Abstract
Dendritic cells (DC) have been characterized recently as having an important role in the initiation and control of immunological response to Mycobacterium tuberculosis infection. Blood DC have been subdivided into myeloid (mDC) and plasmacytoid (pDC) subsets, on the basis of differences in phenotype markers and function. Little is known about the enumeration and functional evaluation of circulating DC in patients with tuberculosis and their correlation with clinical outcome during the course of anti-tuberculous treatment. We assessed circulating mDC and pDC counts measured by a newly developed single-platform flow cytometric assay based on TruCOUNT, as well as the production of interferon (IFN)-α after in vitro stimulation by herpes simplex virus (HSV-1) in 24 patients with active tuberculosis (TB) and 37 healthy donors. Absolute numbers of both DC subsets were decreased significantly in patients with active TB compared to controls. Similarly, the production of IFN-α was highly impaired. In 13 patients these parameters were assessed longitudinally, before and after the specific anti-microbial treatment. Most interestingly, in all nine patients with successful anti-tuberculous therapy there was a significant and marked increase of pDC counts and IFN-α production. In contrast, no significant longitudinal variations in DC counts and IFN-α production were observed in four patients with lack of response to specific treatment. In conclusion, active TB is associated with a defect in blood DC numbers and IFN-α production that is restored after bacterial clearance and clinical improvement, as a result of effective anti-tuberculous treatment.
Keywords: anti-tuberculous treatment, dendritic cells, IFN-α production, M. tuberculosis, TruCOUNT
Introduction
Mycobacterium tuberculosis, the aetiological agent of tuberculosis (TB), affects approximately one-third of the world's population and is responsible for 3 million deaths per year [1]. Despite a wide investigation of the immunopathogenesis of TB, the mechanisms leading to complete clearance, latent infection or chronic disease have not been elucidated fully [2,3]. Both innate and adaptive immunity are involved in the protective immune response against M. tuberculosis [4,5]. The successful elimination of the intracellular pathogen from the host depends mainly on the efficient interaction between dendritic cells (DC), activated macrophages as effectors and antigen-specific CD4 and CD8 T cells with the interplay of a complex network of cytokines, chemokines and their receptors [6–8]. DC are the key antigen-presenting cells (APC) controlling the initiation of T cell-dependent immune response. DC encounter microbial pathogen at the site of infection and then traffic to the lymph nodes, where they present pathogen-derived antigens to naive T cells [9–11].
Many experimental studies have shown that DC play a pivotal role in the immunological response during M. tuberculosis infection [12,13]. M. tuberculosis is phagocytosed by human DC, mainly by the interaction between the mycobacterial lipoarabinomannan and DC-specific ICAM-1-grabbing nonintegrin (DC-SIGN) [14]. Mycobacteria-infected DC accumulate in the spleens of mycobacterium-infected mice and are detectable in granulomas of infected rats and humans. It has also been demonstrated that DC have poor mechanisms to eliminate M. tuberculosis and may provide niches for long-term survival of mycobacteria at the site of infection [15]. On the other hand, recent data have shown a critical role for DC in priming the CD4+ T cell response to early secretory antigen of tuberculosis 6 (ESAT6), thus suggesting that DC are essential in the initiation of adaptive T cell response to human M. tuberculosis infection [16].
Two types of DC that can be identified on phenotypic markers and function are circulating in immature form in human blood: myeloid DC (mDC) and plasmacytoid DC (pDC) [17]. mDC express CD11c, require granulocyte–macrophage colony-stimulating factor (GM-CSF) for growth and survival and perform antigen uptake, T cell activation and secretion of interleukin (IL)-12 and IL-18. pDC express CD123, are dependent on IL-3 for survival and produce high levels of interferon (IFN)-α in response to viral infection [18,19]. Recent studies have documented ex vivo significant reduction in circulating DC counts during viral infections; in particular, a decrease in both mDC and pDC counts are found in individuals infected with human immunodeficiency virus (HIV) and in those infected with hepatitis C virus (HCV) [20,21]. Little is known about the direct enumeration and functional evaluation of circulating DC in patients with TB [22].
In the present study, we performed a direct enumeration of both subsets of circulating DC during human TB by using a newly developed single-platform flow cytometric assay based on TruCOUNT [23]. In addition, functional assessment of pDC was performed by analysing IFN-α production [24,25]. The number and function of DC was evaluated longitudinally at the time of diagnosis and after the beginning of anti-tuberculous specific treatment.
Materials and methods
Study population
The study population included 24 patients with TB (17 males, seven females; age range 15–78 years), admitted to the Department of Infectious and Tropical Diseases of the Azienda Policlinico Umberto I, ‘La Sapienza’ University of Rome. Seventeen patients had pulmonary TB, while seven were affected by extrapulmonary TB (three vertebral, two lymphadenitis, one peritoneal and one meningitis). Diagnosis of TB was made on the basis of clinical and radiological findings and was confirmed by identification of M. tuberculosis by microbiological methods and/or histological examination of affected tissues. After the diagnosis all patients were treated with the four classical anti-tuberculosis drugs (rifampicin, isoniazid, pyrazinamide and ethambutol). All patients were seronegative for HIV infection. The study of circulating DC in blood samples was performed in all 24 patients at the time of diagnosis. In addition, 13 patients with TB were analysed for DC numbers and function after the beginning of treatment and were followed for 6 months. Patients were classified as ‘responders to treatment’ if, after 6 months of specific therapy, the results of microbiological cultures in appropriate specimens were negative for M. tuberculosis and there was a clinical and radiological improvement associated with normal inflammatory parameters (C-protein level and sedimentation rate). Thirty-seven healthy donors (18 males, 19 females; age range 15–66 years) were included as controls. Informed consent was obtained from all subjects before being included in the present study.
Reagents
TruCOUNT™ tubes, FACS lysing solution and monoclonal antibodies (mAbs) for DC staining were purchased from Becton Dickinson (BD Biosciences Pharmingen, Italy).
Quantification of circulating pDC and mDC
For DC enumeration, peripheral blood was collected in ethylenediamine tetraacetic acid (EDTA) tubes. Whole blood (0·1 ml) was stained directly in TruCOUNT tubes containing a calibrated number of fluorescent beads. The following mAbs were added: anti-CD45-peridinim chlorophyll (PerCP), anti-human leucocyte antigen (HLA)-D-related (DR)-APC, a lineage–fluorescein isothiocyanate (FITC) cocktail composed of anti-CD3 (SK7), anti-CD14 (MP9), anti-CD16 (3G8), anti-CD19 (SJ25C1), anti-CD20 (L27), anti-CD56 (NCAM 16–2) and anti-CD123-phycoerythrin (PE) or anti-CD11c-PE. Relevant isotype control mAbs were used. After mixing, a 15-min incubation time in the dark at room temperature (RT) was performed. To each tube, 450 µl of fluorescence activated cell sorter (FACS) lysing solution was added, vortexed and incubated for 15 min at RT. Samples were analysed within 1–3 h of staining using a FACScalibur flow cytometer and CellQuest version 1·0 (Becton Dickinson, Mountain View, CA, USA). All data were collected using identical instrument settings.
Gating strategies and sample analysis were as follows: the threshold was set on PerCP fluorescence (FL3) to reduce debris and include TruCOUNT beads; an R1 gate for lymphocytes and monocytes was defined in a dot plot of CD45 versus side scatter (SSC). For rare event analysis of DC, at least 100 000 cellular events in R1 were acquired from 0·1 ml of blood. These events were displayed in a lineage–FITC versus HLA-DR-APC dot plot in which a second gate was created to identify lineage-negative cells (R2 gate). To define mDC and pDC, events from R1 and R2 were analysed in a contour plot of CD11c versus HLA-DR or CD123 versus HLA-DR, respectively. All HLA-DR positive, CD11chi and CD123hi events were included in this gating strategy; 22 000 bead events were collected on average. To calculate the absolute pDC or mDC numbers (cells/ml blood) the following formula was used: (R3 events × known TruCOUNT beads)/(R4 events × 0·1 ml), where the TruCOUNT bead number (R4) was obtained in an ungated dot plot of FL1 versus FL2.
IFN-α production
After Ficoll separation, peripheral blood mononuclear cells (PBMC) (106 cells/ml) were cultured for 18 h at 37°C, 5% CO2, in round-bottomed polypropylene tubes in RPMI-1640 supplemented with glutamax and 10% fetal calf serum (FCS) in the presence or absence of herpes simplex virus (HSV-1) at a multiplicity of infection of 0·25. Cells were removed by centrifugation, and supernatants were stored at −80°C. IFN-α production was assessed by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (BMS216CE, Bender MedSystems, Vienna, Austria), according to the manufacturer's instructions. The detection threshold was < 3·6 pg/ml.
Statistical analysis
Values are given as median and ranges. The differences of values between the healthy controls and the patients were analysed using the non-parametric Mann–Whitney U-test. Data in the longitudinal analysis were evaluated by the Wilcoxon signed-rank test. The significance of correlation analysis was estimated using the Spearman's rank correlation test. P-value < 0·05 was regarded as significant.
Results
Reduction of circulating plasmacytoid DC and myeloid DC in patients with tuberculosis
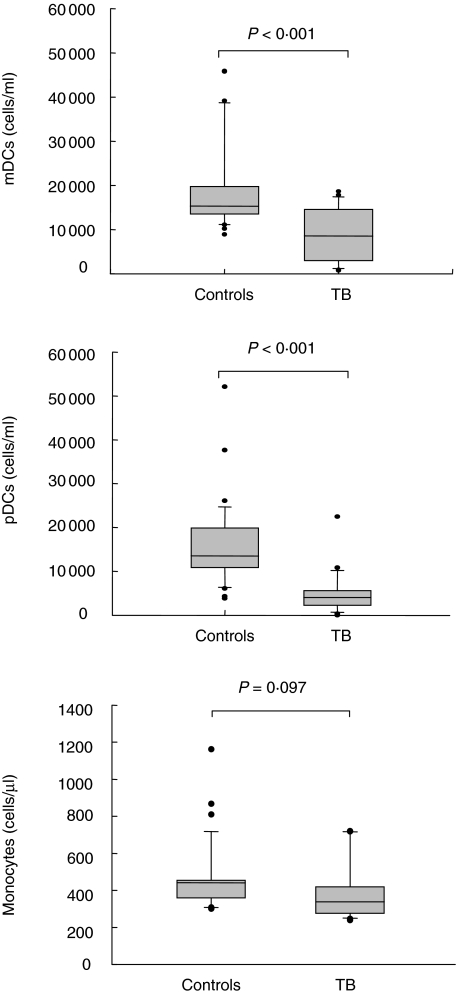
Circulating pDC and mDC were quantified in 24 patients with active TB and 37 healthy donors using the single-platform TruCOUNT assay. Gating strategy used for cytofluorimetric DC subsets definition is shown in Fig. 1: DC subpopulations were distinguished easily from other cells both in controls and patients. A significant reduction in both circulating pDC and mDC was found in patients with TB at the time of diagnosis when compared with healthy donors (P < 0·001 for both) (Fig. 2). Indeed, in TB patients the median (range) DC counts (cells/ml) were as follows: 4014 (2–22 496) for pDC versus 13 553 (3875–52 111) in controls and 8545 (725–18 675) for mDC versus 15 300 (8901–45 917) in controls. As assessed with the non-parametric Spearman's test, a positive significant correlation was found between the two subsets of DC (r = 0·43; P = 0·03). The degree of reduction was more evident for pDC. On the other hand, monocytes count (cells/µl) in TB patients showed no significant differences if compared with healthy donors (median, range: 380, 240–720 versus 440, 290–1150) (P = 0·097) (Fig. 2).
Fig. 1.
Cytofluorimetric dendritic cells (DC) subset definition by TruCOUNT assay. The R1 gate was used for lymphocytes and monocytes. Beads appear at the extreme right of the dot plot (a). The R2 gate was created to identify lineage-negative cells (b). To define myeloid DC (mDC) and plasmacytoid DC (pDC), events from R1 and R2 were analysed in a contour plot of CD11c versus human leucocyte antigen (HLA)-D-related (DR) (c) or CD123 versus HLA-DR (d). All CD11chi-HLA-DR+ and CD123hi-HLA-DR+ were included in this gating strategy. TruCOUNT beads (R4) were obtained in an ungated dot plot of fluorescence ( FL)1 versus FL2 (e).
Fig. 2.
Enumeration of circulating plasmacytoid dendritic cells (pDC), myeloid DC (mDC) and monocytes in 24 patients with active tuberculosis (TB) and 37 healthy donors. Dendritic cells (DC) subsets were measured by a single-platform flow cytometric assay based on TruCOUNT. A significant reduction in both circulating pDC and mDC in patients with TB at the time of diagnosis compared with healthy donors was found. Monocyte count, obtained by automated haematology blood analyser, showed no significant differences in TB patients if compared with healthy controls. Box plots show the 10th, 25th, 50th (median), 75th and 90th percentile and outlying values. Statistical significance was analysed by use of the Mann–Whitney U-test.
When the patients were stratified according to site of infection, no significant differences were found in pDC counts between pulmonary TB and extrapulmonary (median, range: 3956, 2–22 496 and 4073, 974–5754, respectively) (P > 0·05). On the contrary, the absolute number of mDC was significantly lower in patients with extrapulmonary TB than in those with pulmonary TB (median, range: 5894, 725–16 594 versus 8558, 956–18 675, P = 0·013).
Impaired production of IFN-α in patients with tuberculosis
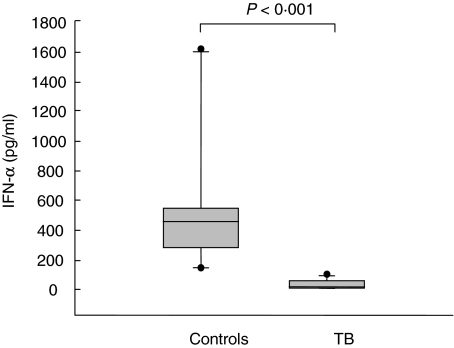
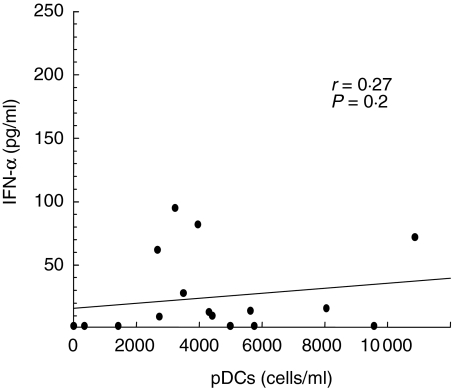
To assess whether the reduction in DC subsets may have functional consequences, the production of IFN-α from PBMC was evaluated in 17 TB patients and in 11 healthy controls. There was a marked decrease of IFN-α production (pg/ml) in patients with TB (median, range: 10, < 3·6–95 versus 450, 138–1612 of controls) (P < 0·001) (Fig. 3). The lowest levels were detected in five patients with extrapulmonary TB (median, range 9·3 pg/ml, < 3·6–14), although no significant differences were found in comparison with the 12 TB pulmonary cases (13, < 3·6–95) (P = 0·291). Moreover, no significant correlation was found between the levels of IFN-α and pDC counts (r = 0·27, P = 0·2), as shown in Fig. 4.
Fig. 3.
Ex vivo interferon (IFN)-α production in patients with active tuberculosis (TB) and controls. The production of IFN-α from herpes simplex virus (HSV-1) stimulated peripheral blood mononuclear cells (PBMC) was evaluated in 17 TB patients and in 11 healthy controls. PBMC (106 cells/ml) were cultured for 18 h in the presence or absence of HSV-1 at a multiplicity of infection of 0·25. Cells were removed and supernatants assessed for IFN-α production by enzyme-linked immunosorbent assay (ELISA). Negligible IFN-α production was found in unstimulated PBMC in both donors and patients (data not shown). In contrast, in stimulated PBMC a marked decrease of IFN-α production was found in patients with TB compared with controls. Statistical significance was analysed by use of the Mann–Whitney U-test.
Fig. 4.
Correlation between circulating plasmacytoid dendritic cells (pDC) count and interferon (IFN)-α production in 17 tuberculosis (TB) patients. The pDC count was assessed ex vivo before the beginning of anti-tuberculous treatment. The IFN-α production was evaluated after in vitro herpes simplex virus (HSV-1) stimulation. Spearman's test showed no significant correlation. Line represents regression; P-value, r correlation coefficient.
Restoration of DC numbers and IFN-α production in TB patients with successful response to specific treatment
DC counts in peripheral blood were assessed longitudinally in 13 patients with TB, before and after starting anti-tuberculous treatment. In nine patients who recovered fully at the end of treatment, the number of pDC increased significantly over time (P = 0·006). In the same group of patients, there was also a marked increase in IFN-α levels (P < 0·001) (Fig. 5a). The apparent increase in mDC counts between values at baseline and after the end of therapy was not statistically significant (P = 0·16). Figure 6 shows cytofluorimetric analysis from a representative TB patient who responded to treatment. On the other hand, no significant changes in numbers of both pDC and mDC and IFN-α production were seen in the four patients who did not improve after complete specific treatment (P > 0·05) (Fig. 5b). Among the patients with poor clinical outcome and treatment failure, two had underlying cancer and two were affected by a severe form of extrapulmonary TB. Thus DC counts and IFN-α production were restored only in patients who had a successful response to specific antibiotic treatment.
Fig. 5.
Longitudinal analysis of circulating dendritic cells (DC) and interferon (IFN)-α production in tuberculosis (TB) patients with different clinical outcome. Thirteen patients with TB were studied before and after 3 months and 6 months of anti-tuberculous treatment. (a) Patients with full recovery at the end of the treatment (n = 9); (b) patients who did not improve after complete specific treatment (n = 4). The number of circulating plasmacytoid DC (pDC) increased significantly over time between the 3th and 6th month only in responder patients (P = 0·006, Wilcoxon's signed-rank test). In addition there was a significant increase in IFN-α levels (P < 0·001, Wilcoxon's signed-rank test).
Fig. 6.
Circulating myeloid dendritic cells (mDC) and plasmacytoid DC (pDC) enumeration in one representative tuberculosis (TB) ‘responder’ patient. Dendritic cells (DC) subsets were identified as in Fig. 1 at diagnosis (month 0) and after 3 months and 6 months of treatment. Contour plots are shown.
Discussion
The importance of DC in the initiation and control of innate and adaptive immune responses is well documented. DC is a system of cells that are specialized for uptake, processing and presentation of antigens to T cells. In the periphery, these cells are in the immature state and have the capacity to internalize and phagocytose microrganisms and other foreign antigens [17,18]. Upon activation in the presence of inflammatory mediators, DC undergo a process of maturation and acquire the capacity to migrate into secondary draining lymph nodes, where they become efficient at presenting antigens to naive T lymphocytes. This process is associated with the up-regulation of co-stimulatory molecules and the secretion of cytokines that polarize the T cell-mediate immunity to a Th1 or Th2 response. In recent years there has been an increased interest in the evaluation of peripheral blood DC subsets, as an improvement in the enumeration methods has been obtained [23,26]. DC counts, as measured by flow cytometry, were first shown to be decreased in HIV-infection [20,27–33] and, more recently, in other viral infectious diseases [34], whereas increased DC counts were found in acute influenza infection in macaques [35]. Variations were also described as a function of age, in surgical and physical stress, in advanced breast cancer, in B lineage acute lymphoblastic leukaemia and in other conditions [23,36,37].
In the present study, we demonstrated that patients with active TB exhibited a specific and significant decrease in the absolute number of circulating DC when compared to healthy donors. The reduction affects the two main subsets of DC circulating in the peripheral blood, i.e. CD11c+ mDC and CD123+ pDC. Uehira et al. [22] reported recently that CD11c+ mDC proportions are decreased in blood of patients with TB, while they observed no significant difference in the numbers of CD1a CD11c– between patients and controls, without fully characterizing those cells. In the present study we used a new whole-blood single-platform TruCOUNT assay that was found recently to be a simple and very precise counting assay, not relying on a haematology blood analyser. The plasmacytoid subpopulation was defined precisely by direct labelling of IL-3 receptor (CD123). We found that among the two fractions of DC examined the most significant reduction was seen for pDC counts, which were decreased both in pulmonary and extrapulmonary forms of TB. The degree of reduction in mDC numbers is more significant in patients affected by extrapulmonary TB, which is associated usually with a profound impairment of anti-mycobacterial cell-mediated immunity.
The interaction of mycobacteria with APC, such as monocytes/macrophages and DC, is a crucial point in the immunopathogenesis of tuberculosis, as it could drive the development of protective immunity, latent infection or chronic disease. Monocytes/macrophages are the prime target cells, which show few phenotype changes but are more effective in the killing and elimination of mycobacteria. No evidence of significant decrease in circulating monocytes was found in our TB patients. On the other hand, DC that are present in high amounts at the site of infection, such as the lung, capture and ingest M. tuberculosis through the binding of lipoarabinomannan to the DC-SIGN receptor [14,38]. After bacterial uptake and up-regulation of the expression of co-stimulatory molecules DC migrate in lymph nodes, where they are very efficient in inducing anti-mycobacterial T cell immunity. The dynamic of DC uptake, migration and presentation may affect the outcome of M. tuberculosis infection. Various possible mechanisms could be involved in DC deficiency during active TB disease. One hypothesis is that DC act as a reservoir for mycobacteria in vivo during the phase of latent infection, while during active TB these cells are probably more susceptible to apoptosis and may undergo peripheral destruction. Another possible explanation for the low number of peripheral DC fractions could be their enhanced recirculation from blood to affected tissues, such as inflamed lung or lymph nodes. In patients with Sjögren's syndrome and systemic lupus erythematosus pDC accumulate in salivary gland or skin lesions, resulting in a decrease of the circulating DC pool. In addition, in patients with TB there is evidence of a selective trafficking and accumulation of CD11c+ DC from blood to tuberculous granulomas, suggesting a predominant Th1 polarization at the site of inflammatory tissues [22]. In addition, mycobacterial-infected patient lymph nodes contain large amounts of pDC [39]. The decrease in the circulating DC pool may have functional consequences on the production of cytokines and antigen presentation to naive T cells, particularly through a loss of type I IFN, because pDC are the natural interferon-producing cells in the immune system [24]. Production decrease of IFN-α by in vitro-stimulated PBMC in HIV infection is found during primary infection, when viral load is very high, and during chronic infection, in association with progression to disease and occurrence of opportunistic infections [25,40,41].
IFN-α production by PBMC was diminished dramatically in all our patients with TB; moreover, no significant correlation was found with the number of pDC. This finding suggests that the impairment in IFN-α secretion could be related to a clear defect in the function of pDC, as found in vitro in monocyte-derived DC infected with M. tuberculosis [42], rather than to a simple numerical reduction of these fundamental APC.
The roles of T cells, NF-κB signalling and the IL-12–IFN-γ axis are crucial to control TB infection [43]. Type I IFN might play a role by balancing IL-12 responses, thus influencing the outcome of infection as it does in viral infection models in mice [44]. Type I IFNs are potent anti-viral cytokines but recent lines of evidence indicate that they play a role during the initiation of the immune response against various microrganisms, including M. tuberculosis [45]. Human DC infected with live M. tuberculosis as well as pDC migrate to mycobacterial-infected lymph nodes, producing large amounts of IFN-α [39,46]. Recent studies have demonstrated that IFN-αβ produced by mycobacterial-infected DC modulate the DC chemoattractive capacity via the chemokine CXCL10, suggesting a protective role of IFN type I in tuberculosis [47]. Interestingly, recent data showed a clinical improvement in TB patients treated with aerosolized IFN-α, even though the mechanisms involved have not been clarified [48,49].
Here the number and function of DC were also investigated longitudinally in a group of patients who were followed-up after the beginning of the specific treatment. In particular, for 13 patients the circulating pDC and mDC numbers and the production of IFN-α were assessed after 3 and 6 months of anti-tuberculous therapy and changes were correlated with clinical, radiological and microbiological response to treatment. Most interestingly, in all nine patients with successful anti-tuberculous therapy there was a significant and marked increase, especially for absolute pDC numbers and IFN-α production. In contrast, no significant variations in DC numbers and function were observed in patients with lack of response to specific treatment. The cell-mediated immune response to M. tuberculosis has been investigated previously during anti-tuberculous treatment. Some authors reported a restoration of mycobacterial antigen-induced T cell proliferation and IFN-γ responses upon efficacious anti-tuberculous therapy [50]. More recently, Carrara et al., using an in vitro assay to measure T cell-mediated IFN-γ response to selected epitopes of M. tuberculosis, found a decrease in IFN-γ-producing specific T cells in patients receiving a successful chemotherapy [51]. To our knowledge, this is the first in vivo study that has analysed DC in patients with different clinical outcome during the course of anti-tuberculous treatment. In particular, we observed that the restoration of DC numbers and function correlates with bacterial clearance and clinical improvement, as a result of effective anti-tuberculous treatment. The persistent impairment in peripheral DC pool and function occurs in patients with underlying cancer or a severe form of extrapulmonary TB.
The counting of M. tuberculosis-specific T cells by using enzyme-linked immunospot (ELISPOT) assay for IFN-γ secretion has been proposed as a valid approach for monitoring response to anti-tuberculous treatment [52]. The enumeration of subsets of circulating DC by a simple whole-blood assay might represent an adjunctive and useful marker for monitoring the clinical outcome and response to treatment, especially in extrapulmonary forms of TB.
Acknowledgments
We thank Professor Pierre Lebon for providing HSV-1 and Concepçión Marañón for her invaluable help in flow cytometric analysis.
References
- 1.Dye C, Scheede S, Dolin P, Pathania V, Raviglione MC WHO Global Surveillance Monitoring Project. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Marino S, Kirschner DE. The human immune response to Mycobacterium tuberculosis in lung and lymph node. J Theor Biol. 2004;227:463–86. doi: 10.1016/j.jtbi.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanism of host and bacillus contribute to persistent infection. Lancet Infect Dis. 2003;3:578–90. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 4.van Crevel R, Otthenhoff TH, van der Meer JWM. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orme I. Adaptive immunity to mycobacteria. Curr Opin Microbiol. 2004;7:58–61. doi: 10.1016/j.mib.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SHE. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61:ii53–8. doi: 10.1136/ard.61.suppl_2.ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boom WH, Canaday DH, Fulton SA, Gehring AJ, Rojas RE, Torres M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis. 2003;83:98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 8.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Reid SD, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr Opinion Immunol. 2000;12:114–21. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- 11.Hope JC, Thom ML, McCormick PA, Howard CJ. Interaction of antigen presenting cells with mycobacteria. Vet Immunol Immunopathol. 2004;100:187–95. doi: 10.1016/j.vetimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Henderson RA, Watkins SC, Flynn JL. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–43. [PubMed] [Google Scholar]
- 13.Jiao X, Lo-Mann R, Guermonprez P, et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol. 2002;168:1294–301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 14.Tailleux L, Schwartz O, Herrmann JL, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–7. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buettner M, Meinken C, Bastian M, et al. Inverse correlation of maturity and antibacterial activity in human dendritic cells. J Immunol. 2005;174:4203–9. doi: 10.4049/jimmunol.174.7.4203. [DOI] [PubMed] [Google Scholar]
- 16.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–72. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 19.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 20.Grassi FR, Hosmalin A, McIlroy D, Calvez V, Debré P, Autran B. CD11c-positive dendritic cells are depleted in the blood of HIV-infected patients. AIDS. 1999;13:759–66. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kanto T, Inoue M, Miyatake H, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 22.Uehira K, Amakawa R, Ito T, et al. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin Immunol. 2002;105:296–303. doi: 10.1006/clim.2002.5287. [DOI] [PubMed] [Google Scholar]
- 23.Vuckovic S, Gardiner D, Field K, et al. Monitoring dendritic cells in clinical practice using a new whole blood single-platform TruCOUNT assay. J Immunol Meth. 2004;284:73–87. doi: 10.1016/j.jim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Siegal F, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 25.Kamga I, Kahi S, Develioglu L, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–10. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 26.Servet C, Zitvogel L, Hosmalin A. Dendritic cells in innate immune responses against HIV. Curr Mol Med. 2002;2:739–56. doi: 10.2174/1566524023361907. [DOI] [PubMed] [Google Scholar]
- 27.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–6. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 28.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ and CD11c+ dendritic cell numbers in primary HIV-1 infection. Blood. 2001;9:3016–21. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 29.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 30.Chehimi J, Campbell DE, Azzoni L, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 31.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 32.Anthony DD, Yonkers NL, Post AB, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 33.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–52. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 34.Pichyangkul S, Endy TP, Kalayanarooj S, et al. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol. 2003;171:5571–8. doi: 10.4049/jimmunol.171.10.5571. [DOI] [PubMed] [Google Scholar]
- 35.Coates PT, Barratt-Boyes SM, Zhang L, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–21. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 36.Ho CS, Lopez JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN. Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood. 2001;98:140–5. doi: 10.1182/blood.v98.1.140. [DOI] [PubMed] [Google Scholar]
- 37.Mami NB, Mohty M, Chambost H, Gaugler B, Olive D. Blood dendritic cells in patients with acute lymphoblastic leukaemia. Br J Haematol. 2004;126:77–80. doi: 10.1111/j.1365-2141.2004.04989.x. [DOI] [PubMed] [Google Scholar]
- 38.Geijtenbeek TB, Van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 40.Lopez C, Fitzgerald PA, Siegal FP. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J Infect Dis. 1983;148:962–6. doi: 10.1093/infdis/148.6.962. [DOI] [PubMed] [Google Scholar]
- 41.Siegal FP, Lopez C, Fitzgerald PA, et al. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J Clin Invest. 1986;78:115–23. doi: 10.1172/JCI112539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prabhakar S, Qiao Y, Hoshino Y, et al. Inhibition of response to alpha interferon by Mycobacterium tuberculosis. Infect Immun. 2003;71:2487–97. doi: 10.1128/IAI.71.5.2487-2497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 44.Dalod M, Salazar-Mather TP, Malmgaard L, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–28. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith PL, Lombardi G, Foster GR. Type I interferons and the innate immune response – more than just antiviral cytokines. Mol Immunol. 2005;42:869–77. doi: 10.1016/j.molimm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Giacomini E, Iona E, Ferroni L, et al. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–41. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 47.Lande R, Giacomini E, Grassi T, et al. IFN-alpha beta released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–82. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 48.Giosue S, Casarini M, Alemanno L, et al. Effects of aerosolized interferon-alpha in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 1998;158:1156–62. doi: 10.1164/ajrccm.158.4.9803065. [DOI] [PubMed] [Google Scholar]
- 49.Palmero D, Eiguchi K, Rendo P, et al. Phase II trial of recombinant interferon-alpha2b in patients with advanced intractable multidrug-resistant pulmonary tuberculosis: long-term follow-up. Int J Tuberc Lung Dis. 1999;3:214–8. [PubMed] [Google Scholar]
- 50.Al-Attiyah R, Mustafa AS, Abal AT, Madi NM, Andersen P. Restoration of mycobacterial antigen-induced proliferation and interferon-γ responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol Med Microbiol. 2003;38:249–56. doi: 10.1016/S0928-8244(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 51.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38:754–6. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 52.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]