Abstract
HIV infection activates abnormally the immune system and the chronic phase is accompanied by marked alterations in the CD8 compartment. The expression of CD127 (IL-7R alpha chain) by memory CD8 T lymphocytes in HIV-infected patients is analysed and reported. The memory CD8 T cell subset was characterized by expression of CD45RA and CD27 markers, and CD127 cell surface expression was measured ex vivo by four-colour flow cytometry. HIV infection was associated with a fall in the proportion of CD127+ cells among memory CD8 lymphocytes that resulted in a higher CD127− CD45RA−CD27+ CD8 T cell count in HIV-infected patients. Diminished CD127 cell surface expression [mean fluorescence intensity (MFI)] by positive cells was also observed in this subset. The data suggest that these defects were reversed by highly active anti-retroviral therapy (HAART). The regulation of CD127 expression was also studied in vitro. Down-regulation of CD127 by interkeukin (IL)-7 was observed in memory CD8 lymphocytes from healthy donors and HAART patients. Expression of CD127 by memory CD8 lymphocytes cultured in the absence of IL-7 confirmed that IL-7R regulation is altered in viraemic patients. Under the same experimental conditions, memory CD8 lymphocytes from HAART patients were shown to express CD127 at levels comparable to cells from healthy individuals. Altered CD127 cell surface expression and defective CD127 regulation in the memory CD8 T lymphocytes of HIV-infected patients are potential mechanisms by which these cells may be impeded in their physiological response to endogenous IL-7 stimulatory signals. Our data suggest that these defects are reversed during the immune reconstitution that follows HAART.
Keywords: CD8 T cells, CD127, HAART, IL-7, memory subset, receptor, viraemic patients
Introduction
Chronic activation leads to numerous abnormalities in the immune system of HIV-infected patients [1–3]. The deleterious effects of the infection extend to the overall CD8 T cell population. Trimble et al. showed that circulating CD8 lymphocytes in HIV-infected patients have impaired function and down-regulate CD3zeta, the signalling chain of the T cell receptor (TCR) [4,5]. We have characterized regulatory dysfunctions in the interleukin (IL)-2/IL-2R system and in the Jak/signal transducer and activation of transcription (STAT) signalling pathway of CD8 lymphocytes from HIV-infected patients [6,7]. Describing the abnormalities that characterize the whole CD8 compartment in HIV-infected patients is still a matter of considerable investigation.
CD8-mediated protective immunity is mediated by effector T cells that perform an immediate effector function, whereas reactive memory is mediated by memory T cells that have little or no effector function, but proliferate readily and differentiate to effector cells in response to antigenic stimulation. Different differentiation pathways have been put forward for human CD8 T cells in their conversion from naive to memory and effector cells. Naive CD8 T cells (CD45RA+/RO− CD28+ CD27+ CCR7+) respond to antigenic stimulation. Memory cells (CD45RA−/RO+ CD28+ CD27+ CCR7+) exhibit self-renewal properties. Expression of CCR7 and CD62L can be used to dissect this subset further. Effector cells (CD45RA−/RO+ CD28− CD27− CCR7−) show a weak proliferative capacity but direct cytolytic functions. Terminally differentiated effector cells (CD45RA+/RO+ CD28−CD27− CCR7−) differ from effector cells by their re-expression of CD45RA [8,9].
The role played by the IL-7/IL-7R system in the pathogenesis of HIV infection is still under investigation. Under physiological conditions IL-7 plays a pivotal role by stimulating thymopoiesis and controlling the homeostasis of peripheral T lymphocytes, including naive and memory CD8 T cells [10–15]. During HIV infection, IL-7 is over-produced in response to CD4 lymphopenia [16]. Our data also suggest that IL-7 is involved in the immune reconstitution that follows highly active anti-retroviral therapy (HAART) [17,18]. On the other hand, CD8 lymphocytes from HIV-infected patients have been reported to show reduced expression of IL-7R alpha chain (CD127) [19–22] and recent studies have established that HIV-specific effector lymphocytes have reduced levels of CD127 [23,24].
In this paper we focus our analysis on CD127 expression by whole memory CD8 lymphocytes taken from HIV-infected, therapy-naive patients and HIV-infected patients treated with HAART. We also analyse the regulation of CD127 expression by memory CD8 T lymphocytes from healthy donors and HIV-infected patients. Our data indicate that memory CD8 lymphocytes underexpress CD127 strongly and point to a CD127 regulatory dysfunction in viraemic patients. We also suggest that HAART reverses these defects. Maintaining an active memory T cell compartment is critical in chronic infections, and our results highlight a new defect that may participate in the long-term failure of CD8 responses in HIV-infected patients.
Patients and methods
Patients
Two groups (n = 10) of chronically HIV-1 infected patients were included in the study (data are presented as medians and quartiles). Viraemic patients with a plasma viral load > 10 000 RNA copies/ml, a CD4 count of 526 (486–597) cells/mm [3] and a CD8 count of 866 (457–1764) cells/mm [3] were studied 23 (12–31) months after infection. Patients receiving HAART for at least 1 year [67 (38–72) months), and showing plasma viral loads < 50 copies/ml over the previous 6 months, a CD4 count of 526 (487–597) and a CD8 count of 1024 (769–1728) were also included in the study. These patients had been infected for 106 (55–127) months. HAART includes at least two reverse transcriptase inhibitors and one protease inhibitor. Healthy donors (n = 10) were also included as controls. Written informed consent was obtained from all patients.
CD127 expression in memory and CD8 lymphocyte subsets
CD8 lymphocyte subsets were analysed for their cell surface expression of CD45RA and CD27. CD127 cell surface expression was measured ex vivo immediately after peripheral blood mononuclear cells (PBMC) preparation or in vitro after 3 and 6 days of culture in the absence or in the presence of IL-7, as described in Figs 1 and 2.
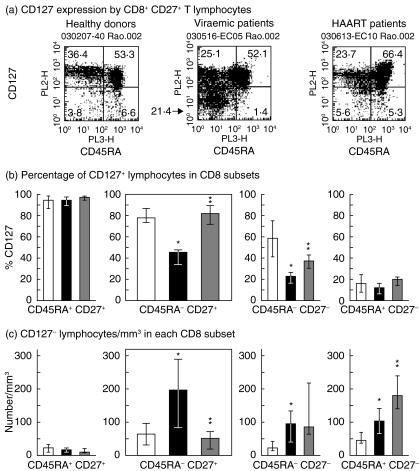
Fig. 1.
Ex vivo expression of CD127 by memory CD8 lymphocyte taken from viraemic and highly active anti-retroviral therapy (HAART)-treated HIV-infected patients. Peripheral blood mononucear cells (PBMC) were incubated with antibody mixtures containing anti-CD8-APC (clone DK25, IgG1k, Dako Cytomation A/S, Glostrup, Denmark), anti-CD27-FITC (clone M-T27, IgG1k, Dako Cytomation S/A), anti-CD45RA Cy5 (clone HI 100, IgG2b, Pharmingen, San Diego, CA, USA) and anti-CD127-RPE (R34·34, IgG1, Immunotech, Marseille, France). The binding of anti-CD127 monoclonal antibodies (mAb) R34·34 to interleukin (IL)-7R alpha was not exposed to competition from IL-7 under our experimental conditions. After 30 min at 4°C, the cells were washed and fixed in phosphate-buffered saline (PBS) paraformaldehyde (1%). An isotype control was performed for each reagent. Staining analyses were performed on a fluorescence-activated cell sorter (FACSCalibur) flow cytometer running cellquest version 3·1 software (Becton Dickinson, Mountain View, CA, USA). Data were expressed as medians and interquartile ranges. Statistical analyses were performed by the Mann–Whitney U-test. The main comparisons were performed between healthy donors and viraemic patients, and between viraemic and HAART patients. (a) CD127 expression by CD8+ CD27+ lymphocytes. Naive cells (CD45RA+) and memory cells (CD45RA−). Results are shown for one representative individual in each group. CD45RA labelling delineated naive cells (CD45RA+) from memory CD8 lymphocytes (CD45RA−). (b) CD45RA and CD27 were used to delineate naive (CD45RA+ CD27+), memory (CD45RA− CD27+), effector (CD45RA− CD27−) and terminally differentiated effector lymphocytes (CD45RA+ CD27−). Proportion of CD127+ lymphocytes [mean fluorescence intensity (MFI) > 50] in the four CD8 lymphocyte subsets in healthy individuals, viraemic patients and HAART patients; n = 10 for each group. (c) Absolute counts per cubic millimetre for total CD127− lymphocytes (MFI < 50) in naive, memory, effector and terminally differentiated CD8 lymphocytes from each study group. □, Healthy donors; ▪ viraemic patients;  , HAART patients. *P < 0·05 when compared to healthy donors; **P < 0·05 when compared to untreated viraemic patients.
, HAART patients. *P < 0·05 when compared to healthy donors; **P < 0·05 when compared to untreated viraemic patients.
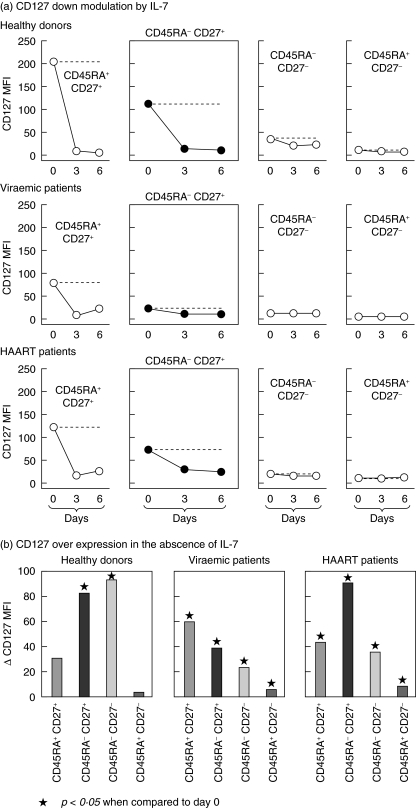
Fig. 2.
Regulation of CD127 expression in memory CD8 lymphocytes taken from viraemic and highly active anti-retroviral therapy (HAART)-treated HIV-infected patients. Peripheral blood mononucear cells (PBMC), prepared as above, were cultured in the absence or presence of interleukin (IL)-7 (10 ng/ml). IL-7 was kindly provided by Cytheris SA (Vanves, France). Cultures (2 × 105 cells/200 microlitre) were performed in 96-well U-bottomed microtitre plates. The medium consisted of RPMI-1640 supplemented with 0·5% human AB serum, 2 mM glutamine, 10 mM HEPES and 50 mM 2-mercaptoethanol. On the days indicated the cells were stained and analysed as described in the legend to Fig. 1. Data were expressed as median [mean fluorescence intensity (MFI)] of all cells in each CD8 lymphocyte subset. For the sake of clarity the figure does not include the interquartile ranges. Statistical analyses were performed by the Wilcoxon matched-pairs signed-ranks test. (a) CD127 down-regulation in the presence of IL-7 was monitored on day 3 and on day 6 was found to be significant when compared to CD127 expression on day 0, and this for all experimental conditions used (P< 0·05). Dotted lines represent the level of CD127 expression on day 0. (b) CD127 over-expression was measured on day 3 in the absence of IL-7. The P-values obtained between day 3 and day 0 are indicated. IL-7 plasma levels were measured in each group by enzyme-linked immunoassay (Quantikine HS kit, RD System, Paris, France): viraemic group [16·2 (13·6–17·2) pg/ml], HAART group [11·0 (8.9–12.8) pg/ml] and healthy donors [1·4 (0·9–2·1) pg/ml]. *P < 0·05 when compared to day 0.
Statistical analysis
Continuous variables were compared between paired groups by the Mann–Whitney U-test. When markers were compared in the same patient, but at two different time-points, the Wilcoxon matched-pairs signed-ranks test was used. P-values < 0·05 were considered significant. All statistical analyses were performed by stata 8 (Stata Corporation, College Station, TX, USA).
Results
Ex vivo expression of CD127 by memory CD8 T lymphocytes from viraemic and HAART-treated HIV-infected patients
Absolute counts for CD45RA− CD27+ memory cells were increased slightly as viraemic patients had more CD8 lymphocytes. HAART restored the memory cell count to levels close to normal.
CD127 expression by memory CD8 lymphocytes was measured in each group and the results obtained for one representative individual in each group are shown (Fig. 1a). The analysis performed on all the individuals in each of the three groups is shown in Fig. 1b. Most memory cells in healthy individuals expressed CD127, but this was reduced significantly in viraemic patients. Furthermore, CD127 expression levels [mean fluorescence intensity (MFI)] were diminished at least threefold in the cells that remained CD127+ 0. HAART reversed these defects (see P-values in Fig. 1b). The proportion of CD127+ cells was then compared between the different CD8 lymphocyte subsets (Fig. 1b). Naive CD8 lymphocytes (CD45RA+ CD27+) showed a high percentage of CD127+ cells both in healthy individuals and viraemic patients. By contrast, the proportion of effector CD8 lymphocytes (CD45RA− CD27−) expressing CD127 was altered significantly in viraemic patients, and HAART only partially reversed this defect. It is noteworthy that CD45RA+ CD27− terminally differentiated CD8 lymphocytes from healthy individuals expressed little CD127 and the consequences of HIV infection could not therefore be measured in this subset.
The absolute CD127− memory lymphocyte count increased dramatically in viraemic patients (Fig. 1c). HAART was able to reverse this defect fully, restoring the CD127− memory CD8 lymphocyte count to levels close to normal. All the differences reported were significant (P < 0·05).
Regulation of CD127 expression in the memory CD8 lymphocytes of viraemic and HAART-treated HIV-infected patients
IL-7-induced down-regulation of CD127 appears to have critical physiological consequences on the regulation of T cell homeostasis [14]. We used this property to determine the function of the IL-7R expressed by memory CD8 lymphocytes. The addition of IL-7 to cultures of PBMC from healthy individuals resulted in pronounced down-regulation of CD127 expression levels (MFI) in the memory CD8 subset (Fig. 2a). This IL-7-mediated effect occurred in memory CD8 lymphocytes from HAART patients, where CD127 expression was found to be partly restored (Fig. 2a). CD127 down-regulation was also observed in naive CD8 lymphocytes from healthy individuals and was preserved in naive CD8 lymphocytes from the two groups of HIV-infected patients.
Surprisingly, memory CD8 lymphocytes from healthy individuals cultured in the absence of IL-7 showed a significant increase in CD127 expression (MFI) (Fig. 2b). This increase peaked on day 3. The phenomenon was less pronounced when the study was performed with PBMC from viraemic patients but remained detectable. When PBMC from HAART patients were cultured in the absence of IL-7, the expression of CD127 by memory CD8 lymphocytes reached levels comparable to healthy individuals. A defect in CD127 expression was also observed in effector CD8 lymphocytes from viraemic patients, but the beneficial effects of HAART were less pronounced in this subset than in the CD8 memory subset. These results indicate that IL-7R function is altered in the memory CD8 lymphocytes of viraemic patients and that HAART reverses these defects.
Discussion
The mechanisms by which HIV infection leads to immunodeficiency that extends beyond the anti-HIV response remains a critical issue in HIV pathogenesis. Defects in long-term memory T lymphocyte responses may participate in these defects. It was therefore critical to investigate further memory CD8 T lymphocytes from chronically HIV-infected patients and to undertake this we conducted a study of CD127 expression and function.
CD127 expression was first measured in memory CD8 lymphocytes from healthy individuals. The proportion of CD127-expressing cells was slightly lower in this subset than in naive CD8 lymphocytes. This proportion was also decreased in the effector subset and was particularly low in terminally differentiated effector cells (Fig. 1b). Similarly, CD127 expression levels (MFI) decreased as the cells progressed to the effector stages (Fig. 2a). Therefore, in physiological terms CD127 expression decreases as the cells move from the naive to the terminal differentiated stage. This appears to be a major feature of CD8 lymphocytes as the vast majority of CD4 lymphocytes express CD127 independently of their naive or memory phenotype (data not shown).
Several studies have already documented the notion that CD127 expression is diminished in CD8 T lymphocytes from HIV-infected patients. As these studies were performed using either whole CD8 lymphocytes or a limited number of cell surface markers (RA/RO or CD28) they do not provide a clear picture of CD127 expression defects among the different CD8 subsets [19–22]. However, more recently Paiardini et al. studied the expression of CD127 by effector CD8 T lymphocytes taken from HIV patients [23]. This question was also addressed by Boutboul et al., using gene expression profiling and flow cytometry analysis of perforin and CD127 expression levels [24]. Both studies established that HIV-specific effector lymphocytes have reduced levels of CD127.
Here the phenotyping of the four major CD8 subsets allows for the first time the pattern of CD127 alteration to be compared in whole memory CD8 lymphocytes from healthy individuals, viraemic individuals and patients receiving HAART. We showed that the percentage and the MFI of CD127+ cells were reduced very significantly in viraemic patients (Figs 1b and 2a). This is consistent with the elevated CD127− memory lymphocyte count in viraemic patients (Fig. 1c). The role played by elevated IL-7 plasma levels [16] (Fig. 2) in the decreased CD127 expression shown by memory CD8 lymphocytes warrants discussion. Because the amplitude of the defect is different from one CD8 subset to the next, IL-7 plasma levels are unlikely to affect this phenomenon directly. Our results in memory CD8 T lymphocytes update those reported by McPherson et al., who studied only CD45RA+ and CD45RO+ CD8 lymphocytes from HIV patients [21]. Similarly, our data are more accurate that those published by Paiardini et al., who considered non-naive cells as memory cells or indicated that they found CD8 lymphocytes with effector memory phenotype among CD127− cells [23]. In our study the direct investigation of CD127 expression by the CD45RA− CD27+ memory CD8 subset establishes unambiguously that CD127 expression was altered in memory CD8 lymphocytes from viraemic patients. Furthermore, our study can be used to compare the magnitude of the defects observed in memory CD8 lymphocytes with those measured simultaneously in naive and effector T cells. Our results also show that CD127 expression is increased in memory CD8 lymphocytes from HAART patients. A comparison of the results obtained in the two HIV+ groups studied here supports the hypothesis that the defects described are linked to viraemia, not the length of the infection. Our cross-sectional analysis suggests that HAART reverses these defects. However, a longitudinal study would be required to draw definitive conclusions in this matter.
To the best of our knowledge, we are also the first to report findings relative to IL-7R regulation on the surface of CD8 lymphocytes from HIV-infected patients. The down-regulation of CD127 by IL-7 was studied in both viraemic and HAART patients. Interestingly, we noted that memory CD8 T lymphocytes taken from HAART patients regained their ability to down-regulate CD127 in the presence of IL-7. It was also noted that the amplitude of CD127 expression in the absence of IL-7 was lower in memory CD8 T cells taken from viraemic patients than in those from healthy donors. By contrast, this ability to increase CD127 expression in the absence of IL-7 was found again in HAART patients. This method of CD127 regulation has already been described in the mouse model [14] and may result from in vivo down-regulation of CD127 by IL-7. In vitro, in the absence of IL-7, CD127 would recover its full expression. Following this hypothesis our results also indicate that the regulation of CD127 expression is altered in HIV-infected patients.
As we have shown that diminished expression of CD127 and a reduced ability to express this receptor when cultured in the absence of IL-7 are characteristics of differentiated CD8 T lymphocytes, our data support further the concept that HIV infection leads to the abnormal activation and differentiation of all CD8 lymphocytes [3,25,26]. This may participate in the skewed maturation and impaired function of HIV-specific CD8 T lymphocytes [27,28]. The results reported in this paper point to a new mechanism that causes defects in long-term memory CD8 responses in HIV-patients.
Acknowledgments
The authors wish to thank M.-T. Rannou for her help in patient recruitment. A. Venet and S. Jacod are also acknowledged for their critical review of the manuscript. Financial support was received from Agence Nationale de Recherche sur le SIDA (ANRS, Clinical Trial EP20) and Cytheris SA.
References
- 1.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 2.Papagno L, Spina CA, Marchant A, et al. Immune activation and CD8(+) T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:173–85. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003;112:821–4. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trimble LA, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downregulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–94. [PubMed] [Google Scholar]
- 5.Trimble LA, Shankar P, Patterson M, Daily JP, Lieberman J. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-regulated CD3zeta and CD28, key signaling molecules for T-cell activation. J Virol. 2000;74:7320–30. doi: 10.1128/jvi.74.16.7320-7330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David D, Bani L, Moreau JL, et al. Regulatory dysfunction of the interleukin-2 receptor during HIV infection and the impact of triple combination therapy. Proc Natl Acad Sci USA. 1998;95:11348–53. doi: 10.1073/pnas.95.19.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryworuchko M, Pasquier V, Keller H, et al. Defective interleukin-2-dependent STAT5 signalling in CD8 T lymphocytes from HIV-positive patients: restoration by antiretroviral therapy. AIDS. 2004;18:421–6. doi: 10.1097/00002030-200402200-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets. function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. IL-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99:2851–8. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- 12.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 13.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Beq S, Delfraissy JF, Theze J. Interleukin-7 (IL-7): immune function, involvement in the pathogenesis of HIV infection and therapeutic potential. Eur Cytokine Netw. 2004;15:279–89. [PubMed] [Google Scholar]
- 16.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 17.Beq S, Fontanet A, Theze J, Colle JH. IL-7 and Flt-3L plasma levels are increased during highly active antiretroviral therapy-associated IL-2 therapy. AIDS. 2004;18:2089–91. doi: 10.1097/00002030-200410210-00016. [DOI] [PubMed] [Google Scholar]
- 18.Beq S, Rannou MT, Fontanet A, Delfraissy JF, Theze J, Colle JH. HIV infection: pre-highly active antiretroviral therapy IL-7 plasma levels correlate with long-term CD4 cell count increase after treatment. AIDS. 2004;18:563–5. doi: 10.1097/00002030-200402200-00025. [DOI] [PubMed] [Google Scholar]
- 19.Carini C, McLane MF, Mayer KH, Essex M. Dysregulation of interleukin-7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. Eur J Immunol. 1994;24:2927–84. doi: 10.1002/eji.1830241202. [DOI] [PubMed] [Google Scholar]
- 20.Vingerhoets J, Bisalinkumi E, Penne G, et al. Altered receptor expression and decreased sensitivity of T-cells to the stimulatory cytokines IL-2, IL-7 and IL-12 in HIV infection. Immunol Lett. 1998;61:53–61. doi: 10.1016/s0165-2478(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 21.MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:454–7. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mussini C, Pinti M, Borghi V, et al. Features of ‘CD4-exploders’, HIV-positive patients with an optimal immune reconstitution after potent antiretroviral therapy. AIDS. 2002;16:1609–16. doi: 10.1097/00002030-200208160-00006. [DOI] [PubMed] [Google Scholar]
- 23.Paiardini M, Cervasi B, Albrecht H, et al. Loss of CD127 expression defines an expansion of effector CD8+ T Cells in HIV-infected individuals. J Immunol. 2005;174:2900–9. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 24.Boutboul FPD, Appay V, Pellé O, et al. Regulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005:1981–6. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 25.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in HIV-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–55. [PubMed] [Google Scholar]
- 26.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 27.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 28.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]