Abstract
We have reported previously that naive T cells from relapsing-remitting multiple sclerosis (RRMS) patients have T cell receptor (TCR) repertoire shifts, but the basis of these TCR repertoire shifts was uncertain. Here, we questioned whether RRMS patients have altered naive CD4 and CD8 T cell homeostasis by studying homeostatic proliferation and thymic production in RRMS patients and healthy controls. We measured thymic production by quantifying signal joint T cell receptor excision circles (sjTRECs). Both naive T subsets from controls showed an age-associated decrease in sjTRECs, i.e. evidence of progressive thymic involution, but we detected no age-associated decrease in sjTRECs in RRMS patients. Instead, naive CD8 T cells from patients had lower sjTRECs (P = 0·012) and higher Ki-67 proliferation levels (P = 0·04) than controls. Naive CD4 T cell sjTRECs did not differ between patients and controls. However, in RRMS these sjTRECs correlated strongly with CD31, a marker expressed by newly generated CD4 T cells but not by naive CD4 T cells that have undergone homeostatic proliferation. HLA-DR2 positivity correlated negatively with naive CD4 T cell CD31 expression in RRMS (P = 0·002). We conclude in RRMS that naive T subsets have homeostatic abnormalities due probably to peripheral (non-thymic) mechanisms. These abnormalities could have relevance for MS pathogenesis, as naive T cell changes may precede MS onset.
Keywords: multiple sclerosis (MS), signal joint T cell receptor excision circles (sjTRECs), T cell homeostasis, T cell receptor (TCR)
Introduction
Multiple sclerosis (MS) is a complex and possibly heterogeneous disorder of the central nervous system (CNS) of uncertain aetiology [1]. An anti-myelin autoimmune response occurs in MS patients and in the animal models of MS. CD4 T cells have a central role in initiating this autoreactivity [2,3]. In the T cell response in MS, the CD4 T cell receptors (TCRs) are oligoclonal and restricted in some MS patients but diverse between patients [4]. In an earlier study, we reported that identical twins discordant for MS have a shift in the overall diversity of their naive CD4 TCR J beta segments, i.e. of their TRBJ repertoires [5]. In a more recent study of discordant MS twins, we observed that both the MS patients and their apparently healthy identical co-twins have a shift in their naive CD4 T cell repertoires affecting the most diverse portions of the TCR, i.e. the CDR3 regions [6]. Because the thymus is probably the only source of naive human CD4 T cells [7], one possible conclusion is that the TCR repertoire shifts occurred during TCR repertoire formation in the thymus in these twins before the onset of MS.
Abnormal T cell generation by the thymus could have relevance for development of MS. Consequently, we studied thymic T cell production further by quantifying signal joint T cell receptor excision circles (sjTRECs) in naive CD4 and CD8 T subsets isolated from patients with relapsing-remitting MS (RRMS) and from age-matched healthy controls; we included naive CD8 T cells in the study in view of the probable role of CD8 T cells in CNS damage in MS [8–10]. The sjTRECs are generated in double-positive (CD4+CD8+) thymocytes by excision of the TCR delta locus from the TCR alpha locus during TCR gene rearrangements. These sjTRECs persist as circular, extrachromosomal, intracytoplasmic fragments in newly generated naive CD4 and CD8 T cells, i.e. in recent thymic emigrants (RTEs). Therefore, some researchers use sjTREC content as an estimate of the number of RTEs [7,11–17]. However, sjTREC levels are not simply a measure of RTEs. For example, the progressive, age-associated decrease in T cell generation by the thymus in healthy individuals is accompanied by homeostatic naive T cell proliferation to maintain the size of the naive T cell compartment or niche. The sjTRECs do not replicate during mitosis. Consequently, homeostatic proliferation dilutes sjTREC content, and the extent of homeostatic proliferation influences sjTREC levels. Similarly, antigen-induced proliferation and entry of naive T cells into the memory T cell compartment reduce T cell sjTREC levels [7,14–21].
Naive T cells and memory T cells occupy different niches that are regulated independently [18,19]. For example, naive CD4 and CD8 T cell numbers remain relatively constant throughout life, whereas the number of memory CD4 and CD8 T cells varies with age [22] and the number of memory CD8 T cells can vary dramatically during some antiviral immune responses [20]. Because of these important differences between naive and memory T cells we decided, unlike others [21], that it is inappropriate to measure sjTRECs in the collective memory and naive T cell populations in order to make inferences about naive T cell subsets in RRMS. Because naive T cells have not been antigen-stimulated [23], we reasoned further that analysis of naive T cell sjTREC levels might elucidate changes that precede the onset of MS and possibly predispose to MS. Furthermore, as there is ample evidence that sjTRECs occur almost entirely in naive T cells and the sjTREC content of the memory T subsets is low [7,11–17], we measured sjTREC levels exclusively in naive CD4 and CD8 T cells. We also included measures of naive T cell homeostatic proliferation. Our overall objective was to determine whether RRMS patients have a significant alteration in naive T cell homeostasis due to either altered T cell generation by the thymus and/or to peripheral mechanisms such as increased homeostatic T cell proliferation.
Kong et al. [24] reported that treatment with glucocorticoids leads to impaired thymic output of naive T cells in chickens. Thus, after a 5–7-week treatment regimen, DNA deletion circle levels fell to as low as 10% of values quantified in healthy controls [24]. With 3 weeks of glucocorticoid treatment, DNA deletion circles declined by 50–60%[24]. This decline in thymic output seemed to be reversible and DNA deletion circles returned to normal levels within a month after discontinuation of the treatment [24]. Although these DNA deletion circles are comparable to sjTRECs in humans, we questioned whether treatment with glucocorticoids or other immunomodulatory agents has long-term effects on sjTREC levels in human T cells. For this reason, our exclusion criterion for patients in this study was treatment with corticosteroids and/or immunomodulatory agents at any time during the natural history of their disease.
Materials and methods
Patients and controls
We recruited 42 RRMS patients (median age = 39 years) from the MS clinics at the Montreal Neurological Hospital over a 9-month period. None of the patients had been treated with either corticosteroids or immunomodulatory agents. All patients had clinically definite MS by the accepted criteria of McDonald et al. [25] and all patients were in clinical remission at the time that blood samples were drawn. Disease duration for patients varied between 1 and 30 years with a median disease duration of 11·2 years (Fig. 1). RRMS patients, treated with corticosteroids or immunomodulatory agents during the natural history of their disease, were excluded from the study. Negative treatment history was confirmed by chart review and patient interview. We recruited an independent set of 48 healthy controls (median age = 40 years) who had no history of autoimmune disease. We studied a greater number of individuals for naive CD4 T cell sjTRECs than for naive CD8 T cell sjTRECs, as we developed the method for naive CD4 T cell isolation before the method for naive CD8 T cell isolation. This study received ethical approval from the Institutional Review Board of the Faculty of Medicine at McGill University in accordance with the published guidelines of the Tri-Council Policy Statement (Canada).
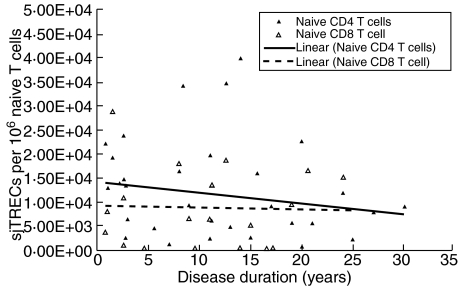
Fig. 1.
Naive T cell subset signal joint T cell receptor excision circles (sjTREC) levels in relapsing-remitting multiple sclerosis (RRMS) patients based on disease duration. There is no relationship between sjTREC levels and disease duration for either naive T cell subset in RRMS.
Isolation of peripheral blood mononuclear cells (PBMCs) and naive T cell subsets
PBMCs were isolated from 60 ml of ethylenediamine tetraacetic acid (EDTA)-treated blood by density-gradient centrifugation using the under-laying method with Ficoll-Paque™ PLUS (Amersham Biosciences, Uppsala, Sweden). We separated PBMC into CD4 and CD8 T cell populations using magnetic anti-CD4 and anti-CD8 microbeads (Miltenyi Biotech, Foster City, CA, USA) according to the manufacturer's instructions for MACS technology. All cells were treated with a release reagent (Miltenyi Biotech) to remove magnetic beads. Isolation of naive CD4+CD45RA+ and naive CD8+CD45RA+ T cells was then achieved by negative selection using magnetic anti-CD45RO microbeads (Miltenyi Biotech).
Immunophenotypic analysis by flow cytometry
For immunophenotypic analysis, the naive CD4 and CD8 T cells were single- and/or double-stained with fluorescent-conjugated monoclonal antibodies (mAbs) specific for cell surface markers and analysed by flow cytometry using a FACSCalibur™ (Becton Dickinson, San Diego, CA, USA) with Cellquest® software. Fluorescein isothiocyanate (FITC) labelled anti-CD4, anti-CD8, anti-CD45RO, anti-CD27 antibodies and phycoerythrin (PE)-labelled anti-CD45RA and anti-CD31 antibodies were purchased from BD PharMingen (San Diego, CA, USA). Single stains were performed using each of these antibodies and double stains were performed using FITC-labelled anti-CD4/PE-labelled anti-CD45RA, FITC-labelled anti-CD8/PE-labelled anti-CD45RA and FITC-labelled anti-CD27/PE-labelled anti-CD45RA.
Naive T cell expression of the Ki-67 proliferation antigen was determined by flow cytometry after nuclear staining with an anti-Ki-67 antibody (BD PharMingen) as described by Hazenberg et al. [12].
Quantification of sjTRECs by real-time polymerase chain reaction (PCR)
DNA was extracted from purified naive CD4 and CD8 T cells using the DNA Isolation Kit (Gentra, Minneapolis, MN, USA), according to the manufacturer's instructions. A genomic DNA sample from thymus tissue was prepared by homogenization. The sjTREC external standard was generated by PCR amplification followed by cloning of a 376 base pairs (bp) fragment into a plasmid, pCR®2·1-TOPO® (Invitrogen, Carlsbad, CA, USA). TOP 10 Escherichia coli containing the plasmid was grown in Luria–Bertani broth and the plasmid isolated by MidiPrep® (Qiagen, Mississauga, ON, Canada), according to the manufacturer's instructions. The absolute number of molecules of sjTREC external standard per 1 µg of plasmid DNA was calculated and then used to prepare stock dilutions of 109, 108, 107, 106, 105, 104, 103 and 102 molecules of sjTREC per 5 µl (stored at −80°C). For real-time PCR analysis of sjTRECs, we used the forward and reverse primers, and a sjTREC probe labelled at the 5′-end with FAM™ and the 3′-end with TAMRA™ described by Pirovano and Mazzolari [22]. One vial of each dilution was thawed immediately before use and run in triplicate to generate an external standard curve for each real-time PCR run. All acceptable runs had curve fits R2 = 0·99.
As an internal control, to normalize for input DNA we used exon 12 of the albumin gene, which has two genomic copies per cell [26] and no pseudogenes [27]. We PCR-amplified and cloned a 72 bp albumin gene fragment into pCR®2·1-TOPO® (Invitrogen). TOP 10 Escherichia coli containing the plasmid was then grown in Luria–Bertani broth and the plasmid was isolated by MidiPrep® (Qiagen), according to the manufacturer's instructions. The absolute number of molecules of the albumin internal standard per 1 µg of plasmid DNA was calculated and then used to prepare dilutions of 109, 108, 107, 106, 105, 104, 103 and 102 molecules of albumin per 5 µl (stored at −80°C). For real-time PCR analysis of the albumin control we used the forward and reverse primers, and a probe labelled at the 5′-end with FAM™ and the 3′-end with TAMRA™, as described by Aarskog and Vedeler [26]. One vial of each dilution was thawed immediately before use and run in triplicate to generate an internal standard curve for each real-time PCR run. All acceptable runs had curve fits R2 = 0·99. In addition, in every experiment, a non-template control which contained all reagents but lacked plasmid or DNA was included for both primer/probe combinations and run in triplicate. To detect sjTRECs a real-time PCR TaqMan® method was used. Each PCR reaction was performed in 50 µl containing 500 ng DNA, 1·0 × buffer A (containing a passive reference dye) (Applied Biosystems, Foster City, CA, USA), 5·0 mM MgCl2, 200 µM dATP, dGTP, dCTP, 400 µM dUTP, 900 nM forward and reverse primer, 200 nM probe, 0·5 U AmpErase (Applied Biosystems) and 0·25 U AmpliTaq Gold (Applied Biosystems). Real-time PCR was performed as a single-plex reaction using the ABI Prism® 7000 (Applied Biosystems) under the following conditions: 50°C for 2 min followed by 95°C for 10 min, after which 50 cycles of amplification were carried out (95°C for 15 s, 60°C for 1 minute).
HLA-DR2 (HLA-DRB5*0101) typing
We used PCR sequence-specific oligonucleotide typing to identify patients and controls positive or negative for DR2 (DRB5*0101). Briefly, we amplified DRB genes by PCR as described previously [28], dot-blotted the PCR-amplified material onto Hybond™-N membranes (Amersham Biosciences, Baie d’Urfe, QC, Canada) and then used a DRB5*0101 probe labelled with a DIG1 oligonucleotide tailing kit, second generation (Roche Applied Science, Laval, QC, Canada). Prehybridization and hybridization were performed with the DIG Easy Hyb Solution (Roche) and post-hybridization washes were performed according to the manufacturer's instructions. Colour detection utilized the DIG nucleic acid detection kit and the DIG wash and block buffer set (Roche) according to the manufacturer's instructions. DR2 (DRB5*0101)-positive and negative controls were included on every membrane, and these controls always gave clear positive and negative results.
Statistical analysis
Statistical analyses were performed using correlation analysis, the Wilcoxon/Mann–Whitney U-test for independent groups from non-normal populations, and regression analysis. All statistical analyses were performed using SPSS® 12·0 for Windows. A value of P < 0·05 was considered to be significant.
Results
Phenotypic studies and verification of naive phenotype
In all lymphocyte preparations more than 95% of isolated naive CD4 T cells were CD4+ and more than 95% of the isolated naive CD8 T cells were CD8+. Co-expression experiments, for each lymphocyte preparation, showed that more than 95% of CD4 and CD8 T cells co-expressed the naive T cell marker CD45RA [29] and also co-expressed CD27, which we used as an additional naive T cell marker [12]. In addition, cases were stained with CD45RO, a surface marker for memory T cells, in order to discriminate further naive versus memory cells as well as to verify that the isolated naive CD4 T cells and naive CD8 T cells were of high purity. In the majority of cases, less than 6% of gated lymphocytes were CD45RO+.
Reproducibility of sjTREC measurements
We confirmed that sjTREC quantification is reproducible by analysing in triplicate, in two separate experiments, the sjTREC levels in the DNA from a randomly selected sample (case 56) using real-time PCR. In experiment 1, the mean result was 4769·87 sjTRECs per 106 naive CD4 T cells and in experiment 2 (performed over a month later) the mean result was 4726·29 sjTRECs per 106 naive CD4 T cells. Because these results differ by less than 1%, we conclude that sjTREC quantification is reproducible. Similarly, in a second representative sample, we confirmed that sjTREC quantification is reproducible at the lower limits of detection; the triplicate analyses of a patient sample with mean sjTRECs of 1379·69 per 106 naive T cells had a standard deviation of 0·132 cycle threshold (Ct).
Naive CD4 T cell sjTREC analyses in RRMS patients versus healthy controls
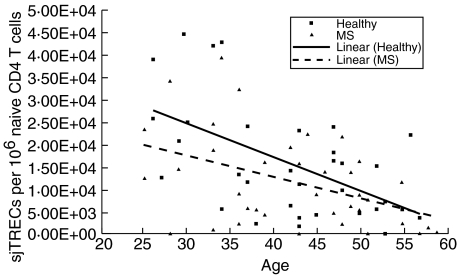
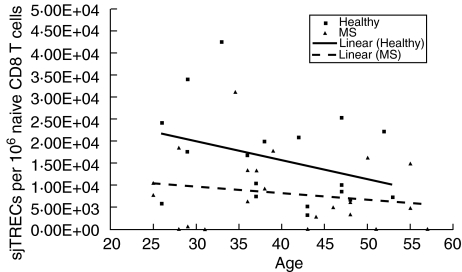
Considerable variation exists in the number of sjTRECs/106 naive CD4 T cells among healthy controls (mean 15 834·60, median 13 341·13, range 52·06–43 264·59) and the RRMS patients (mean 11 560·64, median 8782·32, range 0–39 672·71). As expected from previous reports [11,17], naive CD4 T cell sjTREC levels decrease with increasing age in healthy controls (Fig. 2). Simple linear regression analysis confirms a significant inverse linear relationship between age and naive CD4 T cell sjTREC levels in the group of healthy controls (r = –0·56; P = 0·005). In contrast, naive CD4 T cell sjTREC levels do not decline significantly with increasing age in the RRMS patients (r = –0·44; P = 0·154). Furthermore, RRMS patients and controls do not have significant differences in their naive CD4 T cell sjTREC levels (P = 0·1061) (Wilcoxon/Mann–Whitney U-test).
Fig. 2.
Naive CD4 T cell signal joint T cell receptor excision circles (sjTREC) levels in healthy controls and relapsing-remitting multiple sclerosis (RRMS) patients based on age. sjTREC levels decrease significantly with increasing age in healthy controls.
We considered the possibility that disease duration influences naive CD4 T cell sjTREC levels in RRMS patients, but there is no linear relationship between disease duration and sjTREC levels (r = 0·184; P > 0·1) (Fig. 1).
Ki-67 and CD31 expression on naive CD4 T cells: comparisons of MS patients and controls
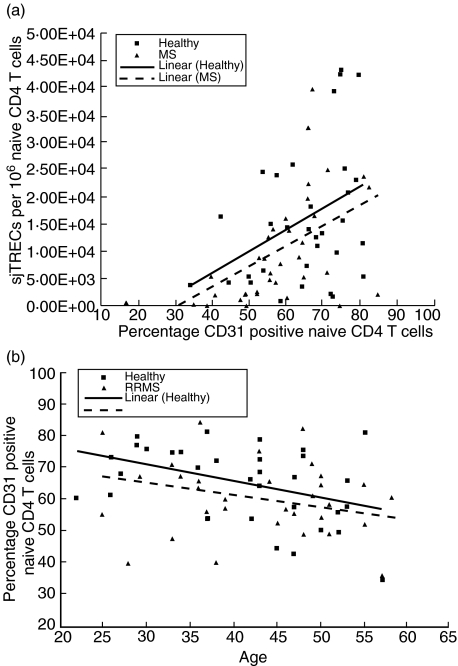
In view of an apparently conflicting report that RRMS patients have significantly lower total CD4 T cell sjTREC levels than do healthy controls [21], we questioned whether RRMS patients might have alterations in measures of naive CD4 T cell homeostasis other than sjTREC levels. We used two approaches to study naive CD4 T cell homeostatic proliferation. First, we used a nuclear stain for the nuclear proliferation antigen Ki-67; proliferation data obtained with this method are comparable to data obtained by in vivo labelling of dividing cells with radiolabelled glucose [12]. Naive CD4 T cells show no statistically significant differences in mean Ki-67 levels between the MS patients (mean 0·20%, range 0·01–0·27) and the unrelated healthy controls (mean 0·23%, range 0·04–0·93). As Ki-67 provides only a ‘snapshot’ of cell proliferation at the time of cell isolation [12], we also analysed CD31 percentage expression in RRMS patients (mean 59·34%, range 16·28–84·77) and controls (mean 64·93%, range 34·14–80·89) (Fig. 3a). CD31 is a differentiation antigen, which is expressed on CD4 T cells that are newly generated by the thymus, i.e. RTEs. These CD31-positive naive CD4 T cells have high sjTREC content. CD31 is no longer expressed on naive CD4 T cells that have undergone homeostatic proliferation [30], and these cells have low sjTREC content. Thus, the percentage of expression of CD31 provides a sensitive measure of the proliferation history of cells within the naive CD4 T cell niche [30]. RRMS patients have significantly lower naive CD4 T cell CD31 counts than do healthy controls (P = 0·03) (Wilcoxon/Mann–Whitney U-test). As expected [30], correlation analysis indicates that healthy controls (r = 0·391; P = 0·027) have a positive linear relationship between sjTRECs and CD31 counts in naive CD4 T cells. Similarly, there is a positive linear relationship between sjTRECs and CD31 counts in naive CD4 T cells from RRMS patients (r = 0·543; P < 0·001), but this linear relationship is stronger in RRMS patients than in controls. This observation led us to question whether there are subtle differences in the proliferation history of naive CD4 T cells from RRMS patients versus controls (see below).
Fig. 3.
(a) Naive CD4 T cell signal joint T cell receptor excision circles (sjTREC) levels and CD31 percentage of expression in relapsing-remitting multiple sclerosis (RRMS) patients compared to healthy controls. There is a positive linear relationship between sjTREC levels and CD31 counts. (b) Naive CD4 T cell CD31 percentage of expression based on age in RRMS patients compared to healthy controls. RRMS patients have significantly lower CD31 percentage of expression than healthy controls.
RRMS patients and healthy controls differ in factors influencing naive CD4 T cell sjTREC content
The strong correlation between naive CD4 T cell CD31 counts and sjTRECs in RRMS suggested that peripheral, non-thymic factors could have a major influence on sjTREC levels in RRMS, particularly as we found no evidence for a significant thymic influence on sjTREC levels in RRMS (see above). We performed multiple linear regression analyses to study further the relationship between naive CD4 T cell sjTREC levels and age and percentage of expression of CD31. The linear relationship between the response variable, i.e. sjTRECs, and the explanatory variables, i.e. both age and percentage of expression of CD31, is significant in both the MS patients (F = 8·712; P < 0·001) and in the healthy controls (F = 8·834; P < 0·001). However, using coefficient analysis we observe that age (t = −3·247; P = 0·003), but not CD31 (t = 1·078; P = 0·29), is the significant explanatory variable in healthy controls, whereas it is CD31 (t = 2·73; P = 0·01) but not age (t = −0·459; P = 0·649), which is the significant explanatory variable in MS patients. Thus, we confirm that the main influence on the naive T cell niche in healthy controls is thymic production [19,20,30,31], which declines exponentially with age [17,31]. In contrast, the linear regression analysis confirms that there is not a significant age-associated decline in thymic production of naive CD4 T cells in RRMS patients (P = 0·154). Instead, the linear regression data, together with the significant differences in patient and control CD31 levels, suggest that the naive CD4 T cell niche is altered in RRMS due to non-thymic factors influencing homeostatic turnover of naive CD4 T cells.
HLA-DR2 (DRB5*0101), sjTREC levels and CD31 counts
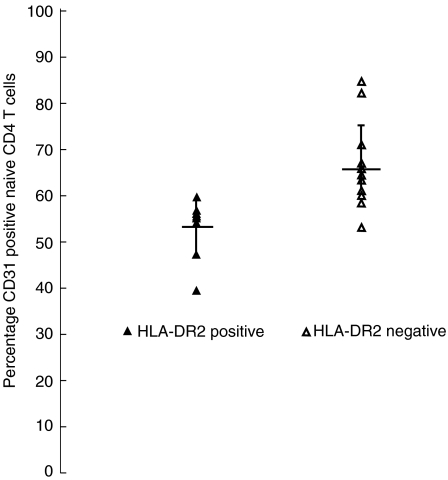
Many studies show an increased frequency of the common Caucasian HLA-DR2 (DRB5*0101) haplotype in MS patients compared with healthy controls [28]. Because thymic selection of CD4 T cells is influenced by HLA class II gene expression, and peripheral naive CD4 T cell survival requires MHC class II recognition [15,16], we investigated the possibility that HLA-DR2 positivity influences naive CD4 T cell sjTREC levels or CD31 counts. Eleven of 48 controls (23%) and 18 of 42 RRMS patients (43%) were DR2+(Fig. 4). The DR2-positivity did not correlate with naive CD4 T cell sjTREC levels either in controls or in RRMS patients (data not shown). In contrast, correlation analysis shows a negative linear relationship between DR2-positivity and naive CD4 T cell CD31 counts in RRMS patients (r = –0·598; P = 0·002) but not in controls (r = 0·208; P = 0·298). The mean CD31 percentage of expression in naive CD4 T cells was significantly lower (P < 0·001) in DR2+ RRMS patients (54·4%) than in DR2− RRMS patients (65·3%). As DR2-positivity predicts low naive CD4 T cell CD31 percentage of expression but does not predict sjTREC levels in RRMS, these findings support further the view that peripheral processes alter naive CD4 T cell homeostasis in RRMS.
Fig. 4.
Naive CD4 T cell CD31 percentage of expression based on HLA-DR2 positivity in relapsing-remitting multiple sclerosis (RRMS) patients. The mean CD31 percentage of expression on naive CD4 T cells is significantly lower in DR2+ patients than in DR2− patients.
Naive CD8 T cell sjTREC levels in healthy controls and in RRMS patients
Considerable variation exists in the number of sjTRECs/106 naive CD8 T cells among healthy controls (mean 15 942·16, median 13 614·13, range 3181·61–42 341·24) and among the RRMS patients (mean 8120·52, median 6131·18, range 0–28 552·20). As expected from previous reports [14,20], sjTREC levels appear to decrease with increasing age in healthy controls (Fig. 5). Moreover, correlation analysis indicates that healthy controls have a significant inverse linear relationship between age and naive CD8 T cell sjTREC levels (r = –0·457; P = 0·025). In contrast, but similar to our findings on sjTRECs in naive CD4 T cells in RRMS, the naive CD8 T cell sjTREC levels do not decline significantly with increasing age in the RRMS patients (r = –0·181; P = 0·421). Furthermore, the RRMS patients have significantly lower naive CD8 T cell sjTREC levels than do healthy controls (P = 0·012) (Wilcoxon/Mann–Whitney U-test).
Fig. 5.
Naive CD8 T cell signal joint T cell receptor excision circle (sjTREC) levels in relapsing-remitting multiple sclerosis (RRMS) patients and in healthy controls based on age. RRMS patients have significantly lower naive CD8 T cell sjTREC levels than healthy controls.
We considered the possibility that disease duration influences naive CD8 T cell sjTREC levels but find no significant linear relationship between disease duration and sjTREC levels (r = 0·038; P >0·1) (Fig. 1).
Ki-67 expression on naive CD8 T cells: comparisons of MS patients and controls
We questioned whether naive CD8 T cells in RRMS patients have evidence of increased proliferation compared with controls. The Ki-67 counts of naive CD8 T cells are significantly higher in RRMS patients (mean 0·34%, range 0·14–0·58) than in healthy controls (mean 0·18%, range 0·01–0·95) (P = 0·04). We did not measure percentage of expression of CD31 on naive CD8 T cells, as we do not know of literature suggesting that this marker is lost from naive CD8 T cells that have undergone homeostatic proliferation.
HLA-DR2 (DRB5*0101) and sjTREC levels in naive CD8 T cells
HLA-DR2 positivity showed a trend towards a positive correlation with sjTREC levels in naive CD8 T cells from RRMS patients (r = 0·382; P = 0·079).
Discussion
Our study identifies novel alterations in homeostasis of the two naive T cell subsets in RRMS patients. First, the untreated patients have a number of significant alterations in their naive CD8 T cells. Thus, the levels of sjTRECs in isolated naive CD8 (CD45RA+) T cells are significantly lower in RRMS than in healthy controls. sjTREC levels are a well-accepted, surrogate measure of thymic production [7,11–17,30]. These levels are also influenced by homeostatic naive T cell proliferation [30] and by antigen-induced proliferation with entry of naive T cells into the memory T cell compartment [11–17,30]. Thus, an important issue is the basis of the low naive CD8 T cell sjTREC levels in RRMS. We think it unlikely that the reduced sjTREC levels in this subset are due to antigen-induced triggering of naive T cells, as the number of autoreactive T cells in the MS autoimmune response represent only a minor component of the peripheral naive T cell repertoire [32]. Our finding of a progressive and significant decrease in naive CD8 T cell sjTREC levels in healthy controls with increasing age confirms that thymic production is the main influence on the naive T cell niche in healthy controls [18,19,33,34]. In contrast, the low levels of sjTRECs in naive CD8 T cells from RRMS patients, independent of age (P = 0·421), suggest that the normal age-associated and progressive decrease in thymic production that we detected (P = 0·025) does not occur to any significant extent in RRMS. One possible explanation is that some process or event early in life, i.e. before the onset of MS, induces premature thymic involution and that the involuted thymus produces a fairly constant level of naive CD8 T cells irrespective of increasing age. An alternative possibility, which we favour, is that some peripheral (non-thymic) process or event in RRMS induces either sudden or sustained loss of naive CD8 T cells at an age when the thymus has limited ability to compensate for T cell lymphopenia, i.e. after puberty [35]. Homeostasis of the naive T cell niche involves a balance between thymic production, delivery of death and survival signals and homeostatic proliferation [15]. The significantly increased expression of the nuclear proliferation antigen Ki-67 by naive CD8 T cells in RRMS indicates that these T cells have increased homeostatic proliferation, presumably in order to maintain the size of the naive CD8 T cell niche. We cannot exclude altered thymic production completely as an explanation for the low naive CD8 T cell sjTREC levels. However, our identification of increased naive CD8 T cell proliferation by Ki-67 staining is more in keeping with some ongoing peripheral process, e.g. reduced delivery of survival signals, which decreases naive CD8 T cell viability and leads to increased homeostatic proliferation in patients with established RRMS. One implication of this increased proliferative activity is that the overall naive CD8 T cell diversity must be reduced in RRMS, analogous to what is described in patients with rheumatoid arthritis [36], who also show reduced sjTREC levels [36]. This reduced diversity could have relevance for MS pathogenesis by affecting the ability of RRMS patients to mount an effective CD8 T cell response to various pathogens.
Hug et al. [21] reported reduced sjTREC levels in CD8 T cells from RRMS patients but concluded, unlike ourselves, that this reduction is due to impaired thymic export of T cells and not to peripheral mechanisms. We think the different conclusions stem from important methodological differences between the two laboratories. Whereas Hug et al. [21] measured sjTRECs in total CD8 T cells, which include naive T cells plus memory and effector T cells, we focused only on naive CD8 T cells. Memory T cells contain few sjTRECs, as these cells have undergone typically several cycles of cell division [37]. sjTRECs do not replicate and are diluted-out during cell division [7,11–17]. Memory T cell numbers increase with age [38]. Furthermore, although we know of no data on the numbers of memory CD8 T cells in RRMS patients versus controls, the size of the memory T cell compartment could vary according to the magnitude of the chronic immune response in MS. Consequently the findings of Hug et al. [21] could be due, in part, to both age-associated and disease-associated differences in memory T cell numbers between RRMS patients and controls. In contrast, our study of naive T cell subsets excludes the possible effects of chronic immune stimulation that occurs in MS [2]. Consistent with that opinion, we find no evidence that the duration of RRMS affects sjTREC levels in either naive T subset (Fig. 1). Importantly, Hug et al. [21] also found no differences in CD8 T cell proliferation between RRMS patients and controls using a telomere assay. We question whether these investigators would have detected homeostatic proliferation of naive CD8 T cells in RRMS, as this proliferation would be masked by the normally occurring and significant proliferation history of memory CD8 T cells within the total CD8 T cell populations from patients and controls.
As reported by others [7,12–17], we find that naive CD4 T cell sjTRECs decrease significantly in healthy controls with increasing age (P = 0·005). Although Fig. 2 suggests that there may be a minor decline in naive CD4 T cell sjTRECs with increasing age in RRMS, we detected no age-associated decrease in sjTRECs in RRMS (P = 0·154). Al-Harthi et al. [39] detected a significant negative correlation between age and sjTRECs in a study of 22 healthy controls. In other words, our number (n = 42) should have been sufficient to detect a significant negative correlation in RRMS, assuming that this correlation is similar in any way to what occurs in healthy individuals. In contrast to Hug et al. [21], we find no significant differences in naive CD4 T cell sjTREC levels between RRMS patients and controls. Our CD4 T cell findings in RRMS reflect our focus on naive T cells which are the only CD4 T cells that have significant sjTREC levels, whereas Hug et al. [21] analysed total CD4 T cells, which include memory and naive CD4 T cells. Our data point instead towards subtle alterations of naive CD4 T cell homeostasis in RRMS. Thus, the linear regression analysis indicates that the percentage of expression of CD31 but not age predicts naive CD4 T cells sjTREC levels in RRMS. CD31 is a differentiation antigen expressed on RTEs, which is lost from naive CD4 T cells that have undergone homeostatic proliferation [21]. By inference, because we exclude a major influence of age on naive CD4 T cell sjTREC levels (Fig. 2) in RRMS, and RRMS patients have lower CD31 counts than controls, we suggest that the predominant influences on naive CD4 T cell sjTREC levels in RRMS are peripheral (non-thymic) events affecting homeostasis.
HLA class II molecules, such as HLA-DR, play a role in positive selection of CD4 T cells in the thymus, but trophic interaction with self-peptide MHC is essential for naive CD4 T cell survival in the periphery [19]. DR2-positivity in RRMS patients (P < 0·001) but not controls significantly predicts a low percentage of CD31 expression but not sjTREC levels in naive CD4 T cells. The strong correlation with CD31 counts indicates that the DR2+ RRMS patient group generally has higher levels of homeostatic proliferation than the DR2− RRMS group. Furthermore, in the absence of evidence of an influence of DR2-positivity on sjTREC levels in RRMS (data not shown), the DR2-CD31 data support the conclusion that peripheral processes alter naive CD4 T cell homeostasis in some RRMS patients. The trend towards prediction of sjTREC levels in naive CD8 T cells by DR2 in RRMS patients suggests further that naive T cells may have altered survival in RRMS, as HLA-DR does not affect thymic selection of CD8 T cells [40]. It remains unclear, however, as to why HLA-DR2-positivity would influence peripheral naive T cell homeostasis in RRMS patients but not in controls.
We have reported previously that naive CD4 T cells isolated from identical twins discordant for RRMS have a shift in their TCR repertoires [5,6]. We postulated that this shift was secondary to altered thymic output of naive CD4 T cells, but had no measures that distinguished between central or peripheral mechanisms affecting the TCR repertoire. We find that our present data point away from a central (thymic) mechanism affecting naive CD4 and CD8 T cells in RRMS patients and suggest instead that peripheral (non-thymic) mechanisms alter naive T cell homeostasis in RRMS patients. Four RRMS patients had undetectable sjTREC levels in both naive T subsets (Figs 2 and 5). Presumably, the same mechanism(s) affected both naive T subsets in these particular individuals and led to our overall findings of altered homeostasis in both naive T subsets in RRMS. The question that remains is the relevance of altered naive T cell homeostasis to RRMS. In our earlier identical twin study, both the healthy members and the MS patients from the discordant MS twins had TR repertoire shifts [6]. Consequently, it seems unlikely that the homeostatic abnormalities are sufficient by themselves to cause MS. Nevertheless, these homeostatic abnormalities may predispose to MS, as we study naive T cells that are largely unaffected by the disease process in MS. Further studies are needed to analyse the extent of the homeostatic abnormalities in MS, including studies on the homeostasis of regulatory T cell subsets in MS. Finally, we propose that the homeostatic abnormalities we describe here may also occur in other autoimmune diseases and play a role in their pathogenesis.
References
- 1.Willer CJ, Ebers GC. Susceptibility to multiple sclerosis: interplay between genes and environment. Curr Opin Neurol. 2000;13:241–7. doi: 10.1097/00019052-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 3.Martino G, Hartung HP. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol. 1999;12:309–21. doi: 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Hafler DA, Saadeh MG, Kuchroo VK, Milford E, Steinman L. TCR usage in human and experimental demyelinating disease. Immunol Today. 1996;17:152–9. doi: 10.1016/0167-5699(96)80611-6. [DOI] [PubMed] [Google Scholar]
- 5.Haegert DG, Cowan T, Murray TJ, Gadag V, O'Connor P. Does a shift in the T cell receptor repertoire precede the onset of MS? Neurology. 1999;53:485–90. doi: 10.1212/wnl.53.3.485. [DOI] [PubMed] [Google Scholar]
- 6.Haegert DG, Galutira D, Murray TJ, O'Connor P, Gadag V. Identical twins discordant for multiple sclerosis have a shift in their T cell receptor repertoires. Clin Exp Immunol. 2003;134:532–7. doi: 10.1111/j.1365-2249.2003.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen M, Cepok S, Quak E, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–50. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- 9.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–9. [PubMed] [Google Scholar]
- 11.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 12.Hazenberg MD, Otto SA, Cohen Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 13.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–86. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye P, Kirschner DE. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J Immunol. 2002;168:4968–79. doi: 10.4049/jimmunol.168.10.4968. [DOI] [PubMed] [Google Scholar]
- 15.Poulin JF, Sylvestre M, Champagne P. Evidence for adequate thymic function but impaired naive T cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003;102:4600–7. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 16.Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001;79:631–40. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 17.Murray JM, Kaufmann GR, Hodgkin PD, et al. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol Cell Biol. 2003;81:487–95. doi: 10.1046/j.1440-1711.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanchot C, Rosado MM, Agenes F, Freitas AA, Rocha B. Lymphocyte homeostasis. Semin Immunol. 1997;9:331–7. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 19.Jameson SC. Maintaining the norm: T cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 20.Callan MF, Fazou C, Yang H, et al. CD8(+) T cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–61. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hug A, Korporal M, Schroder I, et al. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol. 2003;171:432–7. doi: 10.4049/jimmunol.171.1.432. [DOI] [PubMed] [Google Scholar]
- 22.Pirovano S, Mazzolari E, Pasic S, Albertini A, Notarangelo LD, Imberti L. Impaired thymic output and restricted T cell repertoire in two infants with immunodeficiency and early-onset generalized dermatitis. Immunol Lett. 2003;86:93–7. doi: 10.1016/s0165-2478(02)00291-2. [DOI] [PubMed] [Google Scholar]
- 23.Abbas AK, Lichtman AH. Basic immunology: functions and disorders of the immune system. Philadelphia: WB Saunders Co.; 2004. [Google Scholar]
- 24.Kong FK, Chen CL, Cooper MD. Reversible disruption of thymic function by steroid treatment. J Immunol. 2002;168:6500–5. doi: 10.4049/jimmunol.168.12.6500. [DOI] [PubMed] [Google Scholar]
- 25.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 26.Aarskog NK, Vedeler CA. Real-time quantitative polymerase chain reaction. A new method that detects both the peripheral myelin protein 22 duplication in Charcot-Marie-Tooth type 1A disease and the peripheral myelin protein 22 deletion in hereditary neuropathy with liability to pressure palsies. Hum Genet. 2000;107:494–8. doi: 10.1007/s004390000399. [DOI] [PubMed] [Google Scholar]
- 27.Minghetti PP, Ruffner DE, Kuang WJ, et al. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11–22 of chromosome 4. J Biol Chem. 1986;261:6747–57. [PubMed] [Google Scholar]
- 28.Haegert DG, Swift FV, Benedikz J. Evidence for a complex role of HLA class II genotypes in susceptibility to multiple sclerosis in Iceland. Neurology. 1996;46:1107–11. doi: 10.1212/wnl.46.4.1107. [DOI] [PubMed] [Google Scholar]
- 29.Muraro PA, Pette M, Bielekova B, McFarland HF, Martin R. Human autoreactive CD4+ T cells from naive CD45RA+ and memory CD45RO+ subsets differ with respect to epitope specificity and functional antigen avidity. J Immunol. 2000;164:5474–81. doi: 10.4049/jimmunol.164.10.5474. [DOI] [PubMed] [Google Scholar]
- 30.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–94. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol. 2001;125:409–13. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong J, Zang YC, Li S, Rivera VM, Zhang JZ. Ex vivo detection of myelin basic protein-reactive T cells in multiple sclerosis and controls using specific TCR oligonucleotide probes. Eur J Immunol. 2004;34:870–81. doi: 10.1002/eji.200324790. [DOI] [PubMed] [Google Scholar]
- 33.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc Natl Acad Sci USA. 2002;99:2989–94. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr Opin Immunol. 2004;16:775–9. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 36.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:9203–8. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janeway CAJ, Travers P, Walport M, Shlomchik M. Immunobiology. 5. New York: Garland Publishing; 2001. [Google Scholar]
- 38.Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–12. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 39.Al-Harthi L, Marchetti G, Steffens CM, Poulin J, Sekaly R, Landay A. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction–enzyme linked immunosorbent assay (PCR-ELISA) J Immunol Meth. 2000;237:187–97. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 40.Hanson JA, Sohaib SA, Newell-Price J, et al. Computed tomography appearance of the thymus and anterior mediastinum in active Cushing's syndrome. J Clin Endocrinol Metab. 1999;84:602–5. doi: 10.1210/jcem.84.2.5501. [DOI] [PubMed] [Google Scholar]