Abstract
A patient with Waldenstrom's macroglobulinaemia expresses a high titre IgM antibody in serum that binds both mouse and human dendritic cells (DC) in a B7-DC (PD-L2)-dependent manner. We have reported previously that purified antibody from patient serum activates immature and mature DC in vitro, enhancing the ability of these professional antigen-presenting cells to activate naive T cells, take up antigen, resist a cytokine-depleted environment and secrete immunomodulatory cytokines, such as interkeukin (IL)-6 and tumour necrosis factor (TNF)-α. Systemic treatment of experimental animals with this antibody induces potent anti-melanoma immunity and modulates protectively the recall response against antigen challenge through the airway in an experimental model of inflammatory airway disease. Here we describe a monoclonal IgM antibody derived from this serum immunoglobulin that recapitulates each of these earlier observations, providing direct evidence that M protein from the Waldenstrom's patient mediates these potent immunomodulatory effects. Furthermore, cell lines expressing this recombinant form of the human antibody provide the basis for developing this reagent for clinical application.
Keywords: allergy, cancer, dendritic cells, therapy/immunotherapy
Introduction
We have described previously a serum-derived human IgM antibody (IgM12), designated sHIgM12, that has immunotherapeutic activity in mouse models of cancer and asthma. This antibody activates dendritic cells (DC) in vitro [1,2], potentiates anti-tumour immunity when administered systemically to animals bearing transplanted B16 melanoma [3] and modulates the immune response in hypersensitized animals in a model of allergic inflammatory lung disease [4,5]. When applied in vitro to bone marrow-derived murine DC, the antibody induces enhanced antigen-presenting activity, longevity, migration and cytokine production [1,2]. The antibody superactivates DC matured with Toll-like receptor agonists, restoring their ability to acquire exogenous antigen even while maintaining high expression levels of co-stimulatory molecules and class II antigen-presenting molecules [6]. The sHIgM12 antibody binds to both mouse and human DC [1]. We are in the process of investigating the key observation that the sHIgM12 antibody also activates human DC in a B7-DC-dependent manner and are pursuing the hypothesis that the human antibody will potentiate and modulate the human immune response in a similar manner to the way it modulates immunity in the mouse.
Our initial studies used antibodies purified from the serum of a patient with Waldenstrom's macroglobulinaemia and identified the co-stimulatory molecule B7-DC as the cell surface target. We demonstrated that, using IgM monomeric antibody fragments, cross-linking is a critical step in the activation of antibody-treated DC [2]. An important step in defining how this serum-derived antibody works is to develop a monoclonal antibody source, providing a defined reagent that can be used to define mechanisms of action and to provide a renewable source of antibody that can be developed for clinical use. We have generated a cloned antibody by determining the sequence of the antibody from patient serum, constructing a recombinant DNA vector containing synthetic genes encoding the antibody and expressing the antibody in a hybridoma cell line. In the present studies, we have assessed the ability of the recombinantly cloned antibody, designated rHIgM12, to induce the same functional changes in cultured DC and to promote the in vivo immune modulatory events observed originally using the serum-derived antibody, sHIgM12. In so doing, we have provided definitive evidence that the antibody itself mediates the same immune modulatory effects as the serum-derived protein, and have created a reagent that can be developed for immunotherapy in humans.
Materials and methods
Mice
Six to 8-week-old BALBc/J and C57BL6/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in the animal house facility at the Mayo Clinic and used in compliance with the Institutional Animal Use and Care Committee (IACUC).
Amino terminal sequence analysis of immunoglobulin heavy and light chains
IgM antibody was isolated from the serum of a Waldenstrom's macroglobulinaemia patient by a combination of euglobulinic precipitation and size exclusion chromatography on Sepharose 6 matrix (Pharmacia, Upsala, Sweden) equilibrated with 20 nM acetate buffer, 150 nM NaCl, pH 4·0. Fv fragments were isolated by digestion with pepsin followed by chromatography on Superdex-200 equilibrated with phosphate-buffered saline (PBS). Fv fragments were denatured by 2 h incubation at 37°C in 200 mM Tris, 8 M urea, 20 mM dithiothreitiol (DTT), 5 mM ethylenediamine tetraacetic acid (EDTA) and pH 8·0 buffer. The sulph-hydril groups were alkylated by 50 mM iodacetamide for 2 h at room temperature. The Fv fragments were then transferred to 100 mM sodium phosphate, 1 M urea, 10 mM EDTA, 5 mM DTT, 5% glycerol, pH 8·0. The pyroglutamil residues were cleaved by pyroglutamate aminopeptidase (Boehringer Mannheim, Germany) during a 12-h incubation at 4°C followed by 24 h at room temperature. The N-terminally deblocked Fv fragments were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted to polyvinylidene difluoride (PVDF) membrane (Boehringer Mannheim) and sequenced in an automated sequencer (Procise 492 HT, PE Biosystems, Foster City, CA, USA).
Cloning and DNA sequence analysis of antibody variable regions
Peripheral blood lymphocytes (PBL) were obtained from a Waldenstrom's macroglobulinaemia patient and RNA was prepared using Trizol (Tel.Test Inc., Friendswood, TX, USA). Using an oligo-dT primer and an Amersham kit, cDNA was made from the RNA. N-terminal protein sequences were obtained from the paraprotein found to be most abundant in the patient's blood, as described previously [7]. Family-specific primers for both heavy and light chains were designed based on the protein sequences (5′ variable region heavy chain: CTGCAGGAGTCGGGCCCA; 5′ variable region light chain: GACATCCAGATGACCCA). These primers, along with 3′ constant region primers (3′ constant region heavy chain: CGAGGGGGAAAAGGGTT; 3′ constant region light chain: CAACTGCTCATCAGATGGCG) were used to amplify the cDNA with polymerase chain reaction (PCR).
The resulting products (from two separate RNA/cDNA preparations) were then gene-cleaned (BIO 101 Inc., La Jolla, CA, USA), cloned into a TA vector system (Invitrogen, Carlsbad, CA, USA) and transformed into DH5α competent cells (Invitrogen). Ten of the resulting colonies from each transformation (heavy and light chain) were sequenced. Eight of the 10 heavy chain sequences were identical and matched the N-terminal protein sequence. The other two heavy chain sequences did not match the protein sequence completely and were disregarded. Ten different sequences were obtained for the light chain. Of the 10, only two matched the N-terminal light chain protein sequence completely. A CDR1 shared sequence probe and two CDR3 clone-specific oligonucleotide probes were constructed (shared CRD1-AGTATTAGTAGTTATCTAAATT GG; CDR3 clone 1-GAGTTACCATACCCCGTGGACG; CDR3 clone 2-CACGTTTATAATTACCCGATGACCT) and colonies containing the light chain TA vector were screened by hybridization. Of 308 colonies, 48 were positive for the CDR1 shared probe. Of those 48, 43 were positive for clone 1 CDR3, one was positive for clone 2 CDR3 and four were not positive with either probe. The predicted nucleotide sequences from both the heavy chain and the most prevalent light chain (clone 1) were used to predict the size of enzyme degradation products, which were then confirmed in the serum protein by mass spectroscopy.
PAD12 vector construction was performed by inserting the heavy chain variable region cDNA with a leader sequence from the nucleotide database and complete light chain cDNA with attached leader sequence from the nucleotide database in a manner similar to that described previously [8], using the primers described below.
Primers used to make rHIgM12VH and splice it by overlap extension [9] to a leader sequence with an intron (lower case letters) from data base (M29812) are as follows:
5′ primer with BspEI site: TCC GGA CGG TCC GGG A.
TCC GGA CGG TCC G ggacctcct gtgcaagaac atgaaacatc tgtggttctt ccttctcctg gtggcagctc ccagatgtga gtatctcagg gatccagaca.
cagg gatccagaca tggggatatg ggaggtgcct ctgatcccag ggctcactgt gggtctctctgttcacaggg gtcctgtccc aggtgcagct gcaggagtcg ggcccaggac.
3′ primer with PAC I site: CCTTAATTAAGACCTGG AGAGGCCATTCTTACCTGAGGAGACGGTGACCAG GGTTC.
Primers used to make rHIgM12Vk and splice it by overlap extension (soe) to the leader sequence from the data base (lower case letters, accession X59312) are as follows:
5′-Ck primer to soe lym 12 Vk to Ck: CGA ACT GTG GCT GCA C.
3′ Ck primer with Xho 1: CCGCTCGAGTATCTAACA CTCTCCCCTGTT.
5′ Lym 12 Vk with Nhe I: AGCATTACTAGCTAGCTC AAGACTCAGCCTGGAC atggaca tgagggtccc cgctcagctc ctggggctcc tgctactctggctccgag gtgccaga tgt GAC ATC CAG ATG ACC CA
Electroporation and methotrexate (MTX) amplification was performed as described previously [8]. Briefly, 8 million F3B6 mouse/human heterohybridoma cells (American Type Culture Collection: ATCC) were incubated with 10 µg PAD12 vector linearized with Bgl II for 10 min in 800 µl serum free media at which time they were electroporated at 0·2 V in a Biorad Gene PulserTM (Biorad, Hercules, CA, USA). Following a 10-min incubation on ice, the cells were diluted to 24 ml in RPMI-1640 containing 10% fetal calf serum (FC) (Gibco, Carlsbad, CA, USA), plated in a 24-well plate and incubated at 37°C; 48 h later, the cells containing vector were selected using 1 µM methotrexate (Calbiochem, La Jolla, CA, USA). Following a 2-week incubation period, colonies were picked to a new plate and allowed to grow until confluent. At this point, the supernatant was harvested and assayed by enzyme-linked immunosorbent assay (ELISA) for the presence of human IgM. Positive colonies were selected further over the course of 2 months with increasing doses of methotrexate ranging from 1 µM to 200 µM. In this manner, cells were generated that produced greater than 10 µg/ml IgM.
Production of bone marrow-derived DC was accomplished as described previously [1]. Briefly, bone marrow was isolated from the long bones of the hind legs of C57BL/6 J mice. Erythrocytes were lysed by treatment with ACK (0·8% NHyCl, 0·1% KHCO3, 0·003% EDTA). The remaining cells were plated at a density of 106 per ml in six-well plates in RPMI-1640 (GibcoTM/Invitrogen, Carlsbad, CA, USA) supplemented with 10% cosmic calf (Hyclone, Logan, UT, USA) and containing 10 ng/ml of murine granulocyte–macrophage colony-stimulating factor (GM-CSF) and 1 ng/ml of murine interleukin (IL)-4 (Peprotech, Rocky Hill, NJ, USA). The cells were incubated at 37°C with 5% CO2. After 2 days of culture the media was replaced and the cells were cultured for an additional 5 days.
ELISA was performed as described previously [5]. Briefly, MaxisorpTM plates were coated with 20 µg/ml goat anti-human IgA + IgG + IgM (H + l) (ICN Pharmaceuticals, Inc.). Purified human IgM (MP Biomedicals, Irvine, CA, USA) was used as the standard control and supernatants were assayed in duplicate at 1 : 100 and 1 : 1000 dilutions. The detection antibody used was monoclonal anti-human IgM conjugated to alkaline phosphatase (Sigma-Aldrich Co., St Louis, MO, USA). Positive reactions were detected by using p-nitrophenyl phosphate tablets (Sigma-Aldrich Co.) in a pH 9·6 carbonate buffer with magnesium chloride. Adsorbance was read at 405 nM.
Murine tumour necrosis factor (TNF)-α, IL-6 and IL-5 were detected using Ready-Set-Go ELISA kits from e-bioscience (San Diego, CA, USA) as per the manufacturer's instructions. Supernatants were obtained from 106/ml murine bone marrow-derived DC that had been stimulated for 24 h with 10 µg/ml rHIgM12, sHIgM12 or control antibody. Samples were run in triplicate and values are recorded as mean ± standard error of the mean.
IgM purification was performed by PEG 6000 (Fluka, Buchs, Switzerland) precipitation and dialysis against H2O, followed by size fractionation over a Superose 6 column as described previously [8].
Naive T cell stimulation experiments were performed as described previously [2]. Briefly, day 7 bone marrow-derived DC of the appropriate haplotype were treated overnight with 10 µg/ml of sHIgM39, sHIgM12 or rHIgM12. Cells were pulsed concomitantly with 1 mg/ml of ovalbumin (OVA) (Sigma-Aldrich Co.). Cells were then washed and titrated in triplicate against 3 × 105 OT-1 or DO.11 spleen cell targets for 3 days with the addition of tritiated thymidine during the last 18 h. Wells were harvested and counts were read on a Packard Topcount NTX (Perkin Elmer, Boston, MA, USA).
Antigen uptake experiments were performed as described previously [6]. Briefly, murine bone marrow-derived DC were cultured in the presence of 10 ng/ml GM-CSF and 1 ng/ml IL-4 for 5 days, at which time 10 µg/ml of the Toll-like receptor 9 agonist CpG (oligodeoxynucleotide TCC ATG ACG TTC CTG ACG TT) was added. Twenty-four hours later the cells were treated with 10 µg/ml sHIgM12, rHIgM12 or sHIgM39 control antibody and 10 µg/ml OVA-coupled fluorescein isothiocyanate (FITC) was added. Following 24 h incubation the cells were stained with phycoerythrin (PE)-conjugated anti-mouse CD11c, fixed with 2% paraformaldehide and analysed by flow cytometry on a FACSCalibur (BD Biosciences-PharMingen, San Diego, CA, USA). Data were analysed using CellQuest software (BD Biosciences-PharMingen).
Cytokine deprivation experiments were performed as described previously [1]. Briefly, bone marrow-derived DC were plated without GM-CSF and IL-4 (except for one set of control wells which were plated with cytokines) on day 5 into 96-well plates at 2 × 104 cells/well in the presence of rHIgM12, sHIgM12 or control antibody sHIgM39. After 1 h of culture, Alamar blue (Biosource International, Camarillo, CA, USA) was added to a final concentration of 10% (v/v). Readings were taken at 24 h of culture using a CytoFluor Multiplate Reader, Series 4000 (PerSeptive Biosystems, Foster City, CA, USA) set at an excitation wavelength of 520 nm and an emission reading of 590 nm.
B16 malignant melanoma experiments were performed as described previously [3]. Briefly, C57BL/6 mice were injected intravenously (i.v.) with 10 µg sHIgM12, rHIgM12 or control antibody sHIgM39 in 100 µl PBS on days −1, 0 and +1. On day 0, the mice were injected subcutaneously in the flank with 2 × 104 B16 cells in 100 µl PBS. The size of the tumours was measured in two dimensions using calipers (Dyer, Lancaster, PA, USA). Statistical comparisons were made using rank sum anova.
A murine model of allergic inflammatory lung disease was used as depicted in Fig. 1 and as described previously [4]. Briefly, 6–8-week-old BALBc/J mice were injected intraperitoneally (i.p.) with 100 µg chicken OVA (Sigma-Aldrich) in alum on days 0 and 7. On days 13, 14 and 15, 10 µg sHIgM12, rHIgM12 or control antibody sHIgM39 in PBS were injected i.v. On day 14, the mice received their first intranasal challenge of 100 µg OVA in PBS. The mice were rechallenged on days 23, 24, 25 and 26. On day 27, animals were injected with a lethal dose of pentobarbital (Abbott Laboratories, Abbott Park, IL, USA), the lungs were lavaged twice with 0·5 ml Hanks's balanced salt solution (HBSS) and the supernatant was assayed for IL-5 by ELISA (R&D Systems, Minneapolis, MN, USA). After bronchial alveolar lavage (BAL) fluid collection, the lungs were fixed in 10% formalin and embedded in paraffin. Sections were cut and stained with haematoxylin and eosin (H&E).
Fig. 1.
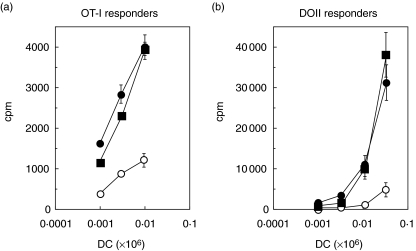
Monoclonal recombinantly cloned human IgM antibody (rHIgM12) stimulates naive class I and class II transgenic T cells. Day 7 bone marrow-derived dendritic cells of appropriate haplotype were treated overnight with serum-derived antibodies, sHIgM39 (open circles), sHIgM12 (filled circles) or recombinant antibody, rHIgM12 (filled squares). Cells were pulsed concomitantly with 1 mg/ml of ovalbumin. Cells were then washed and titrated against OT-I target (a) or DO.11 target (b) in triplicate for 3 days with the addition of tritiated thymidine during the last 18 h before being harvested and counted.
Results
Structure of sHIgM12
The structures of the sHIgM12 heavy and light chains were determined using the protein and cDNA sequencing strategies described in the Methods. The sHIgM12 antibody contains the IgM and kappa constant regions. The antibody is comprised of an IGHV4-59*02 variable region joined to an IGHJ5*02 segment. The light chain includes an IGKV1D-39*01 coding sequence joined to an IGKJ/*01 segment. Comparison of the heavy and light chain variable regions with the closest known genomic coding sequences revealed 14 and six amino acid replacements, indicating that both the heavy and light chains of the sHIgM12 antibody have undergone extensive somatic mutation, despite being of IgM isotype. The variable region amino acid and nucleotide sequences for the heavy and light chains have been deposited in GenBank under the accession numbers DQ146928 and DQ146929, respectively.
Generation of a transfectoma expressing rHIgM12 antibody
The cDNAs from the heavy and light chain variable regions were cloned into a previously described IgM expression vector [8]. The linearized expression vector was introduced by electroporation into the IgM negative heterohybridoma cell line, F3B6. The cells were selected with 1 µM methotrexate. Surviving colonies were monitored for immunoglobulin secretion by ELISA and productive colonies were selected sequentially with increasing amounts of methotrexate to a final concentration of 200 µM. In this manner a transfectoma was obtained that produced rHIgM12 antibody in greater than 10 µg/ml concentration in culture supernatants.
Monoclonal rHIgM12 stimulates cytokine production in murine DC
In order to compare the biological activities of the serum derived and the recombinant monoclonal antibodies, several in vitro and in vivo experiments were performed. In the first experiments, murine-derived DC were treated in vitro with 10 µg/ml sHIgM12 or rHIgM12 for 24 h and evaluated for induction of IL-6 and TNF-α, two cytokines that are induced strongly with the serum-derived B7-DC cross-linking antibody (Xab). Both antibodies caused an approximately 100-fold increase in IL-6 production above the level produced by cells treated with sHIgM39, an isotype control antibody (Table 1). Similarly, an increase in TNF-α production of approximately 45-fold over control was seen with stimulation by either sHIgM12 or rHIgM12.
Table 1.
Cytokine secretion increases upon stimulation of murine dendritic cells (DC) with serum-derived human IgM antibody (sHIgM12) and recombinantly cloned human IgM antibody (rHIgM12). DC derived from murine bone marrow were cultured for 7 days with interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor prior to treatment with sHIgM12, rHIgM12 or the isotype control IgM sHIgM39 that does not bind dendritic cells. Twenty-four hours later, supernatants were harvested and cytokine levels were measured by enzyme-linked immonosorbent assay. Samples were run in triplicate and values recorded as mean ± standard error of the mean.
| Control IgM (pg/ml) | sHIgM12 (pg/ml) | rHIgM12 (pg/ml) | |
|---|---|---|---|
| IL-6 | 95 ± 1·7 | 9595 ± 1273 | 11 920 ± 621 |
| TNF-α | < 15·6 | 600 ± 80 | 662 ± 84 |
Monoclonal rHIgM12 stimulates naive class I and class II transgenic T cells
We have shown previously that serum-derived B7-DC cross-linking antibody enhances the ability of antigen-pulsed DC to stimulate naive antigen-specific CD8 and CD4 T cells [2]. As shown in Fig. 1, naive class I-restricted OT-1 and class II-restricted DO-11·10 T cells incorporated greater quantities of [3H]thymidine when co-cultured with antigen-pulsed DC treated with either serum-derived or recombinant monoclonal B7-DC cross-linking antibody in comparison to cultures with DC treated with isotype control IgM antibody.
Monoclonal rHIgM12 causes antigen uptake in CpG-matured murine DC
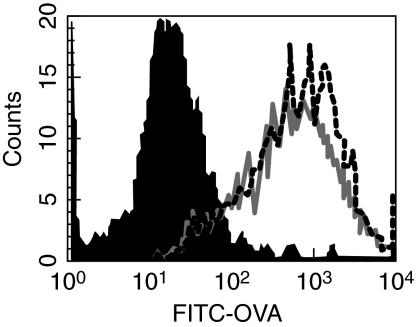
One key phenotype associated with activation of DC following B7-DC cross-linking that contrasts with DC activated using Toll-like receptor agonists is the prolonged increase in antigen acquisition activity extending 24 h after activation [3]. We have found recently that B7-DC cross-linking can induce antigen uptake by DC even after this function has been inhibited following maturation in response to TLR-9 stimulation with CpG-ODN [6]. As shown in Fig. 2, both the recombinantly derived monoclonal and serum-derived B7-DC cross-linking antibody induce the uptake of fluorescently tagged OVA in matured DC.
Fig. 2.
Monoclonal recombinantly cloned human IgM antibody (rHIgM12) promotes antigen uptake in CpG-matured murine dendritic cells. Bone marrow-derived dendritic cells (DC) were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 5 days, at which time they were matured using 10 µg/ml CPG oligodeoxynucleotide. Twenty-four hours later, fluorescein isothiocyanate–ovalbumin and either control antibody (filled), sHIgM12 (solid line) or rHIgM12 (dotted line) was added to the culture. Following a 24-h incubation, the cells were stained with phycoerythrin-conjugated anti-mouse CD11c, fixed with 2% paraformaldehyde and analysed by flow cytometry on a FACSCalibur (BD Biosciences-PharMingen, San Diego, CA, USA). Data were analysed using CellQuest software. Histogram shows cells gated through CD11c.
Monoclonal rHIgM12 prevents cytokine deprivation-mediated cell death in murine DC
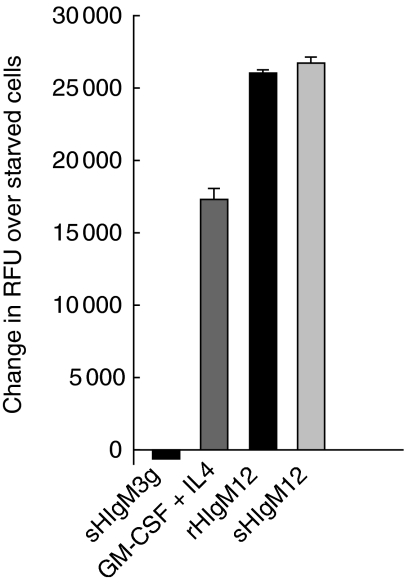
Cellular metabolism is an important parameter of DC viability and differentiation. We have described previously the effects of B7-DC cross-linking on DC metabolism using the metabolic conversion of Alamar blue under cytokine-deprived and -replete culture conditions. DC deprived of the cytokines GM-CSF and IL-4 can be rescued by treatment with the serum-derived sHIgM12 antibody [1]. The current experiment recapitulates this result using the monoclonal recombinant rHIgM12 (Fig. 3). In this experiment, cells deprived of GM-CSF and IL-4 and then treated with either sHIgM12 or rHIgM12 significantly increased the metabolic conversion of Alamar blue relative to cells treated with control antibody.
Fig. 3.
Monoclonal recombinantly cloned human IgM antibody (rHIgM12) prevents cytokine deprivation mediated cell death in murine dendritic cells (DC). Murine bone marrow derived DC were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL-4) for 5 days before being starved of these cytokines. Serum-derived human IgM antibody (sHIgM12), rHIgM12 and control antibodies were added immediately to the starved cells, and metabolism was measured by reduction of Alamar blue at 24 h post-starvation.
Systemic administration of the recombinant monoclonal antibody rHIgM12 potentiates anti-tumour immunity against B16 melanoma. The immunotherapeutic potential of B7-DC cross-linking antibody has been demonstrated previously using a mouse model of malignant melanoma [3]. Animals treated systemically with serum-derived B7-DC cross-linking antibody developed strong cellular immunity against subcutaneous tumour transplants. In order to assess whether the recombinantly cloned antibody retained the ability to induce immunity to transplanted melanoma, C57BL/6 mice were treated with either sHIgM12, rHIgM12 or isotype control antibody in conjunction with the transplanted tumour. A statistically significant treatment effect was observed with the recombinantly cloned antibody, as all the antibody-treated animals displayed strong treatment effects (Table 2). Tumour grew in a delayed fashion in one of five animals that was treated with the cloned antibody rHIgM12, in contrast to robust tumour growth in all five animals receiving isotype control antibody. The tumour resistance induced with rHIgM12 treatments was comparable to that observed with the serum-derived sHIgM12 antibody.
Table 2.
Resistance to B16 melanoma induced by recombinant B7-DC cross-linking antibody. C57BL/6 mice received 10 µg of serum-derived human IgM antibody (sHIgM3) (IgM control antibody), (sHIgM12) or recombinantly cloned human IgM antibody (rHIgM12) intravenously on days −1, 0, and +1. B16 melanoma was injected subcutaneously in the flank on day 0. The width and length of tumours were measured on day 17. The product of width and length was used as an estimate of tumour size. Statistical comparisons were made using rank sum anova (between-group statistical comparisons are shown; the probability statistic for three-group simultaneous evaluation was P = 0·002).
| Treatment | Tumour-free (day 17) | Average tumour size (mm2) | s.e.m. (mm2) | P-value |
|---|---|---|---|---|
| sHIgM39 | 0/5 | 265 | 12 | Reference |
| sHIgM12 | 5/5 | 0 | 0 | P < 0·05 |
| rHIgM12 | 4/5 | 24 | – | P < 0·05 |
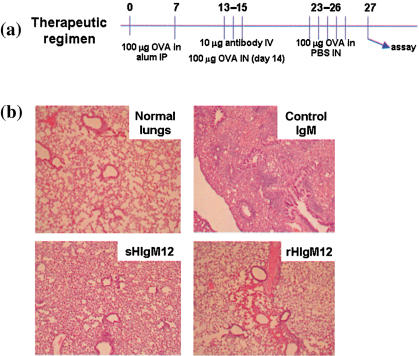
Systemic administration of monoclonal rHIgM12 modulates established immune responsiveness in a murine model of allergic inflammatory lung disease. A second immunotherapeutic application of antibody sHIgM12 is to modify the Th2 recall response of hyperimmune mice upon re-exposure to the offending antigen through the airways. When administered systemically in conjunction with a single exposure to antigen through the airways on day 14 after initial immunization with OVA and alum, the serum-derived antibody completely blocked airway inflammatory disease that followed repeated exposure to airway antigen. A summary of the therapeutic regimen is illustrated in Fig. 4a. To determine whether the recombinantly cloned antibody retained this function, hyperimmunized animals were treated with rHIgM12 antibody in conjunction with a day 14 antigen challenge in the airway. On day 23 after initial immunization, the animals were exposed daily to antigen for 4 days. On day 27 the animals were assessed for inflammatory airway disease. Lungs were analysed histologically for inflammation and pathological changes associated with this syndrome. Bronchial infiltrates and pulmonary cytokine profiles were determined. In all respects, the effects of systemic treatment serum-derived B7-DC cross-linking antibodies were recapitulated with the recombinantly cloned antibody. As shown in Fig. 4b, pulmonary inflammation was blocked completely in animals treated either with the recombinantly cloned or the serum-derived B7-DC cross-linking antibody. No elevation in Th2 cytokines was detected in the lungs of antibody-treated animals. IL-5 levels were 229·5 ± 25 pg/ml in the control antibody-treated mice and 15·5 ± 10 pg/ml and 11·4 ± 6 pg/ml in the lungs of mice treated with rHIgM12 and sHIgM12, respectively. In fact, all cytokine levels in the treated animals were minimal, a finding consistent with the absence of inflammatory lesions in the lungs (data not shown).
Fig. 4.
Absence of lung pathology following therapeutic administration of serum-derived human IgM antibody (sHIgM12) or recombinantly cloned human IgM antibody (rHIgM12) in a murine experimental airway inflammation model. (a) Diagram of the therapeutic regimen. Mice were sensitized to ovalbumin (OVA) protein by two intraperitoneal injections with alum. Intranasal challenge along with antibody administration preceded additional intranasal challenge with the OVA protein. (b) Lungs were harvested, sectioned and stained with haematoxylin and eosin for histological analysis.
Discussion
The human antibody sHIgM12, purified from a Waldenstrom's macroglobulinaemia patient, profoundly modulates the immune response in mouse models when used to cross-link B7-DC, either in vitro or in vivo. The antibody binds to B7-DC and activates both immature [1] and mature DC [6], key regulators of immune responsiveness. Because the effects of antibody treatment are the result of cellular activation, a relatively small amount of antibody (10–30 µg) can elicit biological responses in vivo [3,4,6 and this study]. A key finding in these studies is that the activation state achieved following treatment with sHIgM12 antibody is distinct from the activation and maturation states induced by TLR and CD40 agonists [2,6]. In addition, preliminary experiments looking at the effect of the antibody in TH-1-type disease models such as collagen-induced arthritis (CIA) and experimental autoimmune encephalitis (EAE), in which antibody treatment might be predicted to do harm, have suggested that there are no obvious detrimental effects of the antibody in these systems. The sHIgM12 antibody also binds to cultured human DC [2]. We are currently accumulating evidence that these cells become activated in ways similar to what we have found in the mouse following cross-linking human B7-DC. Because this antibody has potential immunotherapeutic application, we sought to immortalize the source of the B7-DC cross-linking antibody by determining the structure of the antibody and generating cell lines expressing synthetic antibody-encoding genes. By cloning the antibody in this fashion, we have been able to demonstrate definitively that the antibody itself mediates the biological effects observed in vivo and in vitro.
The sHIgM12 antibody is representative of a set of antibodies that binds to both murine and human cells and has demonstrable ability to activate these cells upon binding [2,10]. The characteristics of sHIgM12 antibody are consistent with our previous characterization of a human IgM antibody [7] that has the ability to activate murine oligodendrocytes and induce remyelination when administered systemically to mice displaying extensive demyelination in both viral and toxin models of multiple sclerosis [10,11]. The heavy and light chains of both IgM antibodies contain numerous nucleotide and encoded amino acid differences with respect to their closest germline counterparts. Consequently, it is our view that this set of antibodies, which is capable of remodelling tissue in vivo and of binding to both murine and human cells, is not comprised of germline encoded sequences, but rather represents somatically generated specificities. None the less, these antibodies display autoreactivity similar to natural antibodies, despite the array of somatic mutations that distinguish them from their nearest germline counterparts. Interestingly, the patients from whom these antibodies were isolated displayed no documented unusual immunological side effects due to the specificity of the antibodies. A major unanswered question is whether IgM antibodies that can bind to self cell surface receptors play important physiological roles naturally in the body, or whether the antibodies have meaningful function only when added in concentrations not normally present. The possibility that IgM antibodies have important physiological roles other than their known function against pathogens has been considered widely in the context of development, homeostatic regulation and even enzymatic function [12–14].
The cloned version of the sHIgM12 antibody, designated rHIgM12 (for recombinant DNA-derived human IgM12), was generated by DNA-mediated gene transfer and possesses comparable ability to activate mouse DC in vitro and to modulate immunity in two characterized mouse models, one involving the induction of anti-tumour immunity and a second involving the redirection of an established Th2 immune response to airway antigen. These findings establish definitively that the sHIgM12 M protein enriched from the patient serum is the biological mediator of the immunological modulation of the potent anti-tumour protective response against malignant melanoma, as well as a protective response that prevents inflammatory airway disease in a mouse model of asthma. We also document a renewable source of this immunomodulatory antibody, opening the possibility that this antibody can now be developed as a reagent for modulating immunity in human patients.
Acknowledgments
This work was supported by NIH grants R01 CA096859-01 and R01 CA104996-01.
References
- 1.Nguyen LT, Radhakrishnan S, Ciric B, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–8. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Radhakrishnan S, Nguyen LT, Ciric B, et al. Naturally occurring human IgM antibody that binds B7-DC and potentiates T-cell stimulation by dendritic cells. J Immunol. 2003;170:1830–8. doi: 10.4049/jimmunol.170.4.1830. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan S, Nguyen L, Ciric B, et al. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring anti-tumor immunity. Cancer Res. 2004;64:4965–72. doi: 10.1158/0008-5472.CAN-03-3025. [DOI] [PubMed] [Google Scholar]
- 4.Radhakrishnan S, Iijima K, Kobayashi T, Rodriguez M, Kita H, Pease LR. Blockade of allergic airway inflammation following systemic treatment with a B7-DC (PD-L2) cross-linking human antibody. J Immunol. 2004;173:1360–5. doi: 10.4049/jimmunol.173.2.1360. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease. J Allergy Clin Immunol. 2005;116:668–74. doi: 10.1016/j.jaci.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishnan S, Celis E, Pease LR. B7-DC cross-linking restores antigen uptake and augments APC function by matured dendritic cells. Proc Natl Acad Sci USA. 2005;102:11438–43. doi: 10.1073/pnas.0501420102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Ciric B, Van Keulen V, Rodriguez M, Kyle RA, Gertz MA, Pease LR. Clonal evolution in Waldenstrom's macroglobulinemia highlights functional role of B-cell receptor. Blood. 2001;97:321–3. doi: 10.1182/blood.v97.1.321. [DOI] [PubMed] [Google Scholar]
- 8.Mitsunaga Y, Ciric B, Van Keulen V, et al. Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. FASEB J. 2002;16:1325–7. doi: 10.1096/fj.01-0994fje. [DOI] [PubMed] [Google Scholar]
- 9.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 10.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci USA. 2000;97:6820–5. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieber AJ, Warrington AJ, Asakura K, et al. Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia. 2002;37:241–9. doi: 10.1002/glia.10033. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–18. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 13.Paul S. Natural catalytic antibodies. Mol Biotechnol. 1996;5:197–207. doi: 10.1007/BF02900358. [DOI] [PubMed] [Google Scholar]
- 14.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–79. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]