Abstract
Erythrocyte complement receptor type one (E-CR1) is thought to protect against immune complex (IC) disease through interactions that lead to E-CR1 consumption, and low E-CR1 levels are characteristic of systemic lupus erythematosus (SLE). The purpose of this study was to test the hypothesis that E-CR1 consumption can predict or mark SLE flare. Recurrently active SLE patients [n = 43; 28 with past or present major renal manifestations (SLER) and 15 without (SLENR)], were evaluated every 2 months by detailed protocol testing (mean follow-up 22 months), including direct measurements of E-CR1 levels using a radioimmunoassay. In all patients, detectable E-CR1 levels fluctuated widely through acute periods of consumption and regeneration, preventing the use of any single value as a baseline. However, when individual chronic baseline values were used, determined as the mean of all E-CR1 values 4 months or more from a flare, a clear trend was observed. In 16 of 16 instances of non-renal flare in SLER patients, E-CR1 levels decreased at flare (mean decrease 34%, P < 0·0001). In contrast, no consistent difference was observed for flare in SLENR patients or for renal flare in SLER patients. Changes in E-CR1 levels did not correlate with plasma CR1 levels. In conclusion, single occurrences of E-CR1 consumption did not generally predict or mark SLE flare. However, compared to the average E-CR1 levels measured during no-flare intervals, E-CR1 consumption in SLER patients at flare was strongly associated with freedom from signs of renal involvement. We postulate that E-CR1 consumption reflects E-CR1 function that includes protecting against SLE nephritis.
Keywords: complement, erythrocyte CR1, SLE flare, SLE nephritis
Introduction
The type one complement receptor (CR1, CD35) is a transmembrane glycoprotein that binds the complement proteins C3b, C4b, C3bi and C1q [1]. In humans, approximately 90% of the total circulating CR1 occurs on erythrocytes (E-CR1), where it binds to complement-opsonized immune complexes (IC) through a process known as immune adherence [2]. Non-human primates also have immune adherence receptors, some of which are derived from the CR1-like gene [3]. Studies in both humans and non-human primates have demonstrated that erythrocytes, through the process of immune adherence, shuttle IC through the circulation and target IC disposal to the liver and spleen [4,5]. Interruption of this process accelerates IC clearance through non-discriminatory tissue trapping, and leads to IC deposition in vulnerable tissue sites such as the kidney [6–8]. Thus, E-CR1 can be viewed as a critical component in protecting tissue such as the kidney from IC deposition and subsequent inflammation.
Numerous studies have documented lower E-CR1 function and levels in systemic lupus erythematosus (SLE) populations versus normal controls (reviewed in [9]). A recent report showed that low E-CR1 levels were highly specific for SLE diagnosis relative to healthy controls, and when combined with erythrocyte-bound C4d was highly specific for SLE diagnosis relative to other rheumatic/autoimmune diseases [10]. Although the basis for the low E-CR1 expression in SLE is not understood completely and genetic influences have been implicated, acquired changes are clearly involved. Mechanisms involving proteolytic cleavage, erythrocyte membrane vesiculation and loss of CR1 epitope have been suggested to explain this acquired change [11–16]. Nevertheless, the exact nature of this change in E-CR1 remains unclear.
Studies demonstrating acquired E-CR1 changes show that E-CR1 levels are related inversely to SLE disease activity [17–19] and that E-CR1 levels recover during SLE remission [20,21]. Thus, regardless of the mechanism of E-CR1 alteration, loss of detectable E-CR1 could serve as a biomarker for SLE disease activity or disease relapse. The purpose of this study was to determine if changes in detectable E-CR1 levels correlated with disease flare in a group of recurrently active SLE patients, with or without renal manifestations.
Materials and methods
Patients
The patients in this report were part of the Ohio SLE Study (OSS), a prospective, longitudinal study of recurrently active SLE patients [22,23]. Patients were recruited into the OSS under informed human subjects Internal Review Board (IRB) consent if they had currently active SLE, two or more SLE flares requiring an increase in therapy in the previous 3 years or persistently active SLE, defined as greater than 4 months of activity despite therapy. The OSS patients with renal SLE (SLER) were required to have major renal manifestations past or present taken as: 24-h urine protein/creatinine (Pr/Cr) ratio >1, serum creatinine >1·1 mg/dl (females) or 1·3 mg/dl (males) attributable to SLE, or both. The OSS patients with non-renal SLE (SLENR) were defined as never having shown major renal manifestations attributable to SLE GN. These patients had a normal serum creatinine (1·1 mg/dl females; 1·3 males), a Pr/Cr < 0·3 and urine sediment with <5 RBCs/hpf and no casts. Each patient was evaluated clinically and with laboratories every 2 months.
The patients studied in this report represented all the OSS patients who had been followed for a minimum of 1 year and who had experienced at least one flare, defined as either a renal flare or non-renal flare (see below). In total, 43 patients were identified (28 SLER, 15 SLENR).
Adjudication and classification of SLE flare
After all clinical results were compiled from each 2-month study visit, the patient's study physician determined whether an SLE flare occurred, whether it was renal or non-renal and whether the flare was mild, moderate or severe. Confirmation of the flare was required by independent review of the data by another study physician. Identification of renal flares was based on prespecified criteria of changes in serum creatinine and proteinuria, as we have reported previously [22,23]. A non-renal flare was declared if the patient developed one or more symptoms or signs of non-renal SLE, based on prespecified previously reported criteria [22,23], in the absence of renal flare manifestations, that were attributable to SLE and were of sufficient severity that the managing study physician increased therapy.
Measurements of CR1
Blood samples were collected from the SLE patients every 2 months at the time of their clinical evaluations. From these samples, average E-CR1 levels (mean CR1/E) were determined by radioimmunoassay, as we have described previously [24–26]. This assay uses a high-affinity anti-CR1 monoclonal antibody (mAb), E11 (Accurate Chemical and Scientific Corporation, Westbury, NY, UJSA), that recognizes an epitope that is distinct from the CR1 ligand-binding domain [24]. In our laboratory, this determination has been carried out on 261 healthy normal individuals, with the finding of an average E-CR1 level of 444 CR1/E and a range of 81–1200 CR1/E. The average and range are similar to CR1/E-values reported using dimeric C3b [27], as well as a different anti-CR1 monoclonal antibody [28]. All SLE samples in this study were assayed in duplicate, and every assay included a sample from a healthy normal of a known E-CR1 level to account for assay-to-assay variation. The interassay coefficient of variation, determined from 20 different measurements of this normal sample, was 11·7%.
Plasma samples, collected in ethylenediamine tetraacetic acid (EDTA) at the time of each office visit, were also used to determine relative plasma CR1 concentrations. This determination utilized an enzyme-linked immunosorbent assay (ELISA) as we have reported previously [26], with modifications. Specifically, E11 (2 µg/ml) was coated onto wells of a 96-well plate (Nunc Maxisorb, Nalge Nunc International, Rochester, NY, USA). After blocking with 1% BSA, 50 µl of each EDTA plasma sample, diluted 1/10 in 0·01 M Tris-HCl, pH 7·4, 0·05 M NaCl, 0·01% Tween 20, 1% Igepal, was added and incubated at room temperature for 60 min. Bound CR1 was detected by sequential incubations with a chicken anti-CR1 antibody (Accurate), rabbit anti-chicken-horseradish peroxidase (HRP) (Zymed Laboratories Inc., South San Francisco, CA, USA) and ortho-phenylenediamine (OPD) (Sigma Fast, Sigma Chemical Co., St Louis, MO, USA), with colour development measured at OD490. All samples were run in duplicate, and all plasma samples from a given patient were run together in the same assay and with a normal plasma standard (diluted 1/10). Patient sample values were expressed as the percent of the standard OD490 (OD490 patient sample/OD490 standard × 100). The interassay coefficient of variation was 9·4%.
Some of the plasma samples, corresponding to the same time periods as those demonstrating substantial decreases in detectable CR1, were also assessed for CR1 fragments by unreduced Western blot analysis, as described previously [29], using biotin-labelled E11 followed by strepavidin-HRP (Zymed). Blots were developed using chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ, USA).
Analysis of data
Chronic CR1 levels, both E-CR1 and plasma CR1, were calculated for each patient as the mean of the values determined from samples collected 4 months or more from a lupus flare. Chronic CR1 levels were compared to the CR1 levels 2 months prior to flare (−2), at the time of flare (0) and 2 months after flare (+2) using analysis of variance applicable to repeated measures to determine if differences existed. A mixed model was used where patients were treated as random effects and flare period (chronic, −2, 0, +2) were treated as fixed effects. The response in this model was the natural log of the CR1 levels, C3 levels or C4 levels. These analyses were performed separately for non-renal flare in SLENR patients, for non-renal flare in SLER patients and for renal flare in SLER patients, and an alpha level of P < 0·01 was used for significance due to multiplicity. Multiple regression was also performed to determine the relationship between E-CR1 levels and plasma CR1 levels. Patients were used as random effects, and contributions by patient type (SLENR versus SLER) were tested. The statistical analysis program JMP (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
Number of flares in the SLE population
Fifteen SLENR and 28 SLER patients were identified from the OSS cohort on the basis of having 12 months or more follow-up and experiencing at least one flare. The mean follow-up was 22·3 ± 5·8 months. A total of 66 flares was identified, and the number and type of flares are shown in Table 1. In the SLENR cohort, 13 of 15 patients experienced only one flare, with the other two patients experiencing two flares. In the SLER cohort, the number of flares per patient ranged from one to six, with 13 patients having more than one flare.
Table 1.
Summary of non-renal flares and renal flares in systemic lupus erythematosus (SLE) patients without non-renal flare in systemic lupus erythematosus (SLENR) and with renal SLE (SLER) major renal manifestations.
| SLENR | SLER | |
|---|---|---|
| No. of patients | 15 | 28 |
| No. of patients with only non-renal flare | 15 | 8 |
| No. of patients with only renal flare | 0 | 12 |
| No. of patients with both | 0 | 8 |
| No. of non-renal flares | 17 | 21 |
| ″Mild | 7 | 12 |
| ″Moderate | 9 | 9 |
| ″Severe | 1 | 0 |
| No. of renal flares | 0 | 28 |
| ″Mild | 10 | |
| ″Moderate | 14 | |
| ″Severe | 4 |
CR1 changes and SLE flare
Erythrocyte CR1 levels were measured every 2 months in >85% of these bimonthly study visits. The E-CR1 levels fluctuated widely within each patient, and these fluctuations did not appear to be specific for flare onset, flare type or flare severity (Fig. 1). Neither average individual E-CR1 levels nor average individual E-CR1 level coefficients of variations (a measure of E-CR1 fluctuation) were different between the SLER and SLENR cohort, or between the SLER patients experiencing at least one renal flare and SLER patients experiencing at least one non-renal flare, regardless of flare severity (data not shown).
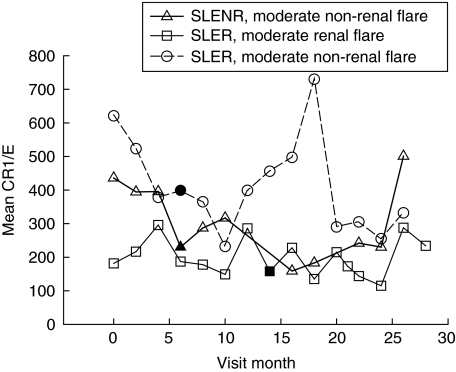
Fig. 1.
Sequential changes in E-CR1 levels in three representative SLE patients. The solid symbols indicate the visit month in which a flare was identified.
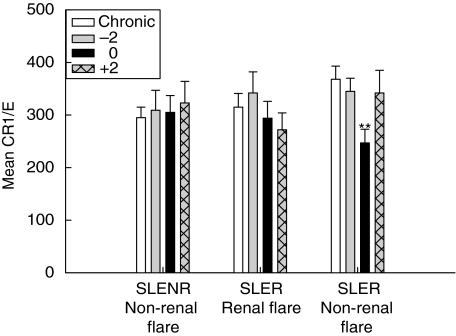
Because of the fluctuations in non-flare E-CR1 levels, no single value could be used as a baseline for comparison to E-CR1 values occurring around a flare. To provide a more reliable baseline for this comparison, ‘chronic’ E-CR1 levels were calculated for each patient as the mean CR1/E of all the samples collected 4 months or more from a flare. This value was then compared to E-CR1 levels 2 months prior to a flare (−2), at the time of flare (0) and 2 months after a flare (+2). These data were analysed after stratifying the flares according to type and severity (Table 1). The results of this analysis, shown in Fig. 2, indicate that while no consistent loss of detectable E-CR1 occurred around non-renal flare in the SLENR patients, or around renal flare in the SLER patient, there was a highly significant reduction in E-CR1 levels at the time of non-renal flare in SLER patients (P < 0·0001). This reduction was observed in 16 of 16 instances of a non-renal flare in which data were available for both chronic E-CR1 levels and E-CR1 levels at the time of flare, and the mean reduction was 34 ± 18%. The severity of the flare did not influence these results.
Fig. 2.
Relationship of E-CR1 levels to SLE flare. Chronic E-CR1 levels were determined for each patient (average of all values 4 months or more from a flare) and compared to E-CR1 levels 2 months prior to (−2), at the time of (0) and 2 months after (+2) flare. This comparison was made for non-renal flare in non-renal SLENR patients, for renal flare in SLER patients, and for non-renal flare in SLER patients. Mean values ± SE are shown. **P < 0·0001 compared to chronic E-CR1 in SLER patients experiencing a non-renal flare.
The loss of detectable E-CR1 at the time of non-renal flare in SLER patients could be related to the type of flare or to the patient type (SLER patients experiencing a non-renal flare). To address these two scenarios, the E-CR1 levels in patients who had experienced at least one renal flare and one non-renal flare were evaluated (n = 7). In this SLER subset, the only significant difference occurred at the time of non-renal flare (P = 0·002, data not shown).
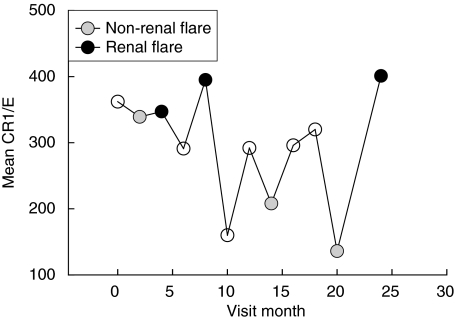
Also relevant to this analysis is a single SLER patient who experienced three non-renal flares and three renal flares, but could not be included in the analysis of Fig. 2 because during follow-up she never experienced a time period that was 4 months or more from a flare, and thus a chronic E-CR1 value could not be calculated. As can be seen in Fig. 3, all three E-CR1 levels at the non-renal flare times in this patient were lower than those occurring at the renal flares times. The mean CR1/E for the three non-renal flares (227 ± 103 s.d.) was significantly lower than the mean CR1/E for the three renal flares (381 ± 30, one-tailed P = 0·034).
Fig. 3.
Sequential changes in E-CR1 levels in a single renal SLER patient who experienced multiple flares, both renal and non-renal. The solid grey symbols indicate the visit months at which non-renal flares were identified, and the solid black symbols indicate the visit months at which renal flares were identified.
Plasma CR1 levels
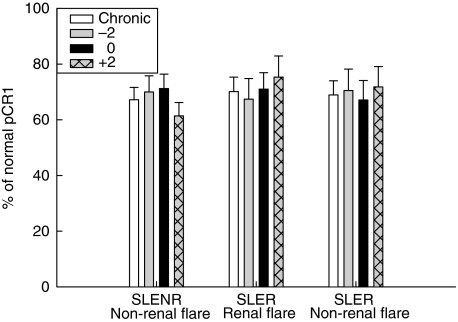
To assess whether the loss of detectable E-CR1 resulted in the shedding of CR1 into the plasma, levels of plasma CR1 (pCR1) were measured by a sandwich ELISA. The degree of pCR1 variability from one study visit to the next was substantially less than that of E-CR1. When chronic pCR1 levels were compared to plasma levels 2 months prior to, at the time of, and 2 months after flare, no differences were identified (Fig. 4). Western blot analysis did not reveal the presence of CR1 fragments at the times that E-CR1 levels decreased (data not shown). Multiple regression analysis of E-CR1 levels and plasma CR1 levels, with the patients accounted for as random effects, indicated that no correlation existed between these two measurements, regardless of the patient type.
Fig. 4.
Relationship of plasma CR1 (pCR1) levels to SLE flare. Relative pCR1 levels, expressed as a percentage of the normal pCR1 standard, were compared for the three types of flare at the indicated times, as described in Fig. 1. Mean values ± SE are shown.
Relationship of C3 and C4 levels to flare and to CR1 levels
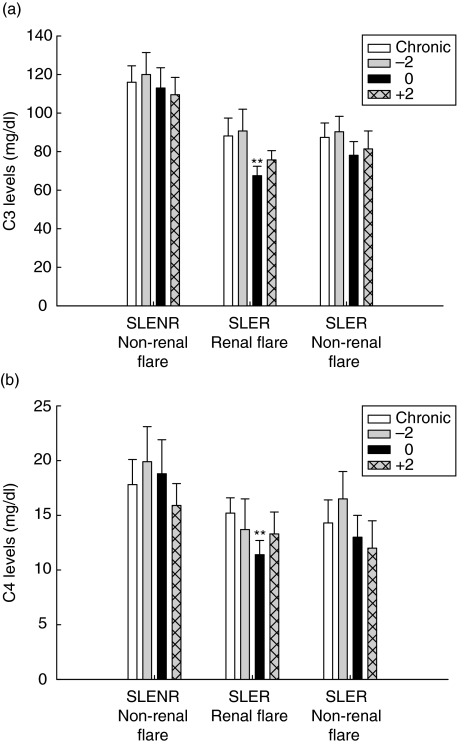
Serum C3 and C4 levels were determined at each study visit. These levels were compared for the same time-periods for each of the three flare groups, as was performed for E-CR1 levels. The only significant differences observed were at the time of renal flare, where both C3 levels (Fig. 5a) and C4 levels (Fig. 5b) were decreased relative to the chronic values (P < 0·0001 for both C3 and C4).
Fig. 5.
Relationship of C3 and C4 concentrations to SLE flare. The C3 levels (a) and C4 levels (b) were compared for the three types of flare at the indicated times, as described in Fig. 1. Mean values ± SE are shown. **P < 0·0001 compared to chronic E-CR1 in SLER patients experiencing a renal flare.
Discussion
Abnormally low E-CR1 levels have long been considered a characteristic of SLE (reviewed in [30]). Previous studies have suggested that loss of detectable E-CR1, referred to herein as E-CR1 consumption, could also serve as a predictor or marker for SLE flare, based on levels that appeared to be inversely related to disease activity [17–21]. However, this question has never been addressed directly and rigorously. The present study was designed to answer this question through a bimonthly analysis of 43 recurrently active SLE patients, followed for an average of 22 months, during which 66 flares were identified (both renal and non-renal).
The results of the present study revealed three unexpected findings concerning E-CR1 and recurrently active SLE. First, E-CR1 levels in a given patient were variable, cycling through periods of consumption and regeneration. Single occurrences of E-CR1 consumption were neither predictive of nor specific for SLE flare. This finding is unexpected because abnormally low E-CR1 levels have been considered a constant phenotype of active SLE patients [17–19,28,31] and single measurements of E-CR1 levels have recently been reported as highly specific and sensitive for SLE [10]. Thus, the cyclic nature of the E-CR1 level changes observed in the current study has never been recognized.
Secondly, in SLE patients with past or present major renal manifestations (SLER), E-CR1 consumption at the time of flare was associated with non-renal flare, i.e. with freedom from renal manifestations of the flare. The association was statistically strong (P < 0·0001) and unique to the SLER patients. This finding appears counterintuitive, as we and others have shown evidence that E-CR1 functions to protect against IC tissue trapping at sites that include the kidney [6–8]. Accordingly, E -CR1 consumption would be expected to result in loss of kidney protection, and thus be associated with renal manifestations during flare in SLER patients.
Thirdly, E-CR1 consumption at non-renal flare did not involve C3 or C4 consumption. In contrast, at renal flare, C3 and C4 were consumed in the face of stable E-CR1 levels. This is an unexpected finding, as previous reports have linked E-CR1 consumption to complement activation [17,18,20]. Thus, greater E-CR1 consumption would be expected to occur concurrent with greater C3 and C4 consumption.
To explain our findings, we propose the following:
The process of E-CR1 consumption is a normal consequence of appropriate E-CR1 function. Thus, under conditions of frequent or continuing exposure to complement-activating IC that occur in recurrently active SLE, frequent episodes of E-CR1 consumption occur.
E-CR1 consumption does not exclusively mean loss of CR1 from the membrane, but also includes changes to the receptor that damage anti-CR1 antibody epitopes. However, these changes are reversible, with periods of epitope restoration (E-CR1 regeneration). We have provided evidence previously that acute ‘consumption and regeneration’ cycles do occur in a monkey erythrocyte complement receptor (the E-CR1 orthologue), following interaction with circulating IC, that are independent of receptor loss from the erythrocyte [15,16].
Enumerating E-CR1 does not completely define the ability of E-CR1 to efficiently bind to complement-opsonized IC and to control complement activation. Changes could occur in functional sites that render them less active, but have minor effects on antibody-based detection of the receptor. Non-numerical changes in E-CR1 could also be related to changes in the clustering of CR1 on the membrane that is thought to be critical for efficient E-CR1 binding of IC [32,33]. In support of this, there is evidence that some E-CR1 clusters are devoid of ligand binding function [34]. Thus, we propose that there are high-function and low-function E-CR1 phenotypes that are changeable and independent of E-CR1 levels.
The greater the ability of E-CR1 to interact with complement-activating IC, the greater is E-CR1 consumption. Thus, E-CR1 of high function that provides the best protection against severe IC injury, such as nephritis, will be the most consumed. In contrast, E-CR1 of low function will not be able to handle the IC storm associated with a flare. This would lead to greater consumption of C4 and C3, and greater deposition of IC in vulnerable organs such as the kidney, leading to a renal flare. E-CR1 consumption would not occur at this time precisely because the patient was largely expressing low function E-CR1.
Using the above hypotheses, we can explain all the findings of the present study. In addition, we come to a new understanding of the role of E-CR1 in the pathogenesis of SLE flare. Most importantly, we are able to construct testable hypotheses regarding new approaches to protection against SLE flare, particularly its nephritis. One key testable hypothesis is that stimulating erythropoiesis in the SLE patient, which leads to higher circulating E-CR1 levels probably by inducing an overall younger erythrocyte population [35], will also lead to an increase in the pool of high-function E-CR1. This could provide an important measure of protection against renal flare, and one that would have the advantage of avoiding the use of immunosuppressive/anti-inflammatory drugs and exposure to their complications.
With regard to the nature of E-CR1 consumption, true loss of CR1 from the erythrocyte membrane, either through proteolysis, vesiculation or another unknown process, should lead to an increase in soluble CR1 in the plasma. Such higher levels of soluble CR1 could, theoretically, increase complement regulation in the plasma and account for the associated protection against renal involvement during flare. However, no differences in plasma CR1 levels were found to be associated with any of the flares, or with acute E-CR1 consumption. Furthermore, regression analysis showed no correlation between E-CR1 levels and relative plasma CR1 levels. These data suggest that an increase in the pool of circulating soluble CR1 is not the cause for protection against renal involvement during flare, and supports further the concept that acute E-CR1 consumption involves mainly changes that render the receptor undetectable. Alternatively, if acute E-CR1 consumption involves CR1 loss from the membrane, the released CR1 are removed from the circulation too quickly to be detected within the 2-month interval in which the patients were followed.
In conclusion, this is the first extensive study of E-CR1 changes during long-term follow-up of recurrently active SLE patients experiencing numerous flares. The present study highlights the ability of E-CR1 levels to fluctuate, indicating that the process of consumption and regeneration occurs continuously in those with recurrently active disease. The present work also suggests that, at various points in time, the SLE patient may express E-CR1 of ‘high function’ or ‘low function’. We postulate that the high function E-CR1 are those that undergo consumption while operating to protect against renal involvement at the time of flare in the SLER patient.
Acknowledgments
This work was supported by P01 DK 55546, M01 RR 00034, and the Lupus Clinical Trials Consortium.
References
- 1.Krych-Goldberg M, Atkinson JP. Structure–function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–22. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RA. The immune-adherence phenomonon: an immunologically specific reaction between microorgansims and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733–7. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 3.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev. 2001;180:100–11. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies KA, Peters AM, Beynon HLC, Walport MJ. Immune complex processing in patients with systemic lupus erythematosus −in vivo imaging and clearance studies. J Clin Invest. 1992;90:2075–83. doi: 10.1172/JCI116090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waxman FJ, Hebert LH, Smead WL, et al. Complement depletion accelerates the clearance of immune complexes from the circulation of primates. J Clin Invest. 1984;74:1329–40. doi: 10.1172/JCI111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waxman FJ, Hebert LA, Cosio FG, et al. Differential binding of immunoglobulin A and immunoglobulin G1 immune complexes to primate erythrocytes in vivo. J Clin Invest. 1986;77:82–9. doi: 10.1172/JCI112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schifferli JA, Ng YC, Paccaud JP, Walport MJ. The role of hypocomplementaemia and low erythrocyte complement receptor type 1 numbers in determining abnormal immune complex clearance in humans. Clin Exp Immunol. 1989;75:329–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Birmingham D. The type one complement receptor (CR1) and human SLE. In: Kammer GM, Tsokos GC, editors. Lupus: cellular and molecular pathogenesis. Totowa, NJ: Humana Press Inc.; 1999. pp. 541–56. [Google Scholar]
- 10.Manzi S, Navratil JS, Ruffing MJ, et al. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3596–604. doi: 10.1002/art.20561. [DOI] [PubMed] [Google Scholar]
- 11.Ripoche J, Sim RB. Loss of complement receptor type 1 (CR1) on ageing of erythrocytes. Studies of proteolytic release of the receptor. Biochem J. 1986;235:815–21. doi: 10.1042/bj2350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa JE, Harrison RA, Barker PJ, Lachmann PJ. An anti-peptide antibody that recognizes a neo-antigen in the CR1 stump remaining on erythrocytes after proteolysis. Clin Exp Immunol. 1992;87:144–9. doi: 10.1111/j.1365-2249.1992.tb06428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual M, Lutz HU, Steiger G, Stammler P, Schifferli JA. Release of vesicles enriched in complement receptor 1 from human erythrocytes. J Immunol. 1993;151:397–404. [PubMed] [Google Scholar]
- 14.Dervillez X, Oudin S, Libyh MT, et al. Catabolism of the human erythrocyte C3b/C4b receptor (CR1, CD35): vesiculation and/or proteolysis? Immunopharmacology. 1997;38:129–40. doi: 10.1016/s0162-3109(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 15.Birmingham DJ, Hebert LA, Cosio FG, VanAman ME. Immune complex erythrocyte complement receptor interactions in vivo during induction of glomerulonephritis in nonhuman primates. J Lab Clin Med. 1990;116:242–52. [PubMed] [Google Scholar]
- 16.Cosio FG, Shen XP, Van Birmingham DJ, AM Hebert LA. Evaluation of the mechanisms responsible for the reduction in erythrocyte complement receptors when immune complexes form in vivo in primates. J Immunol. 1990;145:4198–206. [PubMed] [Google Scholar]
- 17.Ross GD, Yount WJ, Walport MJ, et al. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985;135:2005–14. [PubMed] [Google Scholar]
- 18.Thomsen BS, Nielsen H, Andersen V. Erythrocyte CR1 (C3b/C4b receptor) levels and disease activity in patients with SLE. Scand J Rheumatol. 1987;16:339–46. doi: 10.3109/03009748709102505. [DOI] [PubMed] [Google Scholar]
- 19.de Carvalho Lins CE, Pereira Crott LS, Teixeira JE, Barbosa JE. Reduced erythrocyte complement receptor type 1 in systemic lupus erythematosus is related to a disease activity index and not to the presence or severity of renal disease. Lupus. 2004;13:517–21. doi: 10.1191/0961203304lu1053oa. [DOI] [PubMed] [Google Scholar]
- 20.Iida K, Mornaghi R, Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982;155:1427–38. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holme E, Fyfe A, Zoma A, Veitch J, Hunter J, Whaley K. Decreased C3b receptors (CR1) on erythrocytes from patients with systemic lupus erythematosus. Clin Exp Immunol. 1986;63:41–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16:467–73. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 23.Rovin BH, Tang Y, Sun J, et al. Clinical significance of fever in the SLE patient receiving steroid therapy. Kidney Int. 2005;68:747–59. doi: 10.1111/j.1523-1755.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 24.Birmingham DJ, Cosio FG. Characterization of the baboon erythrocyte C3b-binding protein. J Immunol. 1989;142:3140–4. [PubMed] [Google Scholar]
- 25.Hebert LA, Birmingham DJ, Shen XP, Cosio FG. Stimulating erythropoiesis increases complement receptor expression on primate erythrocytes. Clin Immunol Immunopathol. 1992;62:301–6. doi: 10.1016/0090-1229(92)90107-y. [DOI] [PubMed] [Google Scholar]
- 26.Birmingham DJ, Chen W, Liang G, Schmitt HC, Gavit K, Nagaraja HN. A CR1 polymorphism associated with constitutive erythrocyte CR1 levels affects binding to C4b but not C3b. Immunology. 2003;108:531–8. doi: 10.1046/j.1365-2567.2003.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JG, Wong WW, Schur PH, Fearon DT. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982;307:981–6. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JG, Wong WW, Murphy EE, Schur PH, Fearon DT. Deficiency of the C3b/C4b receptor (CR1) of erythrocytes in systemic lupus erythematosus: analysis of the stability of the defect and of a restriction fragment length polymorphism of the CR1 gene. J Immunol. 1987;138:2706–10. [PubMed] [Google Scholar]
- 29.Birmingham DJ, Logar CM, Shen X-P, Chen W. The baboon erythrocyte complement receptor is a glycophosphatidylinositol-linked protein encoded by a homologue of the human CR1-like genetic element. J Immunol. 1996;157:2586–92. [PubMed] [Google Scholar]
- 30.Birmingham DJ. Erythrocyte complement receptors. Crit Rev Immunol. 1995;15:133–54. doi: 10.1615/critrevimmunol.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- 31.Walport MJ, Ross GD, Mackworth YC, Watson JV, Hogg N, Lachmann PJ. Family studies of erythrocyte complement receptor type 1 levels: reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol. 1985;59:547–54. [PMC free article] [PubMed] [Google Scholar]
- 32.Paccaud JP, Carpentier JL, Schifferli JA. Direct evidence for the clustered nature of complement receptors type 1 on the erythrocyte membrane. J Immunol. 1988;141:3889–94. [PubMed] [Google Scholar]
- 33.Chevalier J, Kazatchkine MD. Distribution in clusters of complement receptor type one (CR1) on human erythrocytes. J Immunol. 1989;142:2031–6. [PubMed] [Google Scholar]
- 34.Taylor RP, Pocanic F, Reist C, Wright EL. Complement-opsonized IgG antibody/dsDNA immune complexes bind to CR1 clusters on isolated human erythrocytes. Clin Immunol Immunopathol. 1991;61:143–60. doi: 10.1016/s0090-1229(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 35.Hebert LA, Birmingham DJ, Dillon JJ, Cosio FG, Shen XP. Erythropoietin therapy in humans increases erythrocyte expression of complement receptor type 1 (CD35) J Am Soc Nephrol. 1994;4:1786–91. doi: 10.1681/ASN.V4101786. [DOI] [PubMed] [Google Scholar]