Abstract
Malignant melanoma is often accompanied by a host response of inflammatory cell infiltration that is highly regulated by multiple adhesion molecules. To assess the role of adhesion molecules, including l-selectin and intercellular adhesion molecule-1 (ICAM-1), in this process, subcutaneous primary growth and metastasis to the lung of B16 melanoma cells not expressing l-selectin, ICAM-1 or their ligands were examined in mice lacking l-selectin, ICAM-1 or both. Primary subcutaneous growth of B16 melanoma was augmented by loss of l-selectin, ICAM-1 or both, while pulmonary metastasis was enhanced by the loss of l-selectin or combined loss of l-selectin and ICAM-1. In both situations, the combined loss of l-selectin and ICAM-1 exhibited the greatest effect. This enhancement was associated generally with a reduced accumulation of natural killer (NK) cells, CD4+ T cells and CD8+ T cells and also with a diminished release of interferon (IFN)-γ and tumour necrosis factor (TNF)-α but not interleukin (IL)-6. Cytotoxicity against melanoma was not defective by the absence of ICAM-1, l-selectin or both, suggesting that the enhancement of tumour growth and metastasis caused by the loss of adhesion molecules results from an impaired migration of effector cells into the tissue rather than from a suppression of the cytotoxic response. The results indicate that l-selectin and ICAM-1 contribute co-operatively to the anti-tumour reaction by regulating lymphocyte infiltration to the tumour.
Keywords: anti-tumour mechanism, l-selectin, ICAM-1, B16 F10 melanoma, mouse
Introduction
Cellular transformation, tumour growth and metastasis of malignant melanoma are complex biochemical processes that involve autonomous tumour cell growth and host–tumour interactions [1]. Leucocytes infiltration to tumour tissues is a critical step in anti-tumour immunity for both primary tumour growth and the metastasis of malignant melanoma [2]. inflammation and immunotherapy can contribute to the regression of solid tumours [2,3]. However, the relationship between inflammation and tumour progression is complex, given that tumour-infiltrating lymphocytes can contribute to cancer growth and spread and to the immunosuppression associated with malignant diseases [4]. Furthermore, there is now evidence that inflammatory cytokines produced, in part, by tumour cells and tumour-infiltrating leucocytes may contribute directly to malignant progression [4]. Indeed, mice lacking tumour necrosis factor (TNF)-α expression are resistant to skin carcinogenesis [5]. In addition, anti-inflammatory drug use is associated with a lower incidence of tumour recurrence in colorectal cancer [6].
Thus, it remains unknown whether leucocytic infiltration and inflammation contribute to tumour regression or tumour progression. In either case, the recruitment of leucocytes from the circulation into tissues, including tumours, is a multi-step process that is highly regulated by multiple cell-surface adhesion molecules [7,8]. Leucocytes first tether and roll on vascular endothelial cells before they are activated to adhere firmly and subsequently emigrate into the extravascular space. Selectins mediate primarily the tethering and rolling of leucocytes while members of the immunoglobulin superfamily, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and their integrin ligands, including lymphocyte function-associated antigen-1 (LFA-1, CD11a/CD18) and very late antigen-4 (VLA-4, CD49d/CD29), are critical for mediating firm adhesion. The selectin family consists of three cell-surface molecules expressed by leucocytes (l-selectin), vascular endothelium (E- and P-selectins) and platelets (P-selectin) [9]. l-Selectin is expressed constitutively on most leucocytes [9]. ICAM-1 is also expressed constitutively at low levels by endothelial cells, but its expression is up-regulated rapidly during inflammation [10].
The involvement of cell-surface adhesion molecules in tumour progression and metastasis has been studied previously; however, almost all studies have focused on the interaction between endothelial cells and cell surface adhesion molecules expressed on tumour cells. For example, mouse B16 melanoma cells that are forced to express sialyl Lewis X, a ligand for P- and E-selectins, produce more lung tumour modules through an efficient interaction with endothelial cells than those without sialyl Lewis X [11]. On the other hand, studies of the role for the adhesion molecule-mediated recruitment of leucocytes in tumour progression and metastasis are limited. Only one previous report has shown that liver metastasis of B16 melanoma is enhanced in ICAM-1-deficient (ICAM-1–/–) mice and LFA-1–/– mice, suggesting that the ICAM-1/LFA-1 interaction is crucial to the immune response to hepatic metastasis [12]. However, the involvement of ICAM-1 in subcutaneous primary tumour growth and pulmonary metastasis was not clarified. Furthermore, the contribution of other cell adhesion molecules to the progression and metastasis of melanoma and their interaction in this process remain unresolved.
In this study, to determine the role of the adhesion molecule-mediated accumulation of leucocytes in the primary growth and metastatic capacity of B16 melanoma, the subcutaneous growth and pulmonary metastasis of B16 F10 melanoma were investigated in mice lacking either l-selectin, ICAM-1 or both adhesion molecules. Subcutaneous injection of B16 F10 melanoma was used to model primary tumorigenesis by establishing a focus of these cells near the skin, the site of origin for melanoma [13]. In contrast, tail vein injection was used to model tumour metastasis by introducing multiple tumour foci throughout the lungs [13]. Our experimental system selectively addressed the role of l-selectin- and ICAM-1-dependent leucocyte recruitment in B16 melanoma cell progression and was not complicated by direct effects on tumour metastasis, as these tumours lacked expression of these adhesion molecules. The results of this study suggest that l-selectin and ICAM-1 contribute co-operatively to the anti-tumour immune reaction by regulating lymphocyte infiltration to B16 melanoma.
Materials and methods
Mice
ICAM-1–/– mice [14], expressing residual amounts of ICAM-1 splice variants in the thymus and spleen but not in other organs, including the skin and lung [15], were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). l-Selectin–/– mice were produced as described [16]. Mice lacking both l-selectin and ICAM-1 (l-selectin/ICAM-1–/–) were generated as described elsewhere [17]. All mice were healthy, fertile and free of signs of infection or disease. All mice were back-crossed between five and 10 generations to the C57BL/6 genetic background. Mice used for experiments were 9–12 weeks old. Age-matched wild-type littermates and C57BL/6 mice (Jackson Laboratory) were used as controls with equivalent results so all control results were pooled. All mice were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science.
B16 melanoma cells
B16 F10 murine melanoma cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in Dulbecco's modified Eagle's medium supplemented with fetal calf serum and penicillin/streptomycin at 37°C in 5% CO2. Cells were passaged twice a week with trypsin. All cell culture reagents were obtained from Sigma-Aldrich (St Louis, MO, USA).
Primary cutaneous tumour growth
B16 F10 cells (1·5 × 106) in 100 µl of phosphate-buffered saline (PBS) were injected subcutaneously into the shaved lateral flank of anaesthetized mice. The size of primary tumours was measured on days 7 and 14. The tumour volume was calculated using the equation: V = 4π(L1×L22)/3, where V = volume (mm3), L1 = longest diameter (mm) and L2 = shortest diameter (mm).
Lung metastasis
B16 F10 cells (1·5 × 106) in 100 µl of PBS were injected intravenously into the tail vein. The mice were killed on days 7 and 14 after injection, and lungs were removed. At these time-points it was not possible to count accurately the number of surface metastatic colonies using a stereomicroscope, because of the small size of the colonies. Therefore, to evaluate lung metastasis, we counted histologically the number of colonies in each section of the three lobes of the right lung. The sections were stained using haematoxylin and eosin (H&E), as described below. When > 60% of the section was occupied with tumour, the colony number was defined as 1000. Each section was examined independently by three investigators in a blinded fashion, and the mean of the results was used for analysis.
Survival curves
B16 F10 cells (1·5 × 106) in 100 µl of PBS were injected intravenously into the tail vein of anaesthetized mice. Survival was recorded over a 28-day period. After 28 days, or at the time of death, dissection was performed to examine the presence of metastasis to organs other than the lung.
Histological examination and immunohistochemistry
Subcutaneous primary tumours were harvested and cut into two segments. Lung tissue was cut into right and left lobes with one piece being fixed in 3·5% paraformaldehyde and paraffin-embedded and the other piece being snap-frozen. For examination of subcutaneous tumours, 6-µm sections were stained using H&E to evaluate cellular infiltration. Extra-tumour neutrophils and mononuclear cells were counted in the sections under a microscopic high power view (0·07 mm2). The mean of numbers of the 10 high-power view fields was calculated. Each section was examined independently by three investigators in a blinded fashion, and the mean of the results was used for analysis.
For immunohistochemistry, frozen sections of the skin and lung were acetone-fixed and incubated with 10% normal rabbit serum in PBS (10 min, 37°C) to block non-specific staining. Sections were then incubated with rat monoclonal antibody specific for mouse CD4, CD8, F4/80 or NK1·1 (BD PharMingen, San Diego, CA). Rat IgG (Southern Biotechnology Associates Inc., Birmingham, AL, USA) was used as a control for non-specific staining. Sections were incubated sequentially (20 min, 37°C) with a biotinylated rabbit anti-rat IgG secondary antibody (Vectastain ABC method, Vector Laboratories, Burlingame, CA, USA), then horseradish peroxidase-conjugated avidin–biotin complexes (Vector Laboratories). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and then counterstained with methyl green. Cells were counted in sections from the skin and lung as above.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from frozen subcutaneous skin tumours and lung tissues with Qiagen RNeasy spin columns (Qiagen Ltd, Crawley, UK) and then reverse-transcribed into cDNA according to the protocol of the reverse transcription system (Promega, Madison, WI, USA). Expression of TNF-α, interleukin (IL)-6 and interferon (IFN)-γ mRNA was analysed using a real-time PCR quantification method, according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Sequence-specific primers and probes were designed by pre-developed TaqMan® assay reagents (Applied Biosystems). Real-time PCR (one cycle of 50°C for 2 min, 95°C for 10 min; 40 cycles of 92°C for 15 s, 60°C for 60 s) was performed on an ABI Prism 7000 sequence detector (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize mRNA. To compare target gene and housekeeping GAPDH gene mRNA expression, the relative expression of real-time PCR products was determined using the ΔΔCt method [18]. Fold induction = 2–[ΔΔCt], where Ct = the threshold cycle, i.e. the cycle number at which the sample's relative fluorescence rises about the background fluorescence and ΔΔCt = [Ct gene of interest (unknown sample) − Ct GAPDH (unknown sample)] − [Ct gene of interest (calibrator sample) − Ct GAPDH (calibrator sample)]. The TNF-α, IL-6 and IFN-γ mRNA levels in wild-type mice were used as the calibrator. Each sample was run in duplicate and the mean Ct was used in the equation.
Cytotoxicity assay
To assess the cytotoxic activity of lymphocytes, B16 F10 murine melanoma cells were used as targets. Donor mice were injected with 1·5 × 106 B16 F10 cells in 100 µl of PBS subcutaneously into the lateral flank for collection of spleen cells and into the left and right lateral flanks for collection of tumour-draining lymph node cells [19–21]. Fourteen days after the injection, mice were killed, spleens and tumour-draining inguinal lymph nodes were harvested and single-cell suspensions were prepared. Splenocytes were isolated by density gradient centrifugation over Ficoll (Atlanta Biologicals, Norcross, GA, USA). To assess antigen-specific T cell cytotoxic activity, tumour-draining lymph node cells were depleted of natural killer (NK) cells using mouse CD49b (DX5) microbeads (Miltenyi Biotec Inc., CA, USA). Beads were added to tumour-draining lymph node cells and incubated at 4°C for 15 min at the ratios recommended. The mixture of cells and beads was placed in a magnetic holder and the supernatant was collected as the negative fraction. The depletion step was repeated twice and flow cytometric analysis showed that > 95% of NK cells were depleted.
Cytotoxicity was assayed using a cell-mediated cytotoxicity kit (Molecular Probes, Eugene, OR, USA), according to the manufacturer's instructions. The target cells were incubated with a staining solution containing DiOC18 for 20 min at 37°C. The stained target cells (1 × 106 cells/ml) were resuspended in complete culture medium and then mixed with splenocytes to yield effector : target (E : T) ratios of 40 : 1, 20 : 1, 10 : 1 and 5 : 1. NK cell-depleted tumour-draining lymph node cells were mixed with the target cells at an E : T ratio of 5 : 1 due to the limited number of lymph node cells. A counterstaining solution containing propidium iodide to detect dead cells was added and the mixture was incubated at 37°C for 2 h. To assess lytic activity, a two-colour flow cytometric analysis was performed using a FACScan flow cytometer (BD PharMingen). The percentage of lysis was calculated by dividing the frequency of dead target cells by the total number of target cells. Spontaneous cell lysis as determined in the absence of effector cells was subtracted from total lysis to calculate the amount of specific lysis.
Statistical analysis
The Mann–Whitney U-test was used for determining the level of significance of differences in sample means and Bonferroni's test was used for multiple comparisons. Survival analysis was performed using a Kaplan–Meier estimate.
Results
Primary tumour growth was augmented by the absence of l-selectin and/or ICAM-1 expression
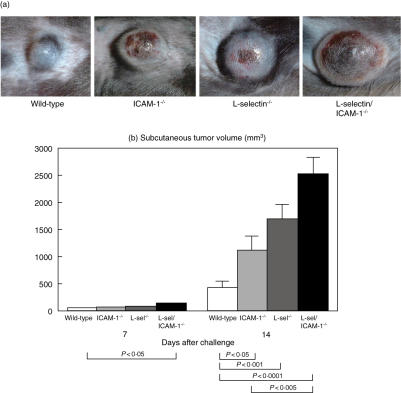
To evaluate the effects of l-selectin and/or ICAM-1 loss on primary tumour growth, B16 F10 melanoma cells were injected subcutaneously into mice lacking l-selectin, ICAM-1 or both and tumour growth was determined on days 7 and 14. In all mice genotypes, the implanted B16 F10 melanoma cells formed solid round tumours with well-defined margins on the lateral flank (Fig. 1a). On day 7, l-selectin/ICAM-1–/– mice exhibited a significant 2·3-fold increase in tumour volume compared to wild-type mice (P < 0·05). There were no other significant differences in tumour size among the groups of mice examined at this time-point. By contrast, after 14 days, the tumour volume in ICAM-1–/– and l-selectin–/– mice was 2·6-fold (P < 0·05) and 3·9-fold (P < 0·001) greater than that in wild-type mice, respectively (Fig. 1b). l-selectin/ICAM-1–/– mice exhibited an even more striking difference. Specifically, tumours from l-selectin/ICAM-1–/– mice were 5·8-fold larger than tumours from wild-type mice (P < 0·0001) and 2·3-fold larger than those from ICAM-1–/– mice (P < 0·005). Tumours from l-selectin/ICAM-1–/– mice also tended to be larger than those from l-selectin–/– mice; however, this difference did not reach statistical significance. In addition to the increased size of the primary tumour, metastatic colonies in the lung were found in one ICAM-1–/– and one l-selectin/ICAM-1–/– mouse at the 14-day time-point. Thus, primary tumour growth was augmented by the loss of l-selectin and/or ICAM-1 expression.
Fig. 1.
Subcutaneous primary growth of B16 melanoma in l-selectin (l-sel)–/–, intercellular adhesion molecule-1 (ICAM-1)–/–, l-sel/ICAM-1–/– and wild-type mice. B16 F10 melanoma cells (1·5 × 106) were injected subcutaneously. Representative subcutaneous tumour nodules from day 14 post-injection in adhesion molecule-deficient and wild-type mice were examined (a). On days 7 and 14, the size of primary tumours was determined (b). All values represent the mean ± s.e.m. These results were obtained from 10 mice in each group.
Enhanced lung metastasis was enhanced in l-selectin–/– and l-selectin/ICAM-1–/– mice
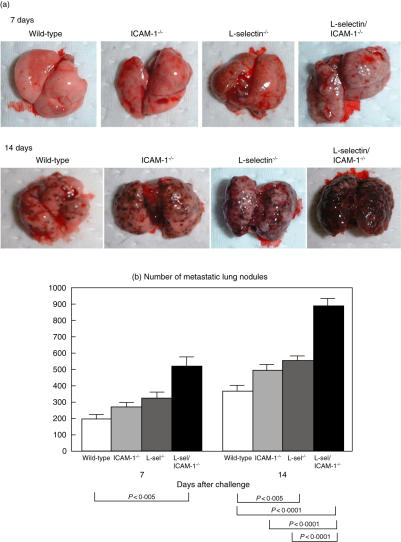
To assess the effects of adhesion molecule loss on the establishment of pulmonary metastasis, B16 F10 melanoma cells were injected via the tail vein into wild-type and adhesion molecule-deficient mice. The cells formed metastatic colonies in the lungs of all genotypes of mice. The metastatic tumours were visible as black pigmented spots that were approximately < 1 mm in diameter after 7 days, increasing to 1–2 mm in diameter, and tended to fuse together after 14 days (Fig. 2a). At the 7-day time-point, loss of both l-selectin and ICAM-1 resulted in a significant 2·6-fold increase in the number of metastatic nodules relative to that of wild-type mice (P < 0·005; Fig. 2b). By contrast, loss of either l-selectin or ICAM-1 alone had no effect on the number of metastatic lung nodules. After 14 days, l-selectin–/– mice exhibited a 50% increase in lung metastasis, which was enhanced compared to wild-type mice (P < 0·005). By contrast, pulmonary metastasis did not differ significantly between ICAM-1–/– and wild-type mice. The l-selectin/ICAM-1–/– mice maintained a 2·4-fold increase in numbers of lung nodules compared to wild-type mice (P < 0·0001). In addition, numbers of lung nodules in l-selectin/ICAM-1–/– mice were increased significantly relative to ICAM-1–/– (P < 0·0001) and l-selectin–/– (P < 0·0001) mice. Expression of l-selectin, ICAM-1 or LFA-1 was not detected on B16 F10 melanoma cells by flow cytometric analysis (data not shown). Furthermore, a previous study has shown that sialyl Lewis X, an epitope expressed on a ligand for l-selectin, is not expressed on B16 F10 melanoma cells either [11]. These findings excluded the possibility that B16 F10 melanoma cells expressed l-selectin or ICAM-1 that may mediate their migration through the endothelium and thereby promote metastasis. Thus, the loss of l-selectin resulted in augmented pulmonary metastasis that was increased further by loss of ICAM-1.
Fig. 2.
Pulmonary metastasis of B16 melanoma in l-selectin (l-sel)–/–, intercellular adhesion molecule-1 (ICAM-1)–/–, l-sel/ICAM-1–/– and wild-type mice. B16 F10 melanoma cells (1·5 × 106) were injected intravenously into the tail vein and after 7 and 14 days, the lungs were removed. Representative metastatic nodules in adhesion molecule-deficient and wild-type mice are shown (a). To evaluate the pulmonary metastasis, the colony number was counted histologically in each section of the three lobes of the right lung after 7 and 14 days (b). All values represent the mean ± s.e.m. These results were obtained from 10 mice in each group.
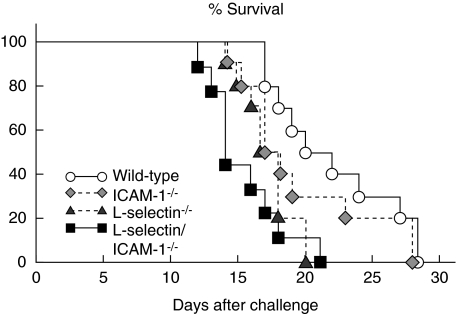
Loss of either l-selectin or l-selectin and ICAM-1 expression reduced survival
To evaluate the effect of losing adhesion molecules on survival, B16 F10 melanoma cells were injected via the tail vein into wild-type and adhesion molecule-deficient mice and deaths were recorded over a 28-day period. Survival was reduced significantly in l-selectin–/– (P < 0·01) and l-selectin/ICAM-1–/– (P < 0·005) mice relative to wild-type mice, but not affected significantly in ICAM-1–/– mice (Fig. 3). l-selectin/ICAM-1–/– mice exhibited a significant decrease in survival compared to ICAM-1–/– mice (P < 0·05), but not l-selectin–/– mice. After 28 days, or at the time of death, none of the wild-type or ICAM-1–/– mice showed any metastasis to organs other than the lung, while one l-selectin–/– mouse displayed metastasis to the heart. By contrast, in l-selectin/ICAM-1–/– mice, the tumour metastasized to the heart in four mice, to the heart and liver in one mouse, to the kidney in two mice, to the liver and kidney in one mouse and to the kidney and intestine in one mouse. These results support the findings that rapid metastasis of melanoma occurs in the absence of either l-selectin or l-selectin and ICAM-1 expression.
Fig. 3.
Effect of loss of l-selectin, intercellular adhesion molecule-1 (ICAM-1) or both on survival after intravenous injection of B16 melanoma cells. Mice were injected intravenously with B16 F10 cells (1·5 × 106) and observed for a period of 28 days. These results were obtained from 10 mice in each group.
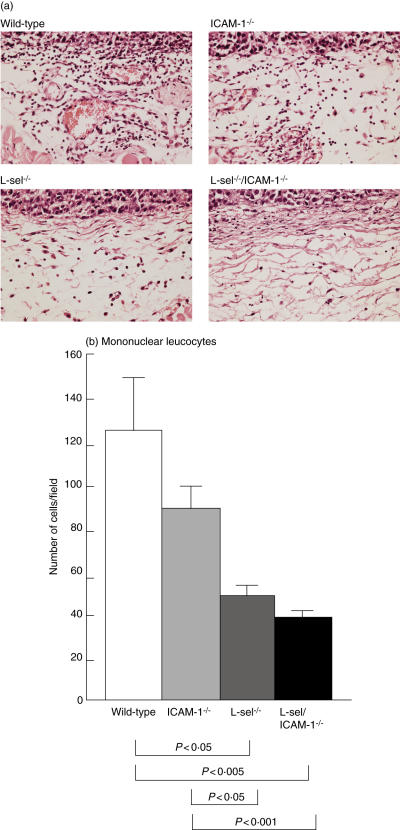
Leucocyte tumour infiltration was reduced with loss of l-selectin and/or ICAM-1 expression
The subcutaneous primary tumours were examined histologically to assess the effects of loss of adhesion molecules on leucocytes infiltration into the tumour. At day 14, neutrophil infiltration into the tumour was modest and there were no differences in numbers of infiltrating neutrophils between genotypes of mice (Fig. 4a and data not shown). By contrast, the numbers of infiltrating mononuclear leucocytes were reduced significantly by 60–70% in l-selectin–/– (P < 0·05) and l-selectin/ICAM-1–/– (P < 0·005) mice compared with wild-type mice. Numbers of infiltrating mononuclear cells also tended to be decreased in ICAM-1–/– mice by 29% relative to wild-type mice (P = 0·09; Fig. 4a,b).
Fig. 4.
The accumulation of leucocytes around the subcutaneous melanoma of l-selectin (l-sel)–/–, intercellular adhesion molecule-1 (ICAM-1)–/–, l-sel/ICAM-1–/– and wild-type mice at 14 days after the injection of B16 cells. Representative light micrographs of skin leucocyte infiltration in adhesion molecule-deficient and wild-type mice was shown. (a) Haematoxylin and eosin stain, magnification × 100. Mononuclear cells were counted under a microscopic high power view [0·07 mm2 (b)]. All values represent the mean ± s.e.m. These results were obtained from 10 mice in each group.
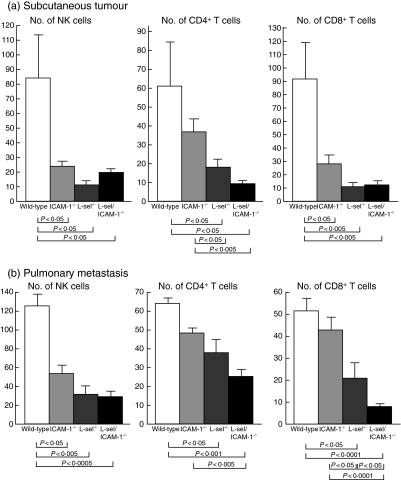
To evaluate which leucocyte subsets were affected by the loss of specific adhesion molecules, immunohistochemical analysis of both the subcutaneous primary tumours and metastatic lung tumours was performed at 14 days after the injection. Numbers of infiltrating F4/80+ macrophages were small and not affected by the loss of adhesion molecules in either the skin or lung tumour (data not shown). In the primary tumour, numbers of NK cells, CD4+ T cells and CD8+ T cells were all decreased significantly by 70–90% in ICAM-1–/–, l-selectin–/– and l-selectin/ICAM-1–/– mice relative to wild-type mice (P < 0·05), with the exception of CD4+ T cell numbers in ICAM-1–/– mice (Fig. 5a). In addition, l-selectin–/– and l-selectin/ICAM-1–/– mice displayed significantly reduced numbers of CD4+ T cells compared to ICAM-1–/– mice (P < 0·05 and P < 0·005, respectively).
Fig. 5.
Accumulation of natural killer (NK) cells, CD4+ T cells and CD8+ T cells around the subcutaneous tumour (a) or pulmonary metastatic tumour (b) of l-selectin (l-sel)–/–, intercellular adhesion molecule-1 (ICAM-1)–/–, l-sel/ICAM-1–/– and wild-type mice 14 days after the subcutaneous or intravenous injection of B16 cells. NK cells, CD4+ T cells and CD8+ T cells were detected immunohistochemically and the numbers of each were determined in one high-power microscopic field (0·07 mm2) in sections from the skin and lung. All values represent the mean ± s.e.m. These results were obtained from five to 10 mice in each group.
In the metastatic tumour, NK cell number was reduced significantly in ICAM-1–/– mice relative to wild-type mice (P < 0·05), while numbers of CD4+ and CD8+ T cells were not diminished significantly (Fig. 5b). By contrast, l-selectin–/– and l-selectin/ICAM-1–/– mice exhibited significantly decreased numbers of NK cells, CD4+ T cells and CD8+ T cells by 40–80% compared to wild-type mice (P < 0·05). There was no significant difference in numbers of NK and CD4+ T cells between l-selectin–/– and l-selectin/ICAM-1–/– mice, while numbers of CD8+ T cells were reduced significantly by 60% in l-selectin/ICAM-1–/– mice relative to l-selectin–/– mice (P < 0·05). Thus, the augmented growth of the primary melanoma was generally associated with a reduction in lymphocyte infiltration.
Effect of the loss of adhesion molecules on local cytokine mRNA expression
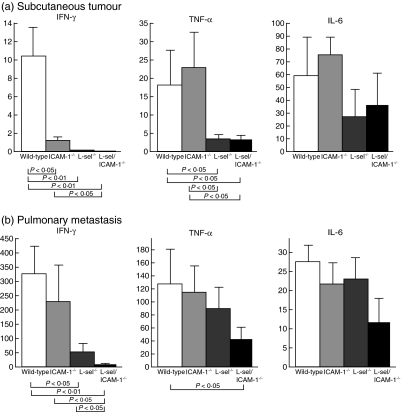
The production and release of proinflammatory cytokines, including TNF-α, IFN-γ and IL-6, by infiltrating leucocytes play a critical role in modulation of the immune response to a tumour [4]. To assess the roles of adhesion molecules in cytokine release during tumour development, TNF-α, IFN-γ and IL-6 mRNA expression levels were measured by real-time PCR in the subcutaneous tumour and pulmonary metastasis models 14 days post-injection in wild-type and adhesion molecule-deficient mice. In subcutaneous tumours, IFN-γ mRNA levels were decreased by > 90% in ICAM-1–/– (P < 0·05), l-selectin–/– (P < 0·01) and l-selectin/ICAM-1–/– (P < 0·01) mice compared with wild-type mice (Fig. 6a). TNF-α mRNA levels were decreased by ∼85% in l-selectin–/– and l-selectin/ICAM-1–/– mice relative to wild-type mice (P < 0·05). By contrast, ICAM-1–/– mice exhibited normal levels of TNF-α mRNA. IL-6 mRNA levels were not significantly different between genotypes of mice.
Fig. 6.
Cytokine mRNA expression in the subcutaneous tumour (a) or pulmonary metastatic tumour (b) of l-selectin (l-sel)–/–, intercellular adhesion molecule-1 (ICAM-1)–/–, l-sel/ICAM-1–/– and wild-type mice at 14 days after the subcutaneous or intravenous injection. The amount of tumour necrosis factor (TNF-α), interferon (IFN)-γ and interleukin (IL)-6 mRNA was measured by real-time polymerase chain reaction (PCR) and normalized to the GAPDH endogenous mRNA control. The TNF-α, IFN-γ and IL-6 mRNA levels in wild-type mice were used as calibrators. All values represent the mean ± s.e.m. of results obtained from five to eight mice in each group.
A similar decrease in IFN-γ and TNF-α mRNA levels was detected in lung tumours (Fig. 6b). However, unlike the primary tumour, loss of ICAM-1 expression did not affect IFN-γ mRNA levels significantly. Similar to the primary tumour, l-selectin–/– mice exhibited significantly reduced IFN-γ mRNA levels compared to wild-type mice (P < 0·05). However, the additional loss of ICAM-1 expression in l-selectin–/– mice resulted in a further decline in IFN-γ mRNA levels (P < 0·05). Moreover, the difference in TNF-α mRNA levels in the lung between groups of mice was smaller than that in the skin, with the only statistically significant difference being found between wild-type and l-selectin/ICAM-1–/– mice (P < 0·05). In sera of mice bearing lung metastatic nodules, TNF-α, IFN-γ and IL-6 were not detected by cytokine-specific enzyme-linked immunosorbent assay (ELISA) (data not shown). Thus, the enhanced tumour progression and metastasis observed with loss of adhesion molecule expression were generally associated with the reduced release of IFN-γ and TNF-α in the tumour microenvironment.
Cytotoxic response to melanoma was not defective in the absence of l-selectin and/or ICAM-1
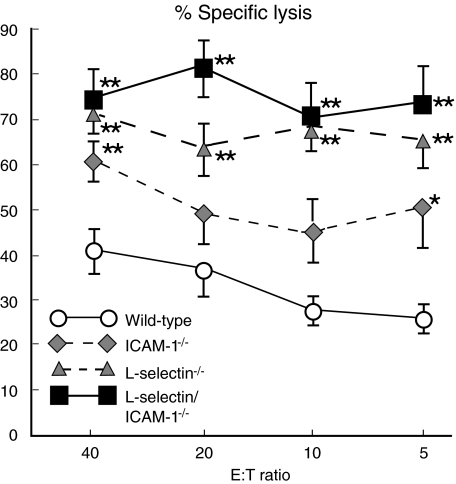
ICAM-1 mediates not only the recruitment of leucocytes to inflammatory sites, but also functions as a co-stimulatory molecule on the surface of antigen-presenting cells [22]. In addition, because l-selectin regulates lymphocyte migration into lymph nodes across high endothelial venules [16], l-selectin function may also be required during the antigen sensitization phase of immune responses [23]. Therefore, the ability of adhesion molecule-deficient mice to generate specific cytotoxic responses against B16 melanoma cells in vitro was assessed. Splenocytes and NK cell-depleted tumour-draining lymph node cells were isolated 14 days after the subcutaneous injection of B16 F10 melanoma cells and were tested for lytic activity against the melanoma cells. NK cell-depleted tumour-draining lymph node cells from l-selectin/ICAM-1–/–, l-selectin–/–, ICAM-1–/– and wild-type mice induced similar levels (mean ± s.e.m.) of lysis (52 ± 7, 47 ± 6, 56 ± 4 and 48 ± 5% at the E : T ratio of 5 : 1, respectively; n = 5). Regarding lysis by splenocytes, l-selectin/ICAM-1–/– and l-selectin–/– mice exhibited significantly more specific killing of melanoma cells than wild-type mice at all E : T ratios (P < 0·01; Fig. 7). Killing activity was generally greater in ICAM-1–/– mice than in wild-type mice, with significant differences being found at E : T ratios of 40 : 1 (P < 0·01) and 5 : 1 (P < 0·05). Thus, the cytotoxic response to melanoma was not defective in adhesion molecule-deficient mice.
Fig. 7.
Effect of loss of intercellular adhesion molecule-1 (ICAM-1), l-selectin or both on the cytotoxic response to B16 melanoma. B16 F10 cells (1·5 × 106) were injected intravenously into the tail vein of mice and after 14 days splenocytes were collected. B16 F10 melanoma cells were used as target cells and stained with DiOC18. The stained melanoma cells were mixed with splenocytes at effector : target (E : T) ratios of 5 : 1, 10 : 1, 20 : 1 and 40 : 1. Propidium iodide was then added and the mixture was incubated at 37°C for 2 h. Two-colour flow cytometric analysis was performed to assess lytic activity. Percentage of lysis was calculated by dividing the frequency of dead propidium iodide-positive target cells by the total target cells stained with DiOC18. All values represent the mean ± s.e.m. of results obtained from five to eight mice in each group.
Discussion
The current study was conducted to define the role of l-selectin and ICAM-1 in primary tumour progression and pulmonary metastasis by recruiting leucocytes to B16 melanoma. This is the first report showing a critical in vivo role for both l-selectin and ICAM-1 in the progression and metastasis of B16 melanoma, using gene-targeted adhesion molecule-deficient mice. In the present study, the primary cutaneous growth of B16 melanoma was augmented by the loss of l-selectin, ICAM-1 or both (Fig. 1), whereas metastasis to the lung was enhanced by the loss of l-selectin or a combined loss of l-selectin and ICAM-1 (Fig. 2). This enhancement was associated generally with a reduced accumulation of NK cells, CD4+ T cells and CD8+ T cells (Fig. 5) and also with a diminished release of IFN-γ and TNF-α (Fig. 6). Therefore, although it remains controversial whether inflammation contributes to the anti-tumour response or promotes tumour progression [4], our results indicate that inflammation and leucocyte recruitment mediated by cell adhesion molecules are involved in anti-tumour immune responses. Consistent with this, the injection of B16 melanoma cells transfected with macrophage inflammatory protein-1α, a chemokine for T cells and immature dendritic cells, resulted in reduced pulmonary metastasis by inducing local inflammation [13]. Furthermore, B16 melanoma-derived factors reduce TNF-α-induced ICAM-1 expression on endothelial cells, leading to diminished leucocyte adhesion that resulted in escape from the immune system [24]. Thus, the present study demonstrates directly the important role for l-selectin and ICAM-1 in anti-tumour immune responses.
A central role for ICAM-1 in the initiation and generation of immune responses raises the issue of whether the current findings with ICAM-1–/– mice result from a lack of sensitization or appropriate generation of cytotoxic T cells [14]. Similarly, the requirement for lymphocytes to express l-selectin to enter lymph nodes across high endothelial venules [16] suggests that l-selectin–/– mice may exhibit impaired sensitization [23]. However, our results demonstrate that the cytotoxic activity against B16 melanoma was augmented in spleens from mice lacking ICAM-1, l-selectin or both (Fig. 7). On the other hand, cytotoxic activity was similar between mutant and wild-type mice in tumour-draining lymph nodes. Consistent with this, a previous study has shown that loss of l-selectin, ICAM-1 or both does not affect the proliferative response of draining lymph node cells to antigen [25]. This suggests that enhanced tumour growth and metastasis with the loss of adhesion molecules results from the impaired migration of effector cells into the tissue rather than the suppression of a cytotoxic response.
In this study, loss of ICAM-1 resulted in enhanced growth of the primary melanoma, but did not affect the pulmonary metastasis significantly (Figs 1 and 2). For growth of primary subcutaneous, l-selectin and ICAM-1 functioned in an independent manner, reflecting the additive effects from blocking a l-selectin-dependent first step and a subsequent independent, non-overlapping ICAM-1-dependent second step (Fig. 1b). By contrast, for the metastasis, the effects from loss of both l-selectin and ICAM-1 were more than would be expected if these molecules functioned independently, suggesting that they functioned synergistically (Fig. 2b). This is consistent with the findings that l-selectin and ICAM-1 function co-operatively to mediate optimal leucocyte rolling as well as to recruit leucocytes to inflammatory sites [26]. Thus, the relative contribution of each adhesion molecule to the inflammatory process is complex and varies according to the site of inflammation and the nature of the inflammatory stimuli. Indeed, ICAM-1–/– mice exhibit delayed skin wound repair and impaired skin contact hypersensitivity reactions, although allogeneic skin graft rejection is normal [14,27–29]. The contribution of ICAM-1 to lung inflammation is also complex. Specifically, radiation-induced, endotoxin-induced and antigen-dependent allergic pulmonary inflammation and bleomycin-induced lung fibrosis are attenuated in ICAM-1–/– mice [30–34], while loss of ICAM-1 increases mortality in Escherichia coli or Klebsilella pneumonia [35,36]. Regarding the interaction of l-selectin and ICAM-1, both molecules function synergistically in skin wound healing [29], while they function in an additive manner in cutaneous immune complex-mediated vasculitis and bleomycin-induced lung fibrosis [34,37]. Thus, the relative contribution of l-selectin and ICAM-1 to anti-tumour immune responses appears to vary according to the site of tumour growth.
Overall, the enhancement of both primary growth and metastasis to the lung was associated with a reduced local recruitment of NK cells, CD4+ T cells and CD8+ T cells (Fig. 5). However, these reductions cannot explain the difference in primary tumour growth between genotypes of mice, as there was no difference in the numbers of NK cells or CD8+ T cells between the groups (Fig. 5a). Therefore, in an immune response to the primary skin tumour growth, CD4+ T cells may play a more important role than NK cells and CD8+ T cells. Similarly, CD8+ T cells appeared to contribute to the immune response to pulmonary metastasis more substantially than NK cells and CD4+ T cells (Fig. 5b). Thus, these results suggest that the relative contribution of lymphocyte subsets to anti-tumour responses varies according to the site of the tumour. It has been recognized generally that NK cells and CD8+ cytotoxic T cells play a crucial role in anti-tumour immunity [38,39]. However, many studies have shown that CD4+ T cells are needed in the effector phase of the anti-tumour immune responses [40,41]. Specifically, mice lacking CD4+ T cells are unable to reject a tumour challenge [40,41]. Furthermore, activated CD4+ T cells are a dominant feature in regressing primary melanoma [42]. Thus, our results indicate that NK cells, CD4+ T cells and CD8+ T cells are involved co-operatively in anti-melanoma immune responses.
In this study, decreased levels of IFN-γ and TNF-α but not IL-6 were related to the enhancement of primary tumour growth and pulmonary metastasis; however, IFN-γ appeared to play the more important role (Fig. 6). Many studies have shown that IFN-γ inhibits tumour cell growth directly. For example, IFN-γ induced cell cycle arrest and/or TNF receptor family-related apoptosis by enhancing CD95/FasL death mechanisms in B16 melanoma [43]. By contrast, the effect of TNF-α on tumour progression is complex [4]. Like IFN-γ, TNF-α can bind to the death receptor and induce apoptosis through adaptor molecules [44]. However, recent studies have shown that when expressed inappropriately, TNF-α promotes the progression of melanomas; anti-TNF-α antibody reduces metastasis to the lung [45] and TNF-α stimulates the migration and invasion of B16 melanoma [46]. Although the effect of IFN-γ and TNF-α could not be assessed separately in the current study, our finding that tumour growth and metastasis were associated with reduced TNF-α expression suggests that TNF-α contributes to anti-tumour immune responses rather than to the promotion of tumour progression.
Acknowledgments
We thank Ms M. Matsubara and Y. Yamada for technical assistance. This work was supported by a Grant-in-Aid from the Ministry of Education, Science and Culture of Japan (to S. Sato) and National Institutes of Health, USA grants CA54464 and CA81776 (to T. F. Tedder).
References
- 1.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 2.Arca MJ, Mule JJ, Chang AE. Genetic approaches to adoptive cellular therapy of malignancy. Semin Oncol. 1996;23:108–17. [PubMed] [Google Scholar]
- 3.Chang AE, Shu S. Current status of adoptive immunotherapy of cancer. Crit Rev Oncol Hematol. 1996;22:213–28. doi: 10.1016/1040-8428(96)00194-1. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 6.Benamouzig R, Yoon H, Little J, et al. APACC, a French prospective study on aspirin efficacy in reducing colorectal adenoma recurrence: design and baseline findings. Eur J Cancer Prev. 2001;10:327–35. doi: 10.1097/00008469-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 9.Tedder TF, Li X, Steeber DA. The selectins and their ligands: adhesion molecules of the vasculature. Adv Mol Cell Biol. 1999;28:65–111. [Google Scholar]
- 10.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–53. [PubMed] [Google Scholar]
- 11.Ohyama C, Tsuboi S, Fukuda M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999;18:1516–25. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marvin MR, Southall JC, Trokhan S, DeRosa C, Chabot J. Liver metastases are enhanced in homozygous deletionally mutant ICAM-1 or LFA-1 mice. J Surg Res. 1998;80:143–8. doi: 10.1006/jsre.1998.5322. [DOI] [PubMed] [Google Scholar]
- 13.van Deventer HW, Serody JS, McKinnon KP, Clements C, Brickey WJ, Ting JP. Transfection of macrophage inflammatory protein 1α into B16, F10 melanoma cells inhibits growth of pulmonary metastases but not subcutaneous tumors. J Immunol. 2002;169:1634–9. doi: 10.4049/jimmunol.169.3.1634. [DOI] [PubMed] [Google Scholar]
- 14.Sligh JE, Ballantyne CM, Jr, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1996;154:6080–93. [PubMed] [Google Scholar]
- 16.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in 1-selectin (CD62L) deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 17.Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci USA. 1998;95:7562–7. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagamu H, Touhalisky JE, Plautz GE, Krauss JC, Shu S. Isolation based on 1-selectin expression of immune effector T cells derived from tumor-draining lymph nodes. Cancer Res. 1996;56:4338–42. [PubMed] [Google Scholar]
- 20.Dohi Y, Sunada S, Aoki M, et al. Eradication of metastatic tumour cells from lymph nodes by local administration of anti-CD3 antibody. Cancer Immunol Immunother. 1993;36:357–63. doi: 10.1007/BF01742251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumura T, Krinock RA, Chang AE, Shu S. Cross-reactivity of anti-CD3/IL-2 activated effector cells derived from lymph nodes draining heterologous clones of a murine tumor. Cancer Res. 1993;53:4315–21. [PubMed] [Google Scholar]
- 22.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–86. [PubMed] [Google Scholar]
- 23.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in 1-selectin-deficient mice. J Exp Med. 1996;183:589–98. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirkx AE, Oude Egbrink MG, Kuijpers MJ, et al. Tumor angiogenesis modulates leukocyte–vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–1329. [PubMed] [Google Scholar]
- 25.Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the 1-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 26.Steeber DA, Tang MLK, Green NE, Zhang X-Q, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interaction between the 1-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 27.Tang MLK, Hale LP, Steeber DA, Tedder TF. 1-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in 1-selectin-deficient mice. J Immunol. 1997;158:5191–9. [PubMed] [Google Scholar]
- 28.Xu H, Gonzalo JA, St Pierre Y, et al. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagaoka T, Kaburagi Y, Hamaguchi Y, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or 1-selectin expression. Am J Pathol. 2000;157:237–47. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamochi M, Kamochi F, Kim YB, et al. P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am J Physiol. 1999;277:L310–9. doi: 10.1152/ajplung.1999.277.2.L310. [DOI] [PubMed] [Google Scholar]
- 31.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci USA. 1997;94:6432–7. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatfield CA, Brashler JR, Winterrowd GE, et al. Intercellular adhesion molecule-1-deficient mice have antibody responses but impaired leukocyte recruitment. Am J Physiol. 1997;273:L513–23. doi: 10.1152/ajplung.1997.273.3.L513. [DOI] [PubMed] [Google Scholar]
- 33.Keramidaris E, Merson TD, Steeber DA, Tedder TF, Tang ML. 1-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol. 2001;107:734–8. doi: 10.1067/mai.2001.114050. [DOI] [PubMed] [Google Scholar]
- 34.Hamaguchi Y, Nishizawa Y, Yasui M, et al. Intercellular adhesion molecule-1 and 1-selectin regulate bleomycin-induced lung fibrosis. Am J Pathol. 2002;161:1607–18. doi: 10.1016/S0002-9440(10)64439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien AD, Standiford TJ, Bucknell KA, Wilcoxen SE, Paine R., III Role of alveolar epithelial cell intercellular adhesion molecule-1 in host defense against Klebsiella pneumoniae. Am J Physiol. 1999;276:L961–70. doi: 10.1152/ajplung.1999.276.6.L961. [DOI] [PubMed] [Google Scholar]
- 36.Zeni F, Parent C, Correa R, et al. ICAM-1 and CD11b inhibition worsen outcome in rats with E. coli pneumonia. J Appl Physiol. 1999;87:299–307. doi: 10.1152/jappl.1999.87.1.299. [DOI] [PubMed] [Google Scholar]
- 37.Kaburagi Y, Hasegawa M, Nagaoka T, et al. The cutaneous reverse Arthus reaction requires intercellular adhesion molecule 1 and 1-selectin expression. J Immunol. 2002;168:2970–8. doi: 10.4049/jimmunol.168.6.2970. [DOI] [PubMed] [Google Scholar]
- 38.Bohm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-γ-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]
- 39.Ito D, Back TC, Shakhov AN, Wiltrout RH, Nedospasov SA. Mice with a targeted mutation in lymphotoxin-α exhibit enhanced tumor growth and metastasis: impaired NK cell development and recruitment. J Immunol. 1999;163:2809–15. [PubMed] [Google Scholar]
- 40.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97:197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 43.Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE. Growth control of melanoma cells and melanocytes by cytokines. Recent results. Cancer Res. 1995;139:169–82. [Google Scholar]
- 44.Nishida S, Yoshioka S, Kinoshita-Kimoto S, et al. Pretreatment with PKC inhibitor triggers TNF-α induced apoptosis in TNF-α-resistant B16 melanoma BL6 cells. Life Sci. 2003;74:781–92. doi: 10.1016/j.lfs.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Waterston AM, Salway F, Andreakos E, Butler DM, Feldmann M, Coombes RC. TNF autovaccination induces self anti-TNF antibodies and inhibits metastasis in a murine melanoma model. Br J Cancer. 2004;90:1279–84. doi: 10.1038/sj.bjc.6601670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu N, Lalla R, Eves P, et al. Melanoma cell migration is upregulated by tumour necrosis factor-α and suppressed by α-melanocyte-stimulating hormone. Br J Cancer. 2004;90:1457–63. doi: 10.1038/sj.bjc.6601698. [DOI] [PMC free article] [PubMed] [Google Scholar]