Abstract
HLA-B27 transgenic (TG) rats develop spontaneous colitis when colonized with intestinal bacteria, whereas athymic nude (rnu/rnu) HLA-B27 TG rats remain disease free. The present study was designed to determine whether or not HLA-B27 expression on T cells is required for development of colitis after transfer of mesenteric lymph node (MLN) cells into rnu/rnu HLA-B27 recipients. Athymic nontransgenic (non-TG) and HLA-B27 TG recipients received MLN cells from either TG or non-TG rnu/+ heterozygous donor rats that contain T cells. HLA-B27 TG rnu/rnu recipients receiving either non-TG or TG MLN cells developed severe colitis and had higher caecal MPO and IL-1β levels, and their MLN cells produced more IFN-γ and less IL-10 after in vitro stimulation with caecal bacterial lysate compared to rnu/rnu non-TG recipients that remained disease free after receiving either TG or non-TG cells. Interestingly, proliferating donor TG T cells were detectable one week after adoptive transfer into rnu/rnu TG recipients but not after transfer into non-TG recipients. T cells from either non-TG or TG donors induce colitis in rnu/rnu TG but not in non-TG rats, suggesting that activation of effector T cells by other cell types that express HLA-B27 is pivotal for the pathogenesis of colitis in this model.
Keywords: T cells, HLA-B27, nude, transgenic rats, commensal bacteria
Introduction
Many animal models of experimental colitis support the concept that inflammatory bowel diseases (IBD) occur due to an overly aggressive immune response to commensal nonpathogenic bacteria in a genetically susceptible host [1]. In these models, the genetically susceptible host develops colitis when housed under specific pathogen-free (SPF) conditions, whereas the germ-free state prevents intestinal inflammation.
A well-characterized model of experimental colitis is the HLA-B27 transgenic (TG) rat model. HLA-B27 TG rats expressing the human MHC Class I gene, HLA-B27, and β2-microglobulin develop spontaneous colitis in SPF conditions [2,3]. Also a single strain of nonpathogenic commensal bacteria, Bacteroides vulgatus, is able to induce colitis in this model [4,5]. However, intestinal inflammation does not occur in germ-free animals [6] or after monoassociation with another commensal intestinal bacterial species, E. coli[4]. Moreover, mesenteric lymph node (MLN) T cells produce interferon (IFN)-γ in response to caecal bacterial lysates [7]. Taken together, these results suggest that commensal luminal bacteria play a crucial role in the induction and perpetuation of colitis in HLA-B27 TG RATS.
T cells, and more specifically CD4+ T cells, are required for the development of inflammation in HLA-B27 TG rats, as shown in cell transfer studies using athymic HLA-B27 TG rats. HLA-B27 TG rats homozygous for the rnu allele (rnu/rnu) are hairless and lack a developed thymus. Rnu/rnu HLA-B27 TG rats do not develop colitis or arthritis [8]. When TG CD4+ T cells were transferred into rnu/rnu TG rats, intestinal inflammation developed [8]. We showed previously that activated CD4+ T cells stimulated with caecal bacterial lysates are required for production of the Th1 cytokines IFN-γ and IL-12 [7]. These findings emphasize the importance of T cells, specifically CD4+ cells, in the pathogenesis of colitis in the HLA-B27 TG model. However, a requirement for expression of HLA-B27 by the T cells has not been evaluated.
Our results show that MLN cells from either non-TG or TG donors induce colitis in rnu/rnu HLA-B27 TG, but not in non-TG recipient rats. These findings suggest that activation of T cells by HLA-B27-expressing accessory cells, defined as non-T cells that either present antigen to T cells or perform costimulatory functions, is pivotal to the pathogenesis of colitis in this model.
Materials and methods
Animals
We used HLA-B27 TG F344 strain rats of the 33–3 line [2,9] and their non-TG littermates that were either homozygous or heterozygous for the rnu allele (rnu/rnu, or rnu/+, respectively) [10,11]. Rnu/rnu rats are athymic and hairless, whereas heterozygous rnu/+ rats are euthymic and have normal T cell function [8]. Animals were all maintained under SPF housing conditions at the University of North Carolina, Chapel Hill. Presence or absence of the HLA-B27 transgene was determined by PCR using DNA isolated from tail clippings. All studies were approved by University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Experimental procedure
Donor cells were obtained from MLN of three to seven month old rnu/+ non-TG and TG rats. After obtaining single MLN cell suspensions by gentle teasing, the cells were washed twice and 1 × 107 unfractionated MLN cells were injected intravenously into the tail vein of four to eight month old rnu/rnu non-TG and TG recipients. After eight weeks, rats were euthanized. Caecal and colonic tissue and MLN were collected. Based on histological findings, similar levels of intestinal inflammation in recipients of different ages were observed.
In separate experiments, donor MLN cells from rnu/+ TG rats were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR, USA) to monitor them after transfer, as described previously [12,13]. After obtaining single MLN cell suspensions by gentle teasing, the cells were washed twice and incubated with CFSE at 5 µM in 1×PBS for 15 min at room temperature. The labelling process was terminated by adding an equal volume of fetal calf serum. Flow cytometry analyses showed greater than 99% of MLN cells were labelled with CFSE. The cells were washed three times, and equal number of cells, either 1·5 or 2 × 107, were injected intravenously into rnu/rnu TG or rnu/rnu non-TG recipients. Rats were euthanized on day two or seven after injection, and flow cytometry was performed on recipient MLN and spleen cells, and recipient MLN cells were stimulated in vitro as described below.
Histology
Tissues from different sections of the intestinal tract (proximal, transverse, and distal colon, and caecum) were collected from each recipient. The tissues were fixed and stained as previously described [3,14]. Histological scoring (range from 0 to 4) was based on well-validated criteria and carried out on blinded samples by one evaluator [3,14].
Preparation of caecal bacterial lysates
Caecal bacterial lysates were prepared as described previously [7,15]. Briefly, caecal contents from several non-TG rats were disrupted using glass beads and the lysate was filter-sterilized (0·22 µm) [7]. Sterility was confirmed by aerobic and anaerobic bacterial culture.
Mesenteric lymph node cell cultures
MLN were removed from the rnu/rnu non-TG and TG recipients, and single cell suspensions were prepared, as described previously [7]. Unseparated MLN cells were washed twice and 4 × 105 cells were stimulated with caecal bacterial lysate cultured in 96 well flat bottom microplates (Costar 3595), in 0·2 ml complete medium (RPMI 1640 plus 5% heat inactivated fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 5 × 10−5 M 2-mercaptoethanol, and 50 µg/ml gentamicin) for three days, as described [7]. Cells were stimulated with concentrations of caecal bacterial lysate that we found to be optimal for the induction of different cytokines. Culture supernatants were collected and stored at −20 °C.
For mixed lymphocyte reactions (MLR), MLN cells (2–5 × 105) from rnu/+ non-TG rats were mixed with equivalent numbers of MLN cells from rnu/rnu TG rats in 0·2 ml complete medium in 96 well flat bottom microplates and incubated for 72 h. During the last 18 h, 1 µCi of 3H-thymidine (Amersham Biosciences, Piscataway, NJ, USA) was added. At the end of the incubation period, the plates were frozen, then thawed and harvested 24–48 h later. 3H-thymidine incorporation was measured using the TopCount NXT Microplate Scintillation Counter system (PerkinElmer, Boston, MA, USA).
Flow cytometry
MLN cell fractions were evaluated by flow cytometry using the following fluorochrome labelled or unlabelled reagents, as previously described [7]. For detection of HLA-B27-expressing cells, we used culture supernatants from the murine hybridoma, designated ME-1, obtained from ATCC (Rockville, MD, USA), followed by either FITC labelled goat anti-mouse IgG (γ-chain specific) antibody (Southern Biotechnology, Birmingham, AL, USA) or APC labelled rat antimouse IgG1 (BD Biosciences Pharmingen, San Diego, CA, USA). For surface immunoglobulin positive B cells, we used FITC labelled goat anti-rat IgG (H + L) antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). For CD4 and CD8, T cell receptor αβ (TCR αβ) and CD45RC expressing cells we used PE-anti-CD4 monoclonal antibody (clone W3/25), and FITC anti-CD8 monoclonal antibody (clone OX-8) (Caltag, Burlingame, CA, USA), FITC or PerCP anti-rat TCRαβ (clone R73), and FITC anti-rat CD45RC (clone OX-22) (BD Biosciences Pharmingen), respectively. Lymphocytes were gated according to forward and side scatter characteristics and analysed using CellQuest™ (Becton Dickinson, San Jose, CA, USA).
Cytokine measurements
Cytokines in cell culture supernatants were measured by ELISA using unlabelled capture antibodies and biotin-labelled detection antibodies, followed by horse-radish peroxidase labelled Streptavidin [7]. The concentration of each cytokine was determined by comparison to a standard curve generated using recombinant proteins. For IFN-γ, we used unlabelled polyclonal anti-IFN-γ antibody and biotin-labelled monoclonal anti-IFN-γ antibody (clone DB-1) (Biosource International, Camarillo, CA, USA). For IL-10 ELISA we used unlabelled monoclonal anti-rat IL-10 antibody (clone A5-7) and biotin-labelled monoclonal anti-rat IL-10 antibody (clone A5-6) (BD Biosciences Pharmingen, San Diego, CA, USA).
Caecal cytokine analysis
Caecal cytokines were measured as described previously [14]. Briefly, caecal tissues were homogenized in PBS containing a cocktail of protease inhibitors [16], after which the homogenate was assayed for IL-1β according to the manufacturer's instructions (National Institute for Biological Standards and Controls, South Mimms, UK).
MPO assay
Homogenized caeca were assayed for MPO activity (units per gram of caecal tissue) as described previously [14,17].
Statistical analysis
Cytokine levels are expressed as mean ± standard error of the mean (SEM) in triplicate measurements. A nonpaired Student t-test was used, in which a two-tailed P-value of < 0·05 was considered statistically significant.
Results
Donor and recipient MLN cell populations before cell transfer
Unseparated MLN cells, containing CD4+ and CD8+ T cell subsets as well as non-T cells, from rnu/+ non-TG or TG rats were used as donor cells for adoptive transfer. The TG donor rats had moderate colitis with histology scores of 1·5–2·0 on a 0–4 scale (data not shown). Non-TG donor MLN cells contained a higher proportion of B cells and a lower proportion of T cells compared to those from their TG littermates that developed colitis (Table 1). Also, both the TG and the non-TG donor cell preparations contained CD4+ cells that express low levels of CD45RC (Table 1). B cells were the predominant cell type in MLN of rnu/rnu non-TG and TG recipients prior to MLN transfer (Table 1). We evaluated cell surface expression of HLA-B27 to confirm the non-TG or TG status of the donor and recipient rats.
Table 1.
Cell populations (%) in mesenteric lymph nodes from donor (rnu/+) and recipient (rnu/rnu) HLA-B27 nontransgenic and transgenic rats before cell transfer.
| Donor | Recipient before transfer | |||
|---|---|---|---|---|
| non-TG n = 6 | TG n = 5 | non-TG n = 2 | TG n = 2 | |
| TCRαβ | 49·4 ± 1·7 | 66·6 ± 1·9† | 1·1 ± 0·1 | 1·9 ± 0·5 |
| CD4 | 30·8 ± 1·8 | 51·8 ± 1·6† | 2·3 ± 0·4 | 2·9 ± 0·1 |
| CD45RClow/CD4+ | 17·1 ± 1·8 | 37·3 ± 4·4† | 1·8 ± 0·1 | 2·5 ± 0·3 |
| CD8 | 17·8 ± 0·5 | 13·7 ± 0·6† | 1·4 ± 0·1 | 1·3 ± 0·1 |
| Surface Ig | 48·9 ± 1·4 | 31·7 ± 2·2† | 91·0 ± 0·5 | 93·5 ± 0·5 |
| HLA-B27 | 0·7 ± 0·2 | 97·9 ± 0·4† | 1·1 ± 0·3 | 98·4 ± 0·1 |
P < 0·005 versus non-TG donor.
Transfer of MLN cells induces inflammation in TG but not in non-TG rnu/rnu recipients
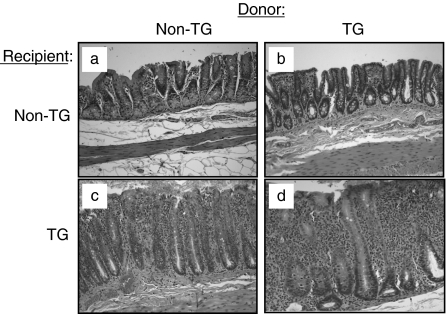
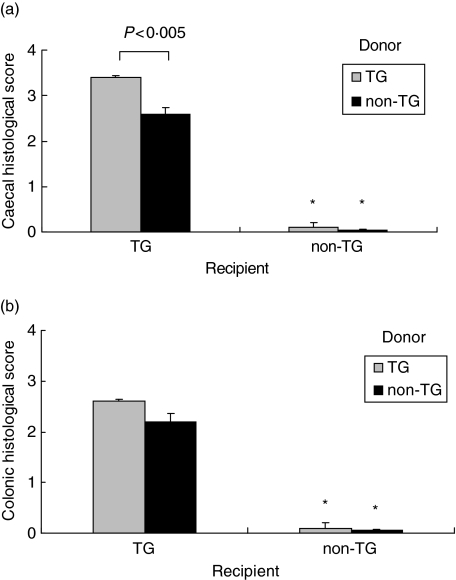
Adoptive transfer of rnu/+ TG MLN cells into rnu/rnu TG recipients induced severe colitis, as shown in Fig. 1d. This colitis is characterized by massive infiltration of granulocytes and mononuclear cells of mucosa and submucosa, bowel wall thickening, crypt abscesses, crypt hyperplasia, and goblet cell depletion. Rnu/rnu non-TG recipients that received TG MLN cells remained disease-free, as shown in Fig. 1b. Interestingly, adoptive transfer of rnu/+ non-TG MLN cells into rnu/rnu TG recipients induced moderate colitis (Fig. 1c). The disease that developed in rnu/rnu TG recipients of non-TG cells differs from the description of graft versus host disease (GVHD), which is characterized by a constellation of severe inflammatory reactions in many tissues including skin, liver, and small intestine but not the large intestine [18]. We did not observe gross or clinical evidence of dermal, hepatic, or small intestinal inflammation. Moreover, in vitro proliferation of rnu/+ non-TG MLN cells cultured with rnu/rnu TG MLN cells, which would be expected if the non-TG cells induced GVHD, was minimal (stimulation indices of 0·8–1·4 in three separate experiments). Rnu/rnu non-TG recipients receiving non-TG MLN cells did not show any signs of colitis (Fig. 1a). Histological inflammatory scores (scale 0–4) confirmed the severe inflammation in both caecum and colon in rnu/rnu TG recipients receiving TG MLN cells, and moderate intestinal inflammation in rnu/rnu TG recipients receiving non-TG MLN cells (Fig. 2a,b). No disease was detected in rnu/rnu non-TG rats receiving either non-TG or TG MLN cells (P < 0·005 versus TG recipients) (Fig. 2a,b).
Fig. 1.
Caecal inflammation in recipients of MLN cell transfers. Representative sections of the caecum of rnu/rnu transgenic and nontransgenic recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic or nontransgenic donors are shown at 10× magnification. Panels represent non-TG donor cells transferred into (a) non-TG or (c) TG recipients, and TG donor cells transferred into (b) non-TG or (d) TG recipients.
Fig. 2.
Caecal and colonic histological scores for recipients of MLN cell transfers. Intestinal tissue was collected from transgenic and nontransgenic rnu/rnu recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic (□) or nontransgenic (▪) donors. Blinded histology scores for (a) caecal and (b) colonic (average of proximal, transverse, and distal colon) tissue are shown. Values represent mean ± SEM, n = 5–8 rats per group. *P < 0·005 for histological scores of non-TG recipient tissue compared to TG recipient tissue.
Flow cytometric analysis of MLN cells in rnu/rnu recipient rats after adoptive transfer
Evaluation of cell subpopulations was carried out on MLN cells collected from all recipients, as shown in Table 2. Eight weeks after MLN cell transfer, cells carrying the HLA-B27 molecule comprised 40% of the total MLN cell population in rnu/rnu TG recipients receiving non-TG MLN cells. In contrast, no HLA-B27 positive cells were detected after transfer of TG MLN cells into rnu/rnu non-TG recipients, a consistent finding observed in three independent experiments. Of interest, equivalent proportions of CD4+ T cells were present in the rnu/rnu TG recipients of either TG or non-TG donor MLN cells. MLN of rnu/rnu non-TG recipients of TG MLN cells contained significantly lower proportions of T cells and higher percentages of B cells than did rnu/rnu non-TG recipients of non-TG MLN cells.
Table 2.
Cell numbers and cell populations in mesenteric lymph nodes from rnu/rnu HLA-B27 transgenic and nontransgenic rats after MLN cell transfer.
| Donor Recipient | TG TG n = 5 | non-TG TG n = 8 | TG non-TG n = 6 | non-TG non-TG n = 5 |
|---|---|---|---|---|
| Total cells (× 106) | 52 ± 12 | 83 ± 21 | 60 ± 7 | 46 ± 9 |
| TCRαβ | 33·8 ± 3·7‡ | 34·2 ± 2·6‡ | 5·3 ± 1·2‡ | 14·9 ± 1·2 |
| CD4 | 30·8 ± 3·4‡ | 29·6 ± 2·5‡ | 4·1 ± 0·8‡ | 13·2 ± 1·2 |
| CD8 | 1·5 ± 0·2 | 4·2 ± 0·6 | 2·8 ± 0·3 | 2·2 ± 0·3 |
| Surface Ig | 56·4 ± 5·4‡ | 60·0 ± 3·3‡ | 89·5 ± 1·0† | 77·7 ± 2·5 |
| HLA-B27 | 92·5 ± 1·6‡ | 40·6 ± 4·8‡ | 0·4 ± 0·2 | 0·5 ± 0·1 |
Values represent mean ± SEM of percentages of the different lymphoid cell populations in MLN of recipients analysed eight weeks after MLN cell transfer.
P < 0·01 versus non-TG donor cells transferred into non-TG recipients
P < 0·005 versus non-TG donor cells transferred into non-TG recipients.
Increased caecal MPO and IL-1β in rnu/rnu TG recipients
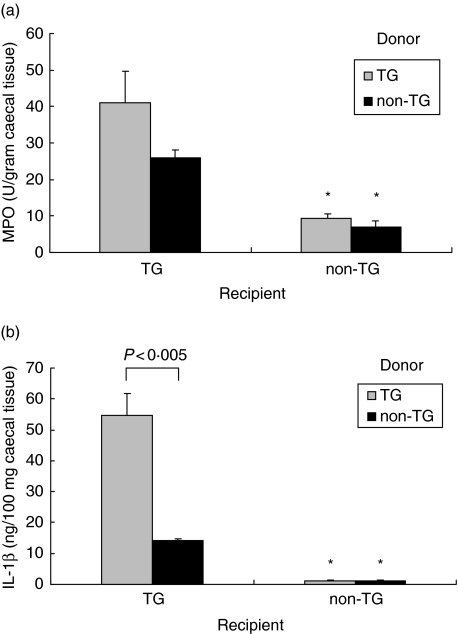
Inflammation in diseased rats was most severe in the caecum. Therefore, we measured myeloperoxidase levels in caecal tissue as a parameter of inflammation, and more specifically, granulocyte and macrophage infiltration. Rnu/rnu TG recipients receiving TG MLN cells had higher caecal MPO levels (albeit not statistically significant) and IL-1β levels (P < 0·005) than rnu/rnu TG recipients of non-TG MLN cells as shown in Fig. 3a,b. However, caecal MPO and IL-1β levels were both significantly higher in TG recipients of non-TG MLN cells than those found in caecal tissue from rnu/rnu non-TG recipients receiving MLN cells from either type of donor.
Fig. 3.
MPO and IL-1β in caecal tissue of cell transfer recipients. Transgenic and nontransgenic MLN cells from rnu/+ rats were transferred into rnu/rnu transgenic (□) or nontransgenic (▪) recipients. After eight weeks, caecal tissue was collected, homogenized, and (a) MPO and (b) IL-1β were determined in duplicate supernatants. Values represent mean ± SEM, n = 5–8 rats per group. *P < 0·005 for caecal tissue from non-TG recipients compared to TG recipients.
Cytokine profile in MLN cell cultures from rnu/rnu TG recipients after MLN cell transfer
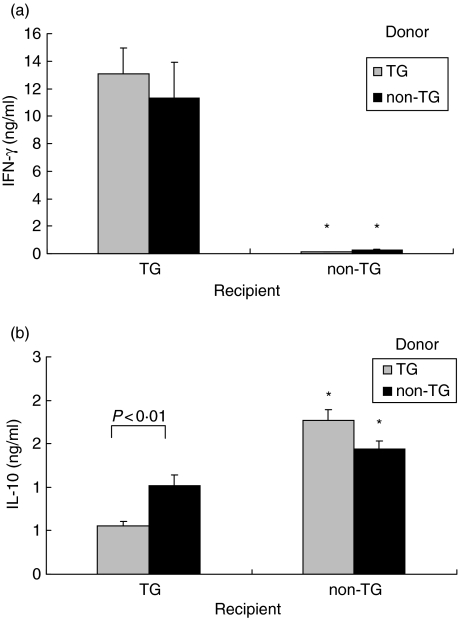
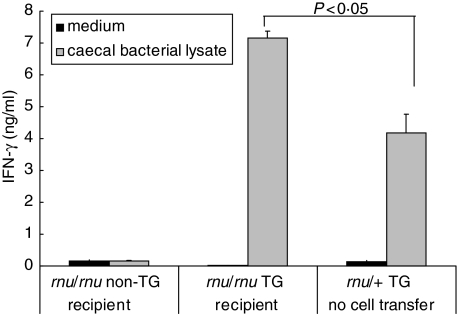
Previously, we have shown that CD4+ T cells are critical for IFN-γ production in HLA-B27 TG rats, whereas B cells are required for IL-10 secretion [7]. In the present study, MLN cells from recipients were isolated and stimulated in vitro with caecal bacterial lysate. IFN-γ secretion in response to stimulation by components of caecal bacteria present in the lysate was significantly higher in rnu/rnu TG recipients compared to rnu/rnu non-TG recipients (P < 0·005), as shown in Fig. 4a. MLN cells collected from rnu/rnu TG recipients as early as one week after transfer of TG donor cells produced higher amounts of IFN-γ in response to caecal bacterial lysate compared to stimulated MLN cells from rnu/rnu non-TG recipients (Fig. 5). Interestingly, the amounts of IFN-γ detectable in the supernatants of the recipient TG MLN cells collected from rnu/rnu TG recipients at this early time point after transfer were higher than the amounts produced by caecal bacterial lysate-stimulated MLN cells from four month old SPF rnu/+ TG rats with colitis (Fig. 5). IL-10 produced in response to caecal bacterial lysate was significantly higher in cultures of rnu/rnu TG recipients of non-TG MLN cells compared to rnu/rnu TG recipients of TG MLN cells (P < 0·01, Fig. 4b).
Fig. 4.
Cytokine production by recipient MLN cells. MLN cells were collected from transgenic and nontransgenic recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic (□) or nontransgenic (▪) donors and cultured in the presence or absence of caecal bacterial lysate. After three days, supernatants were collected and (a) IFN-γ stimulated by caecal bacterial lysate at 50 µg/ml and (b) IL-10 stimulated by caecal bacterial lysate at 10 µg/ml were measured in triplicate supernatants by ELISA. Values represent mean ± SEM, n = 5–8 rats per group. IFN-γ levels were significantly lower (*P < 0·005) and IL-10 levels were significantly higher (*P < 0·005) in supernatants of caecal bacterial lysate stimulated MLN from non-TG recipients compared to TG recipients.
Fig. 5.
IFN-γ production by caecal bacterial lysate stimulated MLN cells. MLN cells from rnu/+ TG rats were transferred into rnu/rnu non-TG or rnu/rnu TG recipients. After seven days, recipient MLN were harvested, and cells were stimulated with caecal bacterial lysate at 50 µg/ml or cultured without stimulation (medium). For comparison, MLN were also obtained from four month old SPF rnu/+ transgenic rats (not transplanted) and stimulated with caecal bacterial lysate or cultured with medium. Supernatants were collected after three days. IFN-γ was measured in triplicate supernatants by ELISA. Values represent mean ± SD of triplicate measurements from MLN cultures of individual animals. Results are representative of two independent experiments.
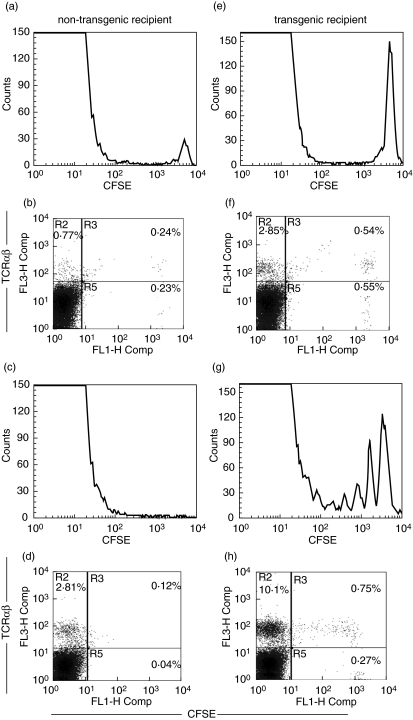
TG MLN donor cells proliferate and accumulate in TG but not in non-TG recipients
To obtain a better understanding of the kinetics of donor cell survival and proliferation after transfer, we evaluated CFSE-labelled TG donor cells after transfer into either rnu/rnu non-TG or rnu/rnu TG recipients. CFSE-labelled dividing cells can be easily monitored due to the incremental reduction in CFSE fluorescence intensity that occurs with each cell division. On day two after transfer, two-fold more CFSE-labelled cells were detectable in the MLN of TG recipients compared to non-TG recipients (Fig. 6bversus6f). Two populations of TCRαβ-expressing cells are present (Fig. 6b,f). One is CFSE positive, identifying donor cells, the other is CFSE-negative. The CFSE-negative cells are either donor cells that divided rapidly after transfer and thus lost detectable CFSE, or alternatively, these cells could represent the minor population of TCRαβ-expressing cells that are present in the MLN of rnu/rnu recipients (see Table 1). At day seven, MLN of non-TG recipients no longer contain CFSE-labelled cells (Fig. 6c,d) while MLN of TG recipients contain CFSE-labelled TCRαβ-expressing cells that had divided, as evidenced by their reduced fluorescence intensity (Fig. 6g,h). It is interesting to note that the transferred cells that do not express TCRαβ are not dividing (Fig. 6h, lower right quadrant). We also evaluated spleen cells of the recipients to determine if donor cells were more or less abundant outside the MLN. More CFSE-labelled donor cells were consistently seen in the MLN than in the spleen of either TG or non-TG recipients (data not shown), excluding the possibility that donor MLN cells home to the spleen in non-TG rats.
Fig. 6.
Equal numbers of CFSE labelled mesenteric lymph node cells from rnu/+ TG rats were transferred into rnu/rnu non-TG (a–d) or rnu/rnu TG recipients (e–h). After two (a,b,e,f) and seven days (c,d,g,h), recipient mesenteric lymph node cells were collected and analysed by flow cytometry to enumerate CFSE-labelled cells (histograms) and cells that express TCRαβ (dot plots). Values represent percent positive cells in the labelled quadrants. Results shown from one transgenic and one nontransgenic recipient evaluated at each time point are representative of two rats of each type evaluated in independent experiments for each time point.
Discussion
The results of the present study show that adoptive transfer of MLN cells from either TG donor rats that develop colitis or from disease-free non-TG donors into athymic TG recipients induces inflammation in the caecum and colon. This finding implicates accessory cells that express the HLA-B27 transgene as the activators of disease-inducing T cell responses.
As demonstrated previously, T cells are required for inflammation in HLA-B27 TG rats, since rnu/rnu TG rats remain disease-free [8]. Moreover, CD4+ T cells have been shown to mediate inflammatory responses in HLA-B27 TG rats [8], and they produce large amounts of IFN-γ when stimulated with components of commensal bacteria [7]. Peptides bound in the peptide binding grooves of MHC class I molecules such as HLA-B27 are usually associated with activation of CD8+, not CD4+ T cells. However, it has been clearly shown that CD4, and not CD8 cells, are crucial for disease-induction in HLA-B27 TG rats. CD4+ T cells were much more efficient than CD8+ T cells in transferring disease into nude HLA-B27 TG rats [8]. Additionally, the minor role for CD8+ T cells in disease was shown by the lack of an effect on colitis by either anti-CD8 treatment or CD8 depletion in HLA-B27 TG rats [19]. In our study, CD8 cells did not proliferate and the percentage of CD8+ cells detected by flow cytometry after MLN cell transfer is low in all rnu/rnu recipients.
CD4+ T cell subpopulations with regulatory functions have been identified. In an elegant study, Powrie et al. [20] showed that transfer of CD4+CD45RBhi T cells from normal mice induced colitis in severe-combined immunodeficient (SCID) mice. This inflammatory process could be prevented by cotransfer of CD4+ cells that express low levels of CD45RB from normal mice. This principle was shown originally in athymic rats, where transfer of T cells that express high levels of the CD45 isoform, designated CD45RC, mediated inflammation in several different organ systems, whereas the severity of inflammation was greatly reduced by cotransfer of a CD4+CD45RClow cell population [21]. Also B cells and intraepithelial lymphocytes (IEL) have been suggested as regulators of inflammation [22,23]. Taken together, analysis of these different models of colitis indicates that disease induction as well as regulation of inflammation are complex processes that require interactions among a variety of different cell types. In our study, we transferred a mixed cell population of non-TG or TG MLN cells into rnu/rnu athymic HLA-B27 TG rats, resulting in moderate to severe colitis in the recipients. Donor cells were unfractionated and contained both CD45RClow CD4+ and CD45RChi CD4+ cells, as well as B cells and CD8+ cells. We have shown previously that MLN-derived rat B cells, but not T cells, produce IL-10 and TGF-β in response to components of commensal bacteria [7]. Our current findings indicate that CD45RClow CD4+cells or B cells, from either TG or non-TG donors, failed to prevent development of inflammation in rnu/rnu TG recipients. Conversely, rnu/rnu non-TG recipients that do not express HLA-B27 remain healthy, even after transfer of MLN cells from TG donors with colitis.
IL-10 is an important immunoregulatory molecule [24]. For example, IL-10 is produced by regulatory T cells and is crucial for the inhibition of colitis in the SCID mouse CD4+CD45RBhi cell transfer model [25]. In another study, IL-10 producing C3H/HeJBir CD4+ T regulatory cells, generated in vitro by sequential stimulation with caecal bacterial antigens in the presence of IL-10, prevented onset of colitis in SCID recipients after cotransfer with colitis-inducing T cells [26]. In HLA-B27 TG and non-TG rats, B cells are the main source of IL-10 [7]. Although less IL-10 is secreted by MLN cells from rnu/rnu TG recipients compared to those from rnu/rnu non-TG recipients, MLN cells of rats from both groups were able to produce IL-10. However, the capacity of these cells to produce IL-10 was not sufficient to down-regulate caecal bacterial lysate-induced IFN-γ secretion by MLN cells from rnu/rnu TG recipients. We have recently identified hyporesponsiveness of TG MLN cells to IL-10 that might also explain the inability of IL-10 to inhibit development of colitis in TG recipients [27].
A major finding in our study is that accessory cells carrying the HLA-B27 molecule determine the outcome of effector T cell responses. This is clearly demonstrated by the finding that non-TG T cells are capable of inducing colitis when transferred to rnu/rnu TG recipients. When the same cells are transferred to rnu/rnu non-TG recipients, no disease develops, indicating that the rnu/rnu host expressing transgenic HLA-B27 orchestrates T cell responses to commensal bacteria. The importance of HLA-B27 in the activation of T cell responses was further underlined by our observation that addition of anti-HLA-B27 antibodies to cocultures of TG CD4+ T cells plus caecal bacterial lysate- pulsed TG antigen presenting cells reduced IFN-γ responses [28].
How can we explain that accessory cells determine disease outcome, but that no disease occurs without T cells? There is growing evidence that T cells are not acting in an independent fashion, but are influenced by the innate immune system [29]. Toll-like receptors (TLR) are crucial for innate immune responses against microbial products [30]. These responses can skew the acquired immune system, including T cells, into regulatory or proinflammatory directions. Therefore, dendritic cells and macrophages can regulate T cell responses, not only by their direct antigen presentation capacities, but also indirectly by the production of cytokines that are induced after TLR-signalling [31]. Once T cells become activated, the cytokines they produce subsequently induce expression of a broad array of genes in antigen-presenting accessory cells, such as IL-12/IL-23, IL-18 and tumour necrosis factor-like 1A (TL1A), thus perpetuating the immune response [32,33].
If CD4+ T cells, and not CD8+ T cells are required, how does the MHC class I molecule HLA-B27 induce development of inflammatory diseases? The molecular basis for the development of spondyloarthrophies in human patients that carry the HLA-B27 allele and for the occurrence of inflammatory conditions in HLA-B27 TG rats has not yet been determined. However, recent reports implicate the unusual biochemical properties of this MHC class I encoded molecule in disease development. The B27 heavy chain can take several different forms in addition to the classical heterodimer of the class I heavy chain noncovalently associated with β-2 microglobulin that presents antigenic peptides to CD8+ T cells [34]. One of the unusual forms results from homodimerization of two B27 molecules. B27 homodimers are capable of activating CD4+ T cells. An intriguing possibility that would explain the development of HLA-B27-associated disease was recently described by Kollnberger et al. [35]. The results of their elegant study using tetramers of B27 homodimers indicate that these molecules are able to activate macrophages to produce TNF and nitric oxide, which would then establish a receptive environment for the development of inflammatory conditions. In separate studies, Turner et al. recently demonstrated the presence of misfolded HLA-B27 molecules accompanied by activation of genes associated with endoplasmic reticulum stress in macrophages from HLA-B27 TG rats [36,37]. Furthermore, IFN-γ, one of the cytokines that is produced by HLA-B27 TG CD4 T cells [7], also activated many of the same ER stress related genes in HLA-B27 TG rat macrophages but not in macrophages derived from wild type rats [37]. HLA-B27 molecules alone are not sufficient to trigger inflammation, since both germ-free HLA-B27 TG rats [3,6] and SPF rnu/rnu TG rats [8] do not develop colitis. Taken together, these observations indicate that the development of disease in HLA-B27 TG rats requires the combination of misfolded HLA-B27 in accessory cells, the presence of intestinal bacteria, and CD4+ T cells that can be induced, as we show here, to produce IFN-γ as early as one week after transfer into SPF HLA-B27 rnu/rnu recipients. Although extra-intestinal manifestations such as arthritis have been reported in HLA-B27 transgenic rats, our specific pathogen free HLA-B27 TG rats rarely develop arthritis. Therefore no conclusions can be drawn from our study about the role of T cells or accessory cells for the development of inflammation outside the intestinal tract.
It is unclear what process determines the poor survival of TG MLN cells in the rnu/rnu non-TG recipients. One possibility is that, for this donor recipient combination, the donor cells proliferate only minimally, and therefore form a minor population relative to the endogenous cell population in the recipients after eight weeks. This is supported by the results of our cell transfer experiment using CFSE labelled cells. Multiple cycles of proliferation were detected in TG recipients but not in non-TG recipients on day seven after MLN cell transfer. Secondly, it is possible that a regulatory cytokine environment predominates in non-TG recipients that directly inhibits TG cell responses. Thirdly, TG donor cells could be killed by NK cells of the non-TG host. Cells carrying high copy numbers of HLA-B27 have reduced levels of endogenous MHC class I molecules [2]. It is possible that this feature makes TG donor cells prone to NK cell-mediated lysis [38,39]. A fourth possibility is that the donor TG MLN cells might undergo apoptosis following transfer into rnu/rnu non-TG recipients, because their activation and subsequent survival depends on interactions with HLA-B27-expressing accessory cells. Finally, other organs such as spleen, liver, or lungs might trap donor cells, thereby preventing these cells from reaching the MLN. Although we cannot rule out trapping of donor cells in nonlymphoid tissues, we detected a higher percentage of CFSE-labelled donor cells in MLN than in the spleen of TG recipients, supporting the notion that MLN constitute an important site for activation, proliferation, and survival of the transplanted cells.
Caecal inflammation was more severe and caecal IL-1β levels were higher in rnu/rnu TG rats after transfer of TG MLN cells than non-TG MLN cells. A number of explanations can be considered, including transfer of greater numbers of T cells (Table 1) as well as previously activated T cells from TG compared to non-TG donors that are capable of responding rapidly to stimulatory signals. Nonetheless, the disease we have documented in rnu/rnu TG recipients of rnu/+ non-TG MLN cells closely resembles the disease that develops in the HLA-B27 transgenic rat model of colitis.Several published reports show reduced capacity of accessory cells, such as dendritic cells, from HLA-B27 TG rats to activate allogenic cells in vitro[40,41]. The results of our study, while not directly addressing costimulatory activity, indicate that cells of rnu/rnu TG recipients are potent stimulators of transferred syngeneic donor MLN cells in vivo. The role of accessory cells in determining the nature of the immune response has been extensively evaluated, and the results indicate that antigen-presenting cells are crucial for both activation and also for subsequent down-regulation of immune responses [42]. The results of our in vivo transfer study indicate that accessory cells in rnu/rnu TG recipients may lack the capacity to inhibit the colitis-inducing abilities of CD4+ T cells from donor rats that interact with HLA-B27 expressing cells.
In conclusion, we have shown that colitis develops in rnu/rnu TG rats receiving TG or non-TG MLN cells. In contrast, rnu/rnu non-TG rats remained disease-free after MLN cell transfer. These observations suggest that the accessory cells of the nude rats that carry HLA-B27 either orchestrate T cell responses to luminal commensal bacteria or fail to adequately regulate these responses. The results of our investigation underscore the importance of HLA-B27 expressing accessory cells in determining pathogenic host mucosal immune responses to commensal bacteria.
Acknowledgments
The authors thank Desmond McDonnell and Lisa Wiltron for expert technical support and Janet Dow for assistance with flow cytometry at the College of Veterinary Medicine, North Carolina State University, Raleigh. The authors also thank Charlotte Walters, Immunoassay Core, Center of Gastrointestinal Biology and Disease at the University of North Carolina at Chapel Hill, and Donna Kronstadt, Gnotobiotic Animal Core, Center of Gastrointestinal Biology and Disease at North Carolina State University for their expert assistance. These studies were supported by U.S. Public Health Service Grants K08 DK02551 (Dieleman), RO1 DK40249 (Sartor, Tonkonogy) and P30 DK34987 (Center for Gastrointestinal Biology and Disease) and AGIKO-grant 920-03-300 (Hoentjen) from the Netherlands Organization for Health Research and Development.
Glossary
List of abbreviations
- B. vulgatus
Bacteroides vulgatus
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cells
- IBD
inflammatory bowel diseases
- IEL
intraepithelial lymphocytes
- IFN
interferon
- GF
germ-free
- GVHD
graft versus host disease
- MLR
mixed lymphocyte reactions
- MLN
mesenteric lymph nodes
- MPO
myeloperoxidase
- non-TG
nontransgenic
- SCID
severe-combined immunodeficient
- rnu/rnu
nude
- SPF
specific pathogen free
- TCR
T cell receptor
- TG
transgenic
- TLR
toll-like receptors
- TL1A
tumour necrosis factor-like 1 A.
References
- 1.Sartor RB. Animal models of intestinal inflammation. In: Sartor RB, Sandborn W, editors. Kirsner's Inflammatory Bowel Diseases. Philadelphia: Elsevier; 2004. pp. 120–37. [Google Scholar]
- 2.Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 3.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rath HC, Wilson KH, Sartor RB. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect Immun. 1999;67:2969–74. doi: 10.1128/iai.67.6.2969-2974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller D, Russo MP, Sartor RB, et al. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–78. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 6.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieleman LA, Hoentjen F, Qian BF, et al. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–9. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breban M, Fernandez-Sueiro JL, Richardson JA, et al. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol. 1996;156:794–803. [PubMed] [Google Scholar]
- 9.Taurog JD, Maika SD, Simmons WA, et al. Susceptibility to inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of B27 expression. J Immunol. 1993;150:4168–78. [PubMed] [Google Scholar]
- 10.Nehls M, Pfeifer D, Schorpp M, et al. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–7. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 11.Hirasawa T, Yamashita H, Makino S. Genetic typing of the mouse and rat nude mutations by PCR and restriction enzyme analysis. Exp Anim. 1998;47:63–7. doi: 10.1538/expanim.47.63. [DOI] [PubMed] [Google Scholar]
- 12.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 13.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieleman LA, Goerres MS, Arends A, et al. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52:370–6. doi: 10.1136/gut.52.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cong Y, Brandwein SL, Mccabe RP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–64. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cominelli F, Nast CC, Clark BD, et al. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86:972–80. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Meth Enzymol. 1990;186:729–42. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- 18.Beschorner WE, Tutschka PJ, Santos GW. Chronic graft-versus-host disease in the rat radiation chimera. I. Clinical features, hematology, histology, and immunopathology in long-term chimeras. Transplantation. 1982;33:393–9. doi: 10.1097/00007890-198204000-00010. [DOI] [PubMed] [Google Scholar]
- 19.May E, Dorris ML, Satumtira N, et al. CD8 alpha beta T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003;170:1099–105. doi: 10.4049/jimmunol.170.2.1099. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F, Leach MW, Mauze S, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 21.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990;172:1701–8. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 23.Laroux FS, Norris HH, Houghton J, et al. Regulation of chronic colitis in athymic nu/nu (nude) mice. Int Immunol. 2004;16:77–89. doi: 10.1093/intimm/dxh006. [DOI] [PubMed] [Google Scholar]
- 24.Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 25.Asseman C, Mauze S, Leach MW, et al. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cong Y, Weaver CT, Lazenby A, et al. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–9. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 27.Qian BF, Tonkonogy S, Hoentjen F, et al. Dysregulated luminal bacterial antigen-specific T cell responses and antigen-presenting cell function in HLA-B27 transgenic rats with chronic colitis. Immunology. 2005;116:112–21. doi: 10.1111/j.1365-2567.2005.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian BF, Tonkonogy SL, Sartor RB. Luminal bacterial antigen-specific CD4+ T cell responses in HLA-B27 transgenic rats with chronic colitis are mediated by both major histocompatibility class II and HLA-B27 molecules. Immunology. 2006 doi: 10.1111/j.1365-2567.2005.02303.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–8. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 31.Sartor RB, Hoentjen F. Proinflammatory cytokines and signaling pathways in intestinal innate immune cells. In: Mestecky J, Mcghee JR, Strober W, editors. Mucosal Immunology. Philadelphia: Elsevier; 2005. pp. 681–702. [Google Scholar]
- 32.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 33.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn's disease. Immunol Rev. 2005;206:277–95. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 34.Boyle LH, Hill Gaston JS. Breaking the rules. the unconventional recognition of HLA-B27 by CD4+ T lymphocytes as an insight into the pathogenesis of the spondyloarthropathies. Rheumatology (Oxford) 2003;42:404–12. doi: 10.1093/rheumatology/keg097. [DOI] [PubMed] [Google Scholar]
- 35.Kollnberger S, Bird LA, Roddis M, et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173:1699–710. doi: 10.4049/jimmunol.173.3.1699. [DOI] [PubMed] [Google Scholar]
- 36.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 37.Turner MJ, Sowders DP, Delay ML, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–48. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 38.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 39.Rolstad B, Naper C, Lovik G, et al. Rat natural killer cell receptor systems and recognition of MHC class I molecules. Immunol Rev. 2001;181:149–57. doi: 10.1034/j.1600-065x.2001.1810112.x. [DOI] [PubMed] [Google Scholar]
- 40.Hacquard-Bouder C, Falgarone G, Bosquet A, et al. Defective costimulatory function is a striking feature of antigen-presenting cells in an HLA-B27-transgenic rat model of spondylarthropathy. Arthritis Rheum. 2004;50:1624–35. doi: 10.1002/art.20211. [DOI] [PubMed] [Google Scholar]
- 41.Stagg AJ, Breban M, Hammer RE, et al. Defective dendritic cell (DC) function in a HLA-B27 transgenic rat model of spondyloarthropathy (SpA) Adv Exp Med Biol. 1995;378:557–9. doi: 10.1007/978-1-4615-1971-3_125. [DOI] [PubMed] [Google Scholar]
- 42.Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin Immunol. 2004;16:99–106. doi: 10.1016/j.smim.2003.12.004. [DOI] [PubMed] [Google Scholar]