Abstract
Whole-cell and soluble extracts of Leishmania promastigotes have both been used as skin test antigens and have also been tested as vaccine candidates. However, the differences in antigenicity between soluble and particulate Leishmania fractions are not known. We evaluated in vitro responses of PBMC from 30 American tegumentary leishmaniasis (ATL) patients and seven noninfected donors to different antigen preparations from Leishmania promastigotes, namely Leishmania amazonensis and L. braziliensis whole-cell extracts, as well as soluble and particulate fractions of L. amazonensis. All Leishmania antigen preparations stimulated significantly higher proliferation and interferon (IFN)-γ production (but not interleukin (IL)-10 production) in PBMC from the leishmaniasis patients than in cells from the control subjects. The L. braziliensis whole-cell extract stimulated significantly higher cell proliferation and IFN-γ production than the L. amazonensis whole-cell extract in the group of patients but not in the control group. This result can be explained by the fact that the patients were infected with L. braziliensis. Again in the group of patients, the PBMC proliferative responses as well as the levels of IFN-γ and IL-10 stimulated by L. amazonensis whole-cell extract were significantly greater than those elicited by the L. amazonensis soluble fraction but were not significantly different from those elicited by the L. amazonensis particulate fraction. We found a higher antigenicity of the particulate fraction as compared to the soluble fraction, what suggests that the antigens present in the particulate fraction account for most of the antigenicity of whole-cell Leishmania promastigote antigen extracts.
Keywords: American tegumentary leishmaniasis, Leishmania amazonensis, Leishmania braziliensis, antigen fractions, cytokines
Introduction
The leishmaniases are a group of diseases caused by protozoan parasites of the genus Leishmania that possess two main stages in their life cycle: intracellular amastigotes in the mammalian host and motile promastigotes in the sandfly vector [1]. Various Leishmania species can cause human infection [2], producing a spectrum of clinical manifestations and a wide epidemiological diversity. It is estimated that 350 million people are at risk, with a global yearly incidence of 1–1·5 million for cutaneous and 500 000 for visceral leishmaniasis [3]. The burden of the leishmaniasis as an international health problem is increasing due to the low effectiveness of control strategies and the emergence of new risk factors [4].
Due to the variety of eco-epidemiological conditions involved in leishmaniasis transmission, it is considered that a vaccine would be the most comprehensive and cost-effective means of prevention. First generation vaccine candidates against leishmaniasis are composed of crude antigen extracts of killed promastigotes [5]. A first generation candidate vaccine was developed in Brazil, consisting of a whole-cell extract of promastigotes killed by ultrasonication and merthiolate [6]. The former version of this candidate vaccine contained five different Leishmania strains [7]. For the sake of standardization, one of them, L. amazonensis IFLA/BR/67/PH8, was selected to constitute a new, single-strain version of the vaccine [8]. The single-strain vaccine has been tested in several clinical trials [8–11]. Although a recent clinical trial did not show a protective effect of this vaccine in Colombia [11], it is well established that this preparation is highly immunogenic [9–11]. The leishmanin skin test is a useful tool for the diagnosis of cutaneous leishmaniasis. It consists of the intradermal injection of an antigen suspension, termed leishmanin, which is also prepared from killed promastigotes [12]. In Brazil, the L. amazonensis vaccine strain (IFLA/BR/67/PH8) is also used in the preparation of leishmanin [13].
Both whole-cell and soluble extracts of Leishmania promastigotes have been arbitrarily used for in vitro studies, as leishmanin antigen for skin tests and as vaccine candidates in preclinical and clinical trials [8–15]. Thus, the main purpose of this study was to compare particulate and soluble Leishmania antigen fractions for their capacity to elicit immune responses in cells from leishmaniasis patients with active disease. This approach may contribute to direct the search for immunogenic molecules of the parasite, which is relevant for the identification of new candidates for subunit vaccines and targets to immunotherapy. We used whole cell extract and fractions (particulate and soluble) from L. amazonensis (IFLA/BR/67/PH8) promastigotes because this strain is currently employed in Brazil in the preparation of leishmanin and candidate vaccines. We also used L. braziliensis whole-cell extract because this species is regarded as the most important cause of ATL in Brazil [2] and it is the only causative agent of the disease in Rio de Janeiro municipality [16], where this study was performed.
Subjects and methods
Study subjects
A group of 30 ATL patients was studied, 25 of them had localized cutaneous leishmaniasis [17], three had mucocutaneous leishmaniasis and the other two had disseminated cutaneous leishmaniasis [18]. Diagnosis was made based on immunological and parasitological criteria, as previously described [19]. All patients came from endemic areas of the municipality of Rio de Janeiro, where only Leishmania braziliensis is transmitted [16]. The parasites isolated from the patients were characterized as L. braziliensis by isoenzyme analysis [20]. A control group of seven healthy subjects coming from nonendemic areas and negative to the leishmanin skin test was also studied. All study subjects gave written informed consent to participate in this study. The Ethics Committee of Fundação Oswaldo Cruz, Brazilian Ministry of Health, approved the study protocol.
Antigens
Promastigotes of L. amazonensis (IFLA/BR/67/PH8) and L. braziliensis (MHOM/BR/75/M2903) were cultured in Schneider's medium supplemented with antibiotics (200 IU penicillin and 200 µg streptomycin/ml) and 10% inactivated foetal calf serum (all from Sigma, St. Louis, MO, USA). Stationary phase promastigotes were washed three times in phosphate buffered saline (PBS), and disrupted by 10 freeze and thaw cycles, followed by ultrasonication (Ultra-tip Labsonic System; Laboratory-Line, Melrose Park, IL, USA), at 40 watts for 15 min in an ice bath to generate the whole-cell extracts of L. braziliensis and L. amazonensis. Part of the L. amazonensis whole-cell extract was centrifuged at 100 000 × g for 1 h at 4 °C to produce sediment (particulate fraction) and supernatant (soluble fraction). All antigen preparations were adjusted to 1 mg/ml protein nitrogen in PBS and all samples were stored at −20 °C until use.
Electron microscopy
Samples of L. amazonensis whole-cell extract as well as particulate and soluble fractions were fixed in 2·5% glutaraldehyde in sodium cacodylate buffer (pH 7·2) for 1 h and then rinsed in the same buffer. They where then postfixed with 1% OsO4, dehydrated in acetone and embedded in Epon resin. After resin polymerization, thin sections were obtained and stained with uranyl acetate and lead citrate. The thin sections where observed with an EM10C Zeiss Microscope.
PBMC proliferation assay
Approximately 20 ml of peripheral blood was obtained from each donor. PBMC were separated from the blood samples in Ficoll-Hypaque gradients (Histopaque-1077, Sigma) and resuspended in RPMI 1640 supplemented with 10 mM Hepes, 1·5 mM/1 l-glutamine, 200 IU/ml penicillin, 200 µg/ml streptomycin and 0·04 mM 2-mercaptoethanol (all purchased from Sigma). Cells (3 × 105) were distributed in triplicate in 96-well flat-bottomed microtiter plates (Nunk, Roskilde, Denmark) in a final volume of 200 µl, along with 10 µg (50 µg/ml) of each antigen preparation or 4 µg of ConA (Sigma, 20 µg/ml). Cells from each donor were left unstimulated in triplicate wells containing medium alone. Cultures were incubated for five days and [3H]-thymidine incorporation was assessed as previously described [7,8,10,19,21,22]. Results were expressed as stimulation indices (SI) defined as the ratios between the mean counts of stimulated and unstimulated cultures.
Cytokine assays
Cytokine levels in supernatants from the PBMC cultures stimulated or not with the antigens or ConA were measured by ELISA. PBMC were cultured in 24-well flat-bottomed plates (Nunc) in a final volume of 1 ml/well and were stimulated in vitro under the conditions described above. The mean of triplicate cultures was compared with standard curves obtained with recombinant IFN-γ and IL-10 (Pharmingen, San Diego, CA, USA). As previously established in kinetics experiments in our laboratory, supernatants were collected on day 3 for IL-10 and on day 5 for IFN-γ [8,10,21,22], and they were all stored at −70°C until use. The detection limit of the assays was 8 pg/ml for both IFN-γ and IL-10.
Statistical analysis
The Kolmogorov-Smirnov, Wilcoxon matched-pairs signed-ranks, Mann–Whitney, Friedman nonparametric repeated measures, Dunn's multiple comparisons and Spearman correlation tests were used for statistical analysis of the data. Nonparametric tests were chosen because the samples did not follow a Gaussian distribution. All tests used were two-tailed. Significance was set at P < 0·05.
Results
Ultrastructural analysis of whole-cell, particulate and soluble extracts of L. amazonensis promastigotes
Ultrastructural analysis of the L. amazonensis antigen preparations with conventional electron microscopy revealed several membrane structures and small granules associated with electron-dense material in the whole-cell extract. The particulate fraction displayed a high concentration of membrane structures associated with electron-dense material. The soluble fraction, on the other hand, had larger dense granules, probably lipids, and very few membrane profiles (data not shown).
Comparison between the groups
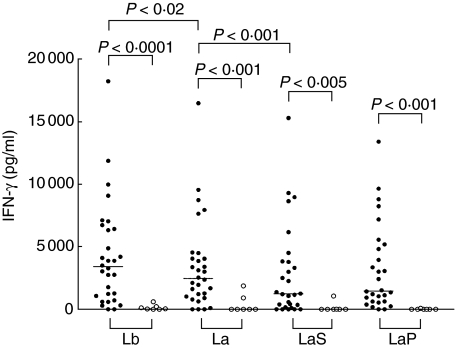
The proliferative responses of PBMC from the ATL patients were significantly stronger than those obtained with cells from the healthy controls after stimulation with the Leishmania antigen preparations (Fig. 1): L. braziliensis whole-cell extract (P < 0·0005), L. amazonensis whole-cell extract (P < 0·005), and both L. amazonensis fractions, particulate (P < 0·05) and soluble (P = 0·01). The IFN-γ responses were also significantly stronger in the group of patients than in the control group (Fig. 2) after stimulation with the Leishmania antigen preparations: L. braziliensis whole-cell extract (P = 0·0001), L. amazonensis whole-cell extract (P = 0·001), L. amazonensis particulate fraction (P = 0·001) and L. amazonensis soluble fraction (P < 0·005). Regarding IL-10, the only difference between patients and controls was observed when the L. amazonensis particulate fraction was used (P < 0·05, data not shown). ConA induced stimulation indices ranging from 3·8 to 184 (median 36·4) in the group of patients and from 24·3 to 52·9 (median 24·3) in the control group. The IFN-γ levels stimulated by ConA were between 1000 and 19 000 pg/ml (median 4050 pg/ml) among the patients and between 2000 and 8000 pg/ml (median 5600 pg/ml) among the controls, whereas ConA-stimulated IL-10 production ranged from 5 to 500 pg/ml (median 130 pg/ml) among the patients and from 5 to 520 pg/ml (median 50 pg/ml) among the controls. The proliferative responses, IFN-γ production and IL-10 production stimulated by ConA were not different between patients and healthy unexposed controls.
Fig. 1.
Proliferative responses stimulated by Leishmania braziliensis (Lb) and L. amazonensis (La) whole-cell extracts, and particulate (LaP) and soluble (LaS) L. amazonensis fractions (all used at 50 µg/ml) in cultures of PBMC from American tegumentary leishmaniasis patients and healthy noninfected control subjects. • patient values; ○ control values. Horizontal lines indicate median values.
Fig. 2.
IFN-γ production stimulated by Leishmania braziliensis (Lb) and L. amazonensis (La) whole-cell extracts, and particulate (LaP) and soluble (LaS) L. amazonensis fractions (all used at 50 µg/ml) in cultures of PBMC from American tegumentary leishmaniasis patients and healthy noninfected control subjects. • patient values; ○ control values. Horizontal lines indicate median values.
Comparison among the stimuli
Comparison between whole-cell extracts from L. braziliensis and L. amazonensis
In the patients group, the L. braziliensis whole-cell extract elicited significantly stronger (P < 0·0001) PBMC proliferative responses than the L. amazonensis whole-cell extract (Fig. 1). A significantly higher production of IFN-γ (P < 0·02) was also observed in the patients’ cell cultures stimulated with the L. braziliensis whole-cell extract, when compared to those stimulated with the L. amazonensis whole-cell extract (Fig. 2). In the group of patients, the indices of cell proliferation stimulated by L. amazonensis and L. braziliensis were highly and positively correlated (r = 0·892, P < 0·0001) and the same was true for IFN-γ levels (r = 0·8723, P < 0·0001). However, in the same group of patients, no significant differences and no correlation were seen between the levels of IL-10 stimulated by L. amazonensis and L. braziliensis whole-cell extracts (data not shown). No differences were observed in the healthy control group between L. amazonensis and L. braziliensis whole-cell extracts with regard to induction of cell proliferation, IFN-γ production or IL-10 production (data not shown).
Comparison among whole-cell, soluble and particulate extracts from L. amazonensis
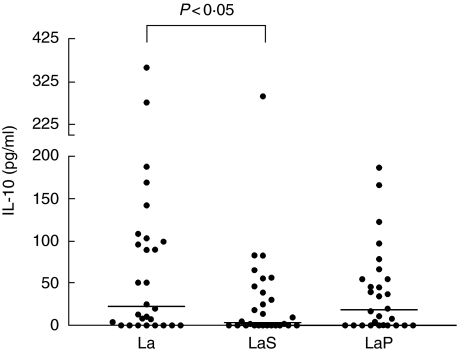
Among the patients, the PBMC proliferative responses and IFN-γ and IL-10 levels stimulated by the L. amazonensis whole-cell extract were significantly higher than those elicited by the L. amazonensis soluble fraction (P < 0·001, P < 0·001 and P < 0·05, respectively) but were not significantly different from those elicited by the L. amazonensis particulate fraction (Figs 1–3, respectively). No other differences were seen among the L. amazonensis antigen preparations in this group. In the patients group, stimulation indices obtained with the L. amazonensis whole-cell extract and fractions were in all cases positively and very significantly correlated and this was also observed with regard to IFN-γ levels (always P < 0·0001), but this was not the case for IL-10 (data not shown). In the control group, the only significant difference observed among the L. amazonensis antigen preparations was between the whole cell extract and the particulate fraction concerning IL-10 production (P < 0·05).
Fig. 3.
Production of IL-10 stimulated by Leishmania amazonensis whole-cell extract (La), soluble (LaS) and particulate (LaP) fractions (all used at 50 µg/ml) in cultures of PBMC from American tegumentary leishmaniasis patients. Dots indicate individual patient values and horizontal lines indicate median values.
Discussion
Localized cutaneous leishmaniasis is the most frequent and benign clinical form of ATL [2,17]. Although it can be caused by all pathogenic Leishmania species with dermal tropism [2,17], clinical and histopathological differences between L. braziliensis and L. amazonensis infections have recently been described [23]. L. amazonensis infection causes large infiltration at the edge of the lesion and its histopathology is characterized as a dense infiltrate of vacuolated macrophages full of amastigotes. In L. braziliensis infection infiltration bordering the ulcer is more modest. Histologically, macrophages and parasites are scanty and, in contrast, lymphocytes and plasma cells are frequent [23]. L. braziliensis causes mucosal leishmaniasis, a clinical form associated with the up-regulation of Th1-type responses [24], whereas L. amazonensis infection can produce diffuse cutaneous leishmaniasis, the anergic form of ATL, characterized by the absence of pathogen-specific cell-mediated immunity and by large numbers of intracellular parasites [25]. All these observations, considered together, suggest differences in cell-mediated immunity against these Leishmania species, being type 1-biased in L. braziliensis infection and type 2-biased in L. amazonensis infection [23]. Based on in vitro studies with cells from BALB/c mice, it has recently been suggested that L. amazonensis antigens by themselves can suppress T cell responses [26].
We found that L. braziliensis antigens elicited significantly higher proliferative responses and IFN-γ (but not IL-10) production, when compared with L. amazonensis antigens, in PBMC cultures from ATL patients (Fig. 2). However, these patients were presumably infected with L. braziliensis, because all came from endemic areas within the municipality of Rio de Janeiro, where L. braziliensis has been the sole Leishmania species isolated from ATL cases [16]. Thus, it is expected that T cells from L. braziliensis-infected patients would respond more strongly to antigens from the homologous species than to antigens from a species belonging to a different subgenus. However, in our previous studies with volunteers immunized with a first generation candidate vaccine composed of whole-cell extracts of killed L. amazonensis promastigotes, either alone or in association with other Leishmania species, we never observed differences between L. braziliensis and L. amazonensis antigens in their ability to elicit T–cell mediated responses [7,8,10]. In spite of the lower levels of cell proliferation and IFN-γ observed after stimulation with L. amazonensis extract as compared to L. braziliensis extract, our data show that both antigen preparations are able to elicit antigen-specific recall responses in PBMC from ATL patients, because significant differences in cell proliferation and IFN-γ production were observed between patients and healthy unexposed controls (Fig. 1).
To our knowledge, this is the first time that particulate and soluble Leishmania antigen fractions have been compared for their capacity to elicit immune responses in cells from leishmaniasis patients with active disease. Electron microscopic observation of the fractions showed that the particulate fraction was characterized by the abundance of membrane fragments, while membrane structures were rare and large lipid granules were numerous in the soluble fraction (data not shown). The cellular responses elicited by soluble and insoluble (membrane rich) fractions were compared in a previous study performed with cells from healthy Swedish donors. The membrane rich fraction induced stronger expression of both IFN-γ and IL-10, as well as a greater proliferative response, when compared to the soluble fraction [27]. The data of cell proliferation and IFN-γ production observed in those normal donors are in agreement with our findings for ATL patients, suggesting that the Leishmania membrane-associated antigens are capable of inducing more potent innate and adaptive immune responses, when compared to the soluble antigens. The difference between the results obtained with those healthy donors and with our L. braziliensis-infected ATL patients with regard to IL-10 production could be due to the Th1-biased response in human L. braziliensis infection [23,24], which is further suggested by our observation that the cells from ATL patients produced more IFN-γ (but not IL-10) than those from healthy unexposed subjects when stimulated with Leishmania antigens. We have tried to assess IL-4 levels in supernatants of Leishmania antigen-stimulated cultures of PBMC from L. braziliensis-infected ATL patients but the results have been poor (our unpublished observations). In contrast with the results obtained with healthy Swedish donors [27], we did not find differences in the ability of the particulate (membrane rich) and the soluble L. amazonensis fractions to elicit IFN-γ or IL-10 production in cells from healthy unexposed subjects (data not shown). In a recent study, both membrane proteins and nonmembranous soluble proteins from L. donovani induced proliferative responses and IFN-γ production in lymphocytes isolated from cured VL patients as well as healthy household contacts. No significant difference was found between the two antigen preparations, but the numbers of subjects in each group were quite low (10 cured patients and five controls). It is noteworthy, however, that the stimulation indices and IFN-γ levels induced by the membrane proteins were always higher than those obtained with the soluble proteins [28].
Several membrane proteins have been considered as putative candidates for subunit vaccine development. Glycoprotein 63 is the most abundant surface protein of Leishmania promastigotes [29]. This antigen and its encoding gene have shown protective efficacy in experimental murine leishmaniasis in a variety of immunization protocols using native protein [30], synthetic peptides [31], DNA [32] or vectored [33,34] vaccines. Kinetoplastid membrane protein-11 (KMP-11), formerly termed lipophosphoglycan-associated protein, stimulates T cells from American tegumentary leishmaniasis patients and from cured visceral leishmaniasis patients to proliferate and produce IFN-γ in an antigen-specific fashion, as such responses were not seen in PBMC from nonexposed donors [35–37]. Moreover, immunization protocols using the KMP-11-encoding gene were shown to be protective against L. major and L. donovani in animal models [38,39]. The higher antigenicity associated with the membrane rich particulate fraction in our study may be due to an intrinsic quality of the antigens present in this fraction or to its particulate nature. Particulate delivery systems have long been used to augment the immune response to antigen [40].
L. amazonensis whole-cell extract has been tested both for immunoprophylaxis and immunotherapy [8,10,41]. Further studies aiming at understanding why the L. amazonensis particulate fraction is more antigenic than the soluble fraction may contribute to the identification of new candidate molecules, adjuvants and antigen delivery systems for the development of prophylactic or therapeutic vaccines against leishmaniasis.
Acknowledgments
Sergio C. F. Mendonça, Maria de Nazareth Meirelles and Armando Schubach are Investigators of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Elaine Telino was a recipient of a M.Sc. Fellowship from CNPq, Denize C. S. Matos is a recipient of a Visiting Scientist Fellowship from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj), and Paula M. De Luca also received a Visiting Scientist Fellowship from Faperj. This study received support from Fundação Oswaldo Cruz, Rio de Janeiro (PDTIS and PAPES 3) and Faperj.
References
- 1.Bates PA, Rogers ME. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med. 2004;4:601–9. doi: 10.2174/1566524043360285. [DOI] [PubMed] [Google Scholar]
- 2.Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz. 2004;99:239–51. doi: 10.1590/s0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 3.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Desjeux P. The increase in risk factors for the leishmaniasis Worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–43. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 5.Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89:83–8. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 6.Mayrink W, da Costa CA, Magalhaes PA, Melo MN, Dias M, Lima AO, Michalick MS, Williams P. A field trial of a vaccine against American dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73:385–7. doi: 10.1016/0035-9203(79)90159-7. [DOI] [PubMed] [Google Scholar]
- 7.Mendonca SC, De Luca PM, Mayrink W, et al. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 8.De Luca PM, Mayrink W, Alves CA, et al. Evaluation of the stability and immunogenicity of autoclaved and non-autoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine. 1999;17:1179–85. doi: 10.1016/s0264-410x(98)00338-7. [DOI] [PubMed] [Google Scholar]
- 9.Marzochi KB, Marzochi MA, Silva AF, Grativol N, Duarte R, Confort EM, Modabber F. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem Inst Oswaldo Cruz. 1998;93:205–12. doi: 10.1590/s0074-02761998000200014. [DOI] [PubMed] [Google Scholar]
- 10.De Luca PM, Mayrink W, Pinto JA, et al. A randomized double-blind placebo-controlled trial to evaluate the immunogenicity of a candidate vaccine against American tegumentary leishmaniasis. Acta Trop. 2001;80:251–60. doi: 10.1016/s0001-706x(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 11.Velez ID, Gilchrist K, Arbelaez MP, Rojas JA, Puerta JA, Antunes CM, Zicker F, Modabber F. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–8. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Salman SM, Rubeiz NG, Kibbi AG. Cutaneous leishmaniasis. clinical features and diagnosis. Clin Dermatol. 1999;17:291–6. doi: 10.1016/s0738-081x(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 13.Da Costa CA, Toledo VPCP, Genaro O, Williams P, Mayrink W. Leishmanin skin test – Evaluation of the composition and stability of the antigen preparation. Mem Inst Oswaldo Cruz. 1996;91:193–4. doi: 10.1590/s0074-02761996000200013. [DOI] [PubMed] [Google Scholar]
- 14.Badaro R, Pedral-Sampaio D, Johnson WD, Jr, Reed SG. Evaluation of the stability of a soluble intradermal skin test antigen preparation in American visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84:226–7. doi: 10.1016/0035-9203(90)90264-f. [DOI] [PubMed] [Google Scholar]
- 15.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–7. [PubMed] [Google Scholar]
- 16.de Oliveira-Neto MP, Mattos MS, Perez MA, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol. 2000;39:506–14. doi: 10.1046/j.1365-4362.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 17.Castes M, Agnelli A, Verde O, Rondon AJ. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol. 1983;27:176–86. doi: 10.1016/0090-1229(83)90068-5. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–25. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 19.Mendonca SC, Coutinho SG, Amendoeira RR, Marzochi MC, Pirmez C. Human american cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil. lymphoproliferative responses and influence of therapy. Clin Exp Immunol. 1986;64:269–76. [PMC free article] [PubMed] [Google Scholar]
- 20.Cupolillo E, Grimaldi G, Jr, Momen H. A general classification of New world Leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296–311. doi: 10.4269/ajtmh.1994.50.296. [DOI] [PubMed] [Google Scholar]
- 21.De Luca PM, Mayrink W, Santiago MA, Nogueira R, Conceição-Silva F, Mélo G, Mendonca SCF. Randomised, double-blind, placebo-controlled study on the immunogenicity of the leishmanin skin test. Trans R Soc Trop Med Hyg. 2003;97:709–12. doi: 10.1016/s0035-9203(03)80109-8. [DOI] [PubMed] [Google Scholar]
- 22.Matos DS, Azeredo-Coutinho RB, Schubach A, Conceição-Silva F, Baptista C, Moreira JS, Mendonca SC. Differential interferon- gamma production characterizes the cytokine responses to Leishmania and Mycobacterium leprae antigens in concomitant mucocutaneous leishmaniasis and lepromatous leprosy. Clin Infect Dis. 2005;40:e5–12. doi: 10.1086/427069. [DOI] [PubMed] [Google Scholar]
- 23.Silveira FT, Blackwell JM, Ishikawa EA, et al. T cell responses to crude and defined leishmanial antigens in patients from the lower Amazon region of Brazil infected with different species of Leishmania of the subgenera Leishmania and Viannia. Parasite Immunol. 1998;20:19–26. doi: 10.1046/j.1365-3024.1998.t01-1-00126.x. [DOI] [PubMed] [Google Scholar]
- 24.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Convit J, Ulrich M, Fernandez CT, Tapia FJ, Caceres-Dittmar G, Castes M, Rondon AJ. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1993;87:444–8. doi: 10.1016/0035-9203(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro RO, Pinto EF, Benedito AB, Lopes UG, Rossi-Bergmann B. The T-cell anergy induced by Leishmania amazonensis antigens is related with defective antigen presentation and apoptosis. An Acad Bras Cienc. 2004;76:519–27. doi: 10.1590/s0001-37652004000300006. [DOI] [PubMed] [Google Scholar]
- 27.Nylen S, Mortbert U, Kovalenko D, Satti I, Engstrom K, Bakhiet M, Akuffo H. Differential induction of cellular responses by live and dead Leishmania promastigotes in healthy donors. Clin Exp Immunol. 2001;124:43–53. doi: 10.1046/j.1365-2249.2001.01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg R, Gupta SK, Tripathi P, Naik S, Sundar S, Dube A. Immunostimulatory cellular responses of cured Leishmania-infected patients and hamsters against the integral membrane proteins and non-membranous soluble proteins of a recent clinical isolate of Leishmania donovani. Clin Exp Immunol. 2005;140:149–56. doi: 10.1111/j.1365-2249.2005.02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao C, Donelson JE, Wilson ME. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol Biochem Parasitol. 2003;132:1–16. doi: 10.1016/s0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- 30.Afrin F, Rajesh R, Anam K, Gopinath M, Pal S, Ali N. Characterization of Leishmania donovani antigens encapsulated in liposomes that induce protective immunity in BALB/c mice. Infect Immun. 2002;70:6697–706. doi: 10.1128/IAI.70.12.6697-6706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsagozis P, Karagouni E, Dotsika E. Dendritic cells pulsed with peptides of gp63 induce differential protection against experimental cutaneous leishmaniasis. Int J Immunopathol Pharmacol. 2004;17:343–52. doi: 10.1177/039463200401700314. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Liew FY. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. Major. Immunol. 1995;84:173–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Connell ND, Medina-Acosta E, McMaster WR, Bloom BR, Russell DG. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–7. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McSorley SJ, Xu D, Liew FY. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect Immun. 1997;65:171–8. doi: 10.1128/iai.65.1.171-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo DM, Turco SJ, Burns JM, Jr, Reed SG. Stimulation of human T lymphocytes by Leishmania lipophosphoglycan-associated proteins. J Immunol. 1992;148:202–7. [PubMed] [Google Scholar]
- 36.Kurtzhals JA, Hey AS, Jardim A, Kemp M, et al. Dichotomy of the human T cell response to Leishmania antigens. II. Absent or Th2-like response to gp63 and Th1-like response to lipophosphoglycan-associated protein in cells from cured visceral leishmaniasis patients. Clin Exp Immunol. 1994;96:416–21. doi: 10.1111/j.1365-2249.1994.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinhas V, Freire M, Bacellar O, Cunha S, Rocha H, Carvalho EM. Characterization of T cell responses to purified leishmania antigens in subjects infected with Leishmania chagasi. Braz J Med Biol Res. 1994;27:1199–205. [PubMed] [Google Scholar]
- 38.Ramirez JR, Gilchrist K, Robledo S, Sepulveda JC, Moll H, Soldati D, Berberich C. Attenuated Toxoplasma gondii ts-4 mutants engineered to express the Leishmania antigen KMP-11 elicit a specific immune response in BALB/c mice. Vaccine. 2001;20:455–61. doi: 10.1016/s0264-410x(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 39.Basu R, Bhaumik S, Basu JM, De Naskar KT, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and – resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–71. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- 40.Copland MJ, Rades T, Davies NM, Baird MA. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Biol. 2005;83:97–105. doi: 10.1111/j.1440-1711.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 41.Machado-Pinto J, Pinto J, da Costa CA, Genaro O, Marques MJ, Modabber F, Mayrink W. Immunochemotherapy for cutaneous leishmaniasis: a controlled trial using killed Leishmania (Leishmania) amazonensis vaccine plus antimonial. Int J Dermatol. 2002;41:73–8. doi: 10.1046/j.1365-4362.2002.01336.x. [DOI] [PubMed] [Google Scholar]