksley RM, Louis JA. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:213–8. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- 29.Louzir S, Salah B, Osman MB, Dellagi K. Leishmanin skin test lymphoproliferative responses and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin Exp Immunol. 1999;116:127–32. doi: 10.1046/j.1365-2249.1999.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa RS, Oliveira AD, Jr, Bacellar O, Carvalho EM. T cell response of asymptomatic Leishmania chagasi infected subjects to recombinant leishmania antigens. Mem Inst Oswaldo Cruz. 1999;94:367–70. doi: 10.1590/s0074-02761999000300015. [DOI] [PubMed] [Google Scholar]
- 31.Bosque F, Saravia NG, Valderrama L, Milon G. Distinct innate and acquired immune response to leishmania in putative susceptible and resistant human population endemically exposed to L. (Viannia) panamensis infection. Scand J Immunol. 2000;51:533–41. doi: 10.1046/j.1365-3083.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho EM, Bacellar O, Brownell C, Regis T, Coffman RL, Reed SG. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 33.Sjölander A, Baldwin TM, Curtis JM, Handman E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response is required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–57. [PubMed] [Google Scholar]
- 34.Sypek JP, Chung CL, Mayor SHE. Resolution of cutaneous leishmaniasis: IL-12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–02. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghalib HW, Whittle JA, Kubin M, et al. Interleukin-12 enhances Th1 type immune response in human Leishmania donovani infection. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 36.Bacellar O, Brodskyn C, Guerreir J, et al. Interleukin-12 restores interferon-γ production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996;173:1515–8. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 37.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–55. [PubMed] [Google Scholar]
- 38.Zwingenberger K, Harms G, Pedrosa C, Omena S, Sandkamp B, Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin-4 over interferon-γ production. Clin Immunol Immunopathol. 1990;57:242–9. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]
- 39.Ghalib HW, Puvezam MR, Skeiky YM, et al. Interleukin-10 production correlates with pathology in human Leishmania donovani infection. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 41.Kemp K, Kemp M, Kharazmi A, et al. Leishmania-specific T cells expressing interferon-gamma (IFN-gamma) and IL-10 upon activation are expanded in individuals cured of visceral leishmaniasis. Clin Exp Immunol. 1999;116:500–4. doi: 10.1046/j.1365-2249.1999.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belkaid Y, Hoffmann KF, Mendez S, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–06. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasim S, El Hassan AM, Khalil EAG. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala azar dermal leishmaniasis. Clin Exp Immunol. 1998;111:64–9. doi: 10.1046/j.1365-2249.1998.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skeiky YA, Coler RN, Brannon M, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in Mpl adjuvant. Vaccine. 2002;20:3292–03. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 45.Gicheru MM, Olobo JO, Anjili CO, Orago AS, Modabber F, Scott P. Vervet monkeys vaccinated with killed Leishmania major parasites and interleukin-12 develop a type 1 immune response but are not protected against challenge infection. Infect Immun. 2001;69:245–51. doi: 10.1128/IAI.69.1.245-251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suffia I, Ferrua B, Stien X, et al. A novel Leishmania infantum recombinant antigen which elicits interleukin-10 production by peripheral blood mononuclear cell of patients with visceral leishmaniasis. Infect Immun. 2000;68:630–6. doi: 10.1128/iai.68.2.630-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 48.Mendez S, Reckling SK, Piccirillo CA, Sacks DL, Belkaid Y. Role for CD4+CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–10. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]