Abstract
Strokes due to transmural vasculitis associated with coccidioidal meningitis result in significant morbidity and mortality. The immunological and inflammatory processes responsible are poorly understood. To determine the inflammatory mediators, i.e. cytokines, chemokines, iNOS, matrix metalloproteinase-9 (MMP-9), that possibly contribute to vasculitis, temporal mRNA expression in brain basilar artery samples and MMP-9 protein in the CSF of male NZW rabbits infected intracisternally with 6·5 × 104 arthroconidia of Coccidioides immitis were assessed. Five infected and 3 sham-injected rabbits at each time point were euthanized 4, 9, 14 and 20 days post infection. All infected rabbits had neurological abnormalities and severe vasculitis in the basilar arteries on days 9–20. In basilar arteries of infected animals versus controls, mRNAs encoding for IL-6, iNOS, IFN-γ, IL-2, MCP-1, IL-1β, IL-10, TNF-α, CCR-1, MMP-9, TGF-β, as well as MMP-9 protein in CSF, were found to be significantly up-regulated. Thus, this study identified inflammatory mediators associated with CNS vasculitis and meningitis due to C. immitis infection. Assessment of the individual contribution of each mediator to vasculitis may offer novel approaches to the treatment of coccidioidal CNS infection. This study also provides unique methodology for immunology studies in a rabbit model.
Keywords: coccidioidomycosis, meningitis, vasculitis, inflammatory mediators, mRNA
Introduction
Infarcts in the central nervous system (CNS) parenchyma are common complications of coccidioidal meningitis in humans [1–3] and in animal models [4–6] and can result in significant morbidity and mortality. The underlying immunological mechanisms responsible are poorly understood but molecules known to be associated with immune response in systemic mycoses, especially those associated with Th-1 responses, are probably involved [1,7]. Several pro-inflammatory cytokines, chemokines, matrix metalloproteinases (MMPs) and molecules associated with nitric oxide production are up-regulated in models of viral and bacterial meningitis as well as other pathological processes affecting the CNS [8–12]. Furthermore, activated MMPs, particularly MMP-9, have been implicated in the pathogenesis of ischaemic brain injury associated with vasculitis in bacterial meningitis [13–16]. The pathogenic mechanisms leading to vasculitis, vasospasm and thrombosis of vessel lumens are not fully understood. In humans with CNS vasculitis associated with bacterial meningitis, compression of vessels, subintimal infiltration of inflammatory cells and loss of autoregulation appear to lead to neuronal injury and infarction are observed [17,18].
To identify pro-inflammatory cytokines, chemokines, nitric oxide derived from inducible nitric oxide synthetase (iNOS) and MMP-9 (termed inflammation-mediating molecules, IMMs) that might contribute to the development of vasculitis in a rabbit model of coccidioidal meningitis, we evaluated the temporal production of the mRNA of these IMMs in brain basilar artery (BA) samples of Coccidioides immitis (CI)-infected male New Zealand White rabbits. The use of the rabbit model allowed the isolation of these relatively large vessels for this analysis. It was previously demonstrated that the brain BA develops arteritis following intracisternal administration of 5·0−5·4 × 104 arthroconidia [4,6]. To isolate the brain BAs, we removed as much of the leptomeningeal inflammatory exudate on their outer surfaces as possible using careful dissection and irrigation. We then used reverse-transcription PCR-amplification to test for IMM mRNA production in BA samples at defined time points after infection. We also evaluated the temporal production of MMP-9 protein in the CSF over the same 20-day period using zymography. The findings indicate potential involvement of numerous IMMs in the pathogenesis of BA vasculitis in CI meningitis.
Materials and methods
Use of research animals
The animal studies presented in this manuscript were conducted according to the guidelines of the Institutional Animal Care and Use Committee of the California Institute for Medical Research.
Disease induction
Non-immunosuppressed New Zealand White rabbits (Myrtle's Rabbitry, Thompson Station, TN, USA) were inoculated with 6·5 × 104 arthroconidia (infected) or saline (sham infection control) administered intracisternally on day 0 as described previously [6]. Five groups of animals were established according to day of euthanasia after inoculation. Group 1 consisted of 5 control animals euthanized on day 0. Groups 2, 3, 4 and 5 consisted of 3 control animals and 5 infected animals euthanized on days 4, 9, 14 and 20, respectively. Rabbits were assessed daily for fever, weight and neurological abnormalities, including paresis, gait disturbance and abnormal mobility and posture [6].
Sample acquisition
At the experimental completion day for each group, rabbits were euthanized via intravenous injection of a concentrated pentobarbital solution as described previously [6]. Prior to euthanasia, and while under a surgical plane of inhalation anaesthesia, CSF was collected from each animal and evaluated for WBC count, protein, lactic acid and MMP-9 concentration by zymography, as previously described [6,19]. After euthanasia, brain and proximal spinal cord were harvested and processed for histological assessment and quantitative fungal cultures, as previously described [6].
Brain basilar arteries were removed and washed immediately after necropsy. Tissues were divided and one half of each sample was placed into 10% formalin for histopathological evaluation. The other half was quick-frozen on dry ice and stored at −80 °C until it was processed for RNA extraction and purification as described below.
Molecular methods
Due to the lack of reagents or published protocols available to perform immunology studies in the rabbit, all RT-PCR tests in this study were developed in-house using published rabbit gene sequences and Oligo 5·0 primer design software (Molecular Biology Insights, Inc., Cascade, CO, USA). Table 1 shows the primer sequences, annealing temperatures and PCR product sizes for each IMM mRNA tested. The specificity of each amplicon was verified by direct sequencing (data not shown).
Table 1.
RT-PCR Primers used for IMM mRNA Assessment.
| IMM | Sequence (5′– 3′) | Annealing temp (°C) | PCR product size (base pairs) |
|---|---|---|---|
| GAPDH | TCA CCA TCT TCC AGG AGC GA | 59 | 293 |
| CAC AAT GCC GAA GTG GTC GT | |||
| iNOS | CTG CTT TGT GCG GAG TG | 60 | 174 |
| CAC CCG AAC ACC AGG AT | |||
| MMP-9 | GCA GGG TAG GGG GTA TGG A | 60 | 630 |
| ACA GGG CTT GGC TTT GGA | |||
| IL-2 | TGC TGG ATT TAC AGG TGC TTT TGA | 55 | 197 |
| GGT ATT TCC CCC ATG AGA GTT TTT G | |||
| IFN-γ | ATC TTG GGT TCT TAC GGC TGT T | 56 | 363 |
| TTC ACT TAC TGC TTT ACG CTG GAC | |||
| TNF-α | GGC TCA GAA TCA GAC CTC AG | 57 | 297 |
| GCT CCA CAT TGC AGA GAA GA | |||
| IL-1β | TCC AGC TGC GCA TCT CCT GC | 58 | 354 |
| CTT CTC CTT GCA CAA AAC TC | |||
| IL-6 | GCT CCT GGT GGT GGC TAC | 58 | 449 |
| GGG TGG CTT CTT CAT TCA AA | |||
| IL-10 | CTA TGT TGC CTG GTC TTC CTG | 60 | 397 |
| CTG CCT TGC TCT TGT TTT CAC | |||
| CCR-1 | ATC GTC ACC AGC ATC GTC A | 57 | 189 |
| CAG CAG GGG CAG AAC AAG | |||
| MCP-1 | CCA GCC AGA TGC CGT GAA T | 56 | 155 |
| AGA TCC CCT TGG CCA GTT TG | |||
| TGF- β | CGG CAG CTG TAC ATT GAC TTC C | 62 | 271 |
| GCG CAC GAT CAT GTT GGA C | |||
| PDGF- β | GAC TAC CTG CAC CGC AAC A | 60 | 153 |
| CAT GTA GCC GCC GTC ACT |
Total RNA was extracted from BA samples using mechanical homogenization of thawed tissue and purified with the mini-RNA extraction kit of Qiagen, Inc (Valencia, CA, USA). Purified RNA was stored as single use (2 µl) aliquots at −80 °C until RT-PCR assays were performed.
RT-PCR reactions were performed on all rabbits’ mRNA at the same time for each individual IMM tested. Assays were in a total volume of 25 µl using the One-Step RT-PCR kit of Qiagen and 0·10 µM of each IMM-specific primer set (forward and reverse primers). Thirty cycles of amplification were performed on all primer sets, except the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control set (22 cycles) in a PE Biosystems GeneAmp 9700 thermocycler. Cycle parameters for all assays were 50 °C for 30 min (RT step), 95 °C for 15 min, 30 (or 22) cycles of 95 °C for 30 s, specific annealing temperature for 30 s and 72 °C for 10 s and the last cycle was followed by 10 min at 72 °C. Under these conditions, we were able to reproducibly demonstrate a linear correlation between amplicons produced and cycle number as well as with increasing amounts of RNA added (data not shown).
Assessment of MMP-9 in CSF
MMP-9 in the CSF was quantified by electrophoretic gelatin zymography as described previously [19].
Statistical analysis
The overall normality of the RT-PCR data for each group was assessed using the Shapiro-Wilk test [20]. For those differences between control and infected groups for each day postinfection, the Kruskal–Wallis nonparametric test was utilized for continuous factors and the χ2 test for categorical data (i.e. paresis and gait performance). The Spearman rank-correlation coefficient was used for the correlations. P-values ≤ 0·05 were considered significant.
Results
Clinical and neurological abnormalities
All infected rabbits showed intermittent fever and weight loss starting on day 1 postinfection. In addition, starting on day 9 postinfection, infected rabbits showed increased paresis, gait disturbance, posturing and decreased mobility as compared to control animals.
Histopathological findings
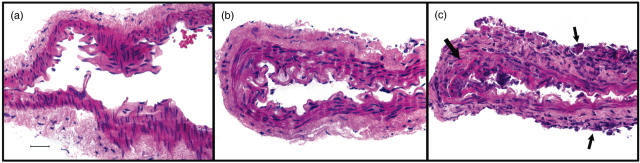
Representative histopathological findings for normal rabbit basilar arteries and arteries with mild and severe meningitis are shown in Fig. 1. Brains and spinal cords were histologically normal in six controls. Mild nonspecific findings, i.e. parenchymal ischaemia or minimal meningitis without granulomas or evidence of fungal infection and attributable to the normal saline injections were present in brain or spinal cord sections of five of the controls (two from day 14 postinfection and one each from the other time points). Eight of nine basilar artery samples from controls that were analysed separately were entirely normal (Fig. 1a). Minimal meningitis was present in the adventitia of one of the control samples from Day 14. Mild or moderate meningitis was present in the brain, spinal cord and separate basilar artery samples of five infected rabbits on Day 4 postinfection (Fig. 1b). All 15 of the infected rabbits sampled on days 9, 14 and 20 had severe granulomatous meningitis and arteritis in brain and spinal cord samples and arteritis in separately analysed basilar artery segments. In these segments, there were variable patterns, i.e. either focal fibrinoid necrosis associated with adherent adventitial inflammation or complete circumferential fibrinoid necrosis associated with more marked adventitial mononuclear cell accumulations. Neutrophils and karyorrhectic cells were also seen in some of the vessel walls (Fig. 1c). None of these samples included adjacent coccidioidal organisms. Because of the variability and the small sizes of the samples, no temporal progression of inflammatory cell subset infiltration patterns could be identified.
Fig. 1.
Histopathological findings in rabbit basilar arteries. (a) Normal histologic appearance of isolated basilar artery from a control rabbit. (b) Isolated basilar artery from a rabbit with mild meningitis on Day 4 after infection. There is minimal inflammation in the adventitia. Small numbers of inflammatory cells adhere to the intimal surfaces. The muscularis and intima are intact. (c) Isolated basilar artery from a rabbit with severe meningitis on Day 9 of infection. Mononuclear inflammatory cells and neutrophils accumulate on the adventitia (small arrows) and infiltrate all layers of the artery wall. There is focal disruption of the internal elastic lamina and fibrin deposition (large arrow). Inflammatory cells accumulate in the adjacent lumen. Original magnifications for all figures are ×80. Bar in (a) = 50 microns.
Spinal fluid characteristics of control and infected rabbits
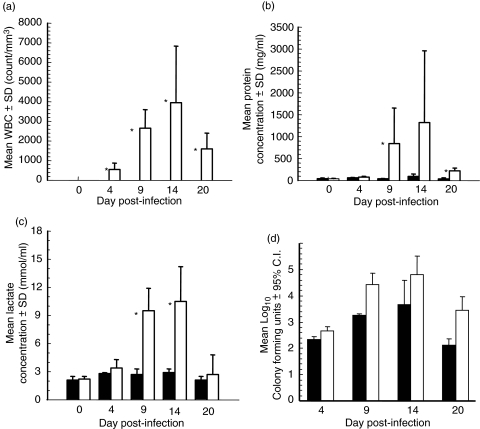
Figure 2 shows the results of assays performed on CSF collected at necropsy. The total WBC count (Fig. 2a) shows a significant increase (P < 0·05) in the infected rabbits starting at day 4 postinjection and continuing to day 20, with the highest numbers of WBC noted on day 14. None of the control animals showed an elevated WBC count in the CSF. The levels of both protein (Fig. 2b) and lactate (Fig. 2c) were significantly elevated (P < 0·05 and P < 0·03 for protein and lactate, respectively) in the infected animals as compared to controls on days 9 and 20 postinfection. Protein levels on day 20 remained significantly (P < 0·03) higher in the infected group, although at a much lower overall level as compared to days 9 and 14. Both protein and lactate analyses revealed peak levels at day 14 postinfection.
Fig. 2.
Spinal fluid characteristics of control and infected rabbits. All rabbits were tested for (a) WBC count, (b) protein and (c) lactate at necropsy. *P < 0·05 infected versus control rabbits using Kruskal–Wallis nonparametric anova. ▪ Control animals (n = 3 per time point); □ Infected animals (n = 5 per time point). (d) Mean C. immitis colony forming units in the brain (▪) and spinal cord (□) of infected rabbits (n = 5). All rabbit brain and cord tissue were cultured for C. immitis at necropsy.
Fungal cultures in brain and spinal cord tissues
The results of quantitative tissue fungal cultures for C. immitis are shown in Fig. 2d. Both brain and spinal cord tissues from infected animals grew C. immitis on all days postinfection, with peak numbers of CFU noted on day 14 for both tissue sources. No C. immitis was recovered from the brains or spinal cords of control rabbits at any day postinfection.
Temporal assessment of mRNA for IMMs in brain basilar arteries
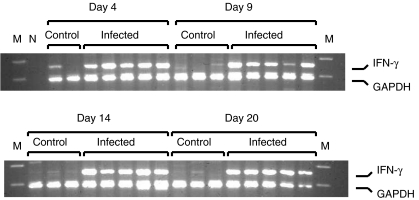
Figure 3 shows an example of an ethidium bromide-stained agarose gel after separation of the RT-PCR products for interferon-γ and the GAPDH internal control. The relative level of each IMM RT-PCR product was calculated as a fraction of the constitutively expressed GAPDH control RT-PCR product after densitometric analysis. The reagent blank (water) RT-PCR displayed no PCR products, as expected.
Fig. 3.
Agarose gel electrophoresis of RT-PCR products for GAPDH and IFN-γ. Ten microliters of each PCR reaction were separated on 3% NuSieve agarose in 1× TBE buffer for 3 h at 100 V. Gels were stained with ethidium bromide. Gels were photographed with Polaroid 665 film and negatives were processed for densitometric analysis. Rabbit groups, days postinfection and specific RT-PCR products are indicated on the figure. M, Molecular weight markers; N, Negative control PCR (reagent blank).
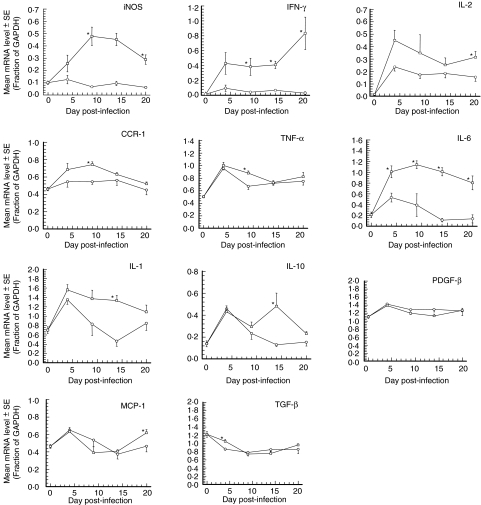
Temporal analysis of the mRNA levels for 12 IMMs in brain BA samples of all rabbit groups is shown in Fig. 4. Many of the IMM mRNAs show an up-regulation in the control groups, likely as a result of the sham infection protocol alone as observed in a previous study in the same model [19]. With the exception of PDGF-β, which shows no difference in mRNA levels between control and infected animals at any time, all IMM mRNAs from infected rabbits display significantly higher levels than the corresponding control animals on at least one time point postinfection. The most significantly up-regulated IMM mRNA was IL-6, which displayed higher mRNA levels in infected rabbits at all days postinfection. Levels of iNOS and IFN-γ mRNAs were significantly elevated in infected animals on days 9, 14 and 20 postinfection. All remaining IMMs display elevated mRNA levels at only a single day postinfection as compared to controls. Significant IMM up-regulation in brain BA samples (mRNA) or CSF (MMP-9) is summarized in Table 2.
Fig. 4.
Mean IMM mRNA levels in the basilar arteries of control and infected rabbits. All rabbit basilar arteries were tested for IMM mRNA levels by qualitative RT-PCR. Each IMM mRNA level is expressed as a fraction of the constitutively expressed GAPDH control level. Each specific IMM is indicated on the figure. *P < 0·05 Infected versus control rabbits using Kruskal–Wallis nonparametric test. ○ Control animals (n = 3); □ Infected animals (n = 5).
Table 2.
Summary of IMM mRNA or protein up-regulation in the brain basilar artery or CSF.
| Time post-infection (days) | ||||
|---|---|---|---|---|
| IMMs | 4 | 9 | 14 | 20 |
| IL-6 mRNA | + | + | + | + |
| iNOS, IFN-γ mRNA | + | + | + | |
| IL-2, MCP-1 mRNA | + | |||
| IL-1β, IL-10 mRNA | + | |||
| TNF-α, CCR-1, MMP-9 mRNA | + | |||
| TGF-β mRNA | + | |||
| CSF MMP-9 Protein | + | + | + | |
P < 0·05/day compared to controls using Kruskal–Wallis nonparametric test.
MMP-9 mRNA in brain basilar arteries and MMP-9 concentrations in CSF
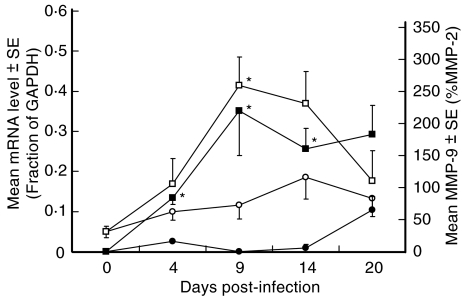
Brain BA MMP-9 mRNA levels and CSF concentrations of MMP-9 as measured by zymography are shown in Fig. 5. Control animals displayed relatively low levels of both MMP-9 mRNA and MMP-9 protein up to day 14. In contrast, infected rabbits showed a marked increase in MMP-9 mRNA starting at day 4 postinfection and continuing to day 14, and in MMP-9 protein starting at day 4 postinfection and continuing to day 20. MMP-9 protein levels in infected animals were significantly (P < 0·05) higher than controls on days 4, 9 and 14. Although day 20 MMP-9 protein levels remained higher in infected animals than in controls, they failed to reach statistical significance due to an increase in MMP-9 protein levels in the control group on this day. MMP-9 mRNA levels in brain BA generally paralleled MMP-9 protein levels in the CSF, but significantly (P < 0·05) higher levels of MMP-9 mRNA in infected animals versus controls was only observed on day 9. The kinetics of MMP-9 protein shown in Fig. 5 is distinct from that of the CSF total protein levels shown in Fig. 2b. It is therefore unlikely that the observed increase in MMP-9 protein in this study is simply related to the increase in total protein levels.
Fig. 5.
Mean MMP-9 mRNA levels in the basilar arteries and MMP-9 in CSF of control and infected rabbits. All rabbit basilar arteries were tested for MMP-9 mRNA levels by qualitative RT-PCR. Each MMP-9 mRNA level is expressed as a fraction of the constitutively expressed GAPDH control level. MMP-9 protein was tested in the CSF of all rabbits by zymography and is expressed as a percentage of MMP-2. *P < 0·05 Infected versus control rabbits using Kruskal–Wallis nonparametric test. MMP-9 protein in CSF, control animals (•, n = 3) and infected animals (▪, n = 5); MMP-9 mRNA, control animals (○, n = 3) and infected animals (□, n = 5).
Comparison of the MMP-9 mRNA data in the BA samples and CSF MMP-9 in individual rabbits demonstrated a high degree of correlation (r = 0·78, P < 0·001) between the two assays (data not shown).
Discussion
Several IMM mRNAs were up-regulated in BA samples from rabbits with CNS coccidioidomycosis. This study was designed to assess the temporal profile of IMM mRNA expression over three weeks of infection. Such studies [21] demonstrate the utility of RT-PCR in mapping the immune response in fungal CNS infections, and enable comparisons with other pathogens. Because of the relatively low numbers of animals examined at individual time points, the power of the statistical analysis at each time point was lessened and it is possible that biologically significant up-regulation may have been underestimated. Furthermore, due to the inclusion of adherent fibrinous inflammatory exudate on the surface of the BA in the mRNA expression analysis, the data generated may represent a composite of signals from both the wall of the artery and from the fibrinous exudate on the surface. Thus, both pathomorphologic correlates of vasculitis, the vessel and the adjacent inflammation, were included in the mRNA analysis. It should also be noted that although several IMM mRNAs (notably IL-6, iNOS and interferon-γ) were increased on more days than other IMM mRNAs (IL-1β, IL-2, TNF- α), it does not necessarily follow that they contribute more to vasculitis development. Nevertheless, the present results indicate the likely involvement of specific IMMs in the pathogenesis of vasculitis in this model over the course of the infection.
We found that iNOS mRNA was dramatically up-regulated over the course of the infection. Nitric oxide is a free radical that is constitutively expressed throughout the CSF under normal physiological conditions. When produced in high quantity via stimulation of iNOS in inflammatory states, it exhibits both potentially deleterious effects due to direct cytotoxicity and proinflammatory action and potential beneficial effects due to prevention of ischaemia by its vasodilatatory effect [22–25]. The nitric oxide metabolite peroxynitrate can directly damage DNA within the CNS via tyrosine nitration, resulting in lipid peroxidation associated with increased intracerebral pressure, BBB disruption and increased CSF leucocyte extravasation [23].
Evidence for a beneficial role of NO at the level of the cerebral vasculature during meningitis is provided by studies in rodent models of bacterial meningitis, where both the lack of endothelial nitric oxide synthetase and inhibition of the iNOS significantly aggravated the disease and increased the extent of neuronal injury in the brain, respectively [26,27]. These results suggest that NO produced in the vasculature has a protective effect against ischaemia in vasculitis by counteracting the decline in cerebral blood flow under the influence of vasoconstrictive factors. Thus, attempts to down-regulate NO production during meningitis are potentially dangerous, despite evidence that NO contributes to some of the potentially harmful changes such as CSF inflammation, brain oedema and intracranial pressure.
Messenger RNA encoding the pro-inflammatory interleukins IL-1β, IL-2 and IL-6 were up-regulated on different days of infection. These interleukins are also up-regulated in CNS trauma, ischaemia, atherosclerosis, lupus and infections in both humans and animals [28–32]. Their effects include enhancement of endothelial cell adhesion molecule expression, thereby facilitating WBC trafficking into the CNS [29] and induction of other pro-inflammatory IMMs, including iNOS, IL-8, IL-6, MCP-1, TNF-α and IFN-γ[28–30]. In particular, IL-2 appears to be important in the development of Th-1 immune responses that are critical to controlling fungal infections [33,34]. Transgenic mice that over-express IL-6 have brain perivasculitis [8]. CSF IL-1β levels are closely correlated with clinical symptoms and WBC counts in patients with C. immitis meningitis [35].
TNF-α, the mRNA of which was significantly up-regulated on day 5 postinfection, is another proinflammatory cytokine produced in response to ischaemic injury and infection. It also up-regulates endothelial cell adhesion molecules [30] and when directly administered into the CSF, elicits perivascular oedema, increased leucocyte adhesion and extravasation and vascular damage [36]. It has previously been shown in a rat model of pneumococcal meningitis that TNF-α modulates the expression and regulation of MMPs. In return, MMPs and related metalloproteinases can act as sheddases or convertases as they transform membrane-bound cytokines, cytokine receptors and adhesion molecules to their soluble forms [13].
IFN-γ mRNA progressively increased over the course of the infection. This Th1-associated cytokine also enhances adhesion molecule expression [30] and is crucial in immune responses to C. immitis infection [37] and other fungal infections [7]. IFN-γ also stimulates other IMMs including IL-12, MCP-1, RANTES and TNF-α[28,38] and may contribute to infiltration of inflammatory cells into the brain [39]. Interferon-γ is a key cytokine in giant cell arteritis [40] but its specific role in C. immitis-associated vasculitis is presently unclear.
The mRNA encoding TGF-β was also up-regulated in our study on day 4 postinfection and is a multifunctional cytokine shown to be both stimulatory and anti-inflammatory under various conditions [28,41,42]. Weyand et al. [43] have shown TGF-β to be a proinflammatory effector molecule related to its ability to induce expression of adhesion molecules and affect chemotaxis of mononuclear cells in temporal arteritis.
Previously, elevated levels of MMP-9 protein were demonstrated over the course of experimental C. immitis meningitis [19]. We studied MMP-9 protein in CSF to correlate with our mRNA studies, and confirm the earlier results in the CSF and demonstrate up-regulation of MMP-9 mRNA in the BAs. MMPs appear to play a central role in the development of brain injury in meningitis. MMP-9 protein is up-regulated in experimental pneumococcal meningitis and it has also been implicated in BBB disruption leading to brain oedema and facilitation of inflammatory cell extravasion and is a potential risk factor for the development of CNS parenchymal damage [13–15]. We observed a significant correlation between the temporal expression of MMP-9 protein in the CSF and MMP-9 mRNA in the brain BA (Fig. 5). It may be possible to monitor CSF levels in patients and be forewarned of an impending vasculitis-induced stroke when a significant elevation from baseline is observed. This would allow for pre-emptive preventative measures when these treatments become available. We also observed slight elevations at different time points of mRNA for the chemokine MCP-1 and the chemokine receptor CCR-1, suggesting possible roles for these molecules in the vasculitis.
With the possible exception of the Th-2 cytokine IL-10 (the mRNA of which was up-regulated on day 14 only) and TGF-β (the mRNA of which was slightly up-regulated on day 4), all of the IMM mRNAs discussed above could contribute to the complex molecular cascades that result in the development of vasculitis associated with C. immitis meningitis. We do not observe direct invasion of the BA walls by the fungus and we have not observed deposition of complement or immune complexes in the vessel walls (unpublished data). Therefore, the vasculitis appears to be secondary to the proximity of organisms and a florid inflammatory response in the subarachnoid space, rather than requiring direct invasion of the organisms into the wall. The IMM mRNAs within the BA walls and immediately surrounding leptomeninges likely promote the transmural inflammatory cell infiltration and fibrinoid necrosis of arterial walls characteristic of vasculitis.
In summary, this study represents a significant advance in knowledge with regards to inflammatory markers in the lagomorph. To our knowledge, this is the only model of a consistently reproducible CNS vasculitis induced by infection. The present findings represent an insight into which IMM mRNAs may contribute to CNS vasculitis associated with experimental C. immitis infection. Moreover, the methodology and reagent specifications used in this study importantly enhance the capabilities of investigators to perform any future immunologic studies in rabbits, as there has been a paucity of resources for this species. The results could facilitate future research efforts using gene knock-out mice [5], as transgenic animals are available in this model, allowing determination of which IMM may be critical for vasculitis development. Although multiple IMMs likely participate in the pathogenesis of vasculitis, it may be possible to inhibit specific vasculitis-associated IMMs, e.g. using blocking agents or antibodies, without inducing broader immunosuppression. This targeted approach to specific immune inhibition may lead to new treatments of vasculitis associated with C. immitis meningitis.
Acknowledgments
This work was supported by Community Outreach Funds; granting agency Kaweah Delta Health Care District, Visalia, California 93291. Additional funding was granted by the Swiss National Science Foundation (632–066057); the Bank of Stockton, Stockton, California; The Valley Foundation, Los Gatos, California; and Children's Hospital Central California, Madera, California.
References
- 1.Williams PL. Vasculitic complications associated with coccidioidal meningitis. Sem Resp Infect. 2001;16:270–9. doi: 10.1053/srin.2001.29319. [DOI] [PubMed] [Google Scholar]
- 2.Williams PL, Johnson R, Pappagianis D, et al. Vasculitic and encephalitic complications associated with Coccidioides immitis infection of the central nervous system in humans: report of 10 cases and review. Clin Infect Dis. 1992;14:673–82. doi: 10.1093/clinids/14.3.673. [DOI] [PubMed] [Google Scholar]
- 3.Sobel RA, Ellis WG, Nielsen SL, Davis RL. Central nervous system coccidioidomycosis: a clinicopathologic study of treatment with and without amphotericin B. Hum Pathol. 1984;15:890–5. doi: 10.1016/s0046-8177(84)80128-8. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen KN, Sobel RA, Clemons KV, et al. Comparative efficacies of terbinafine and fluconazole in treatment of experimental coccidioidal meningitis in a rabbit model. Antimicrob Agents Chemother. 2000;44:3087–91. doi: 10.1128/aac.44.11.3087-3091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamberi P, Sobel RA, Clemons KV, Stevens DA, Pappagianis D, Williams PL. A murine model of coccodioidal meningitis. J Infect Dis. 2003;187:453–60. doi: 10.1086/367961. [DOI] [PubMed] [Google Scholar]
- 6.Williams PL, Sobel RA, Sorensen KN, et al. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J Infect Dis. 1998;178:1217–21. doi: 10.1086/515689. [DOI] [PubMed] [Google Scholar]
- 7.Clemons KV, Stevens DA. Overview of host defence mechanisms in systemic mycoses and the basis for immunotherapy. Sem Resp Infect. 2001;16:60–6. doi: 10.1053/srin.2001.22729. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis PD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157:647–58. doi: 10.1016/S0002-9440(10)64575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus CM, Liu JS, Hahn MT, Hua LL, Brosnan CF, Berman JW, Lee SC. Differential induction of chemokines in human microglia by type I and II interferons. Glia. 2000;29:273–80. [PubMed] [Google Scholar]
- 11.Pelidou SH, Kostulas N, Matusevicius D, Kivisakk P, Kostulas V, Link H. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–42. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Fujii S, Tominaga T, Yoshimoto T, Fujii S, Akaike A, Maeda T, Yoshimura T. Direct evidence of in vivo nitric oxide production and inducible nitric oxide synthase mRNA expression in the brain of living rat during experimental meningitis. J Cereb Blood Flow Metab. 1999;19:1175–8. doi: 10.1097/00004647-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, Tauber MG, Leppert D. Inhibition of matrix metalloproteinases and tumor necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain. 2001;124:1734–42. doi: 10.1093/brain/124.9.1734. [DOI] [PubMed] [Google Scholar]
- 14.Leib SL, Leppert D, Clements JM, Tauber MG. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun. 2000;68:615–20. doi: 10.1128/iai.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood–brain barrier damage and neurological sequelae. Clin Infect Dis. 2000;31:80–4. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- 16.Meli DN, Christen S, Leib SL. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J Infect Dis. 2003;187:1411–5. doi: 10.1086/374644. [DOI] [PubMed] [Google Scholar]
- 17.Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 2002;25:38–45. doi: 10.1016/s0166-2236(00)02024-5. [DOI] [PubMed] [Google Scholar]
- 18.Pfister HW, Borasio GD, Dirnagl U, Bauer M, Einhaupl KM. Cerebrovascular complications of bacterial meningitis in adults. Neurol. 1992;42:1497–504. doi: 10.1212/wnl.42.8.1497. [DOI] [PubMed] [Google Scholar]
- 19.Williams PL, Leib SL, Kamberi P, Leppert D, Sobel RA, Bifrare YD, Clemons KV, Stevens DA. Levels of matrix metalloproteinase-9 within cerebrospinal fluid in a rabbit model of coccidioidal meningitis and vasculitis. J Infect Dis. 2002;186:1692–5. doi: 10.1086/345365. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrica. 1965;52:591–611. [Google Scholar]
- 21.Maffei CML, Mirels LF, Sobel RA, Clemons KV, Stevens DA. Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningitis. Infect Immun. 2004;72:2338–49. doi: 10.1128/IAI.72.4.2338-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–64. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastenbauer S, Koedel U, Pfister HW. Role of peroxynitrite as a mediator of pathophysiological alterations in experimental pneumococcal meningitis. J Infect Dis. 1999;180:1164–70. doi: 10.1086/315048. [DOI] [PubMed] [Google Scholar]
- 24.Luvara G, Pueo ME, Philippe M, Mandet C, Savoie F, Henrion D, Michel JB. Chronic blockade of NO synthase activity induces a proinflammatory phenotype in the arterial wall: prevention by angiotensin II antagonism. Arteriosclerosis Thrombosis Vasc Path. 1998;18:1408–16. doi: 10.1161/01.atv.18.9.1408. [DOI] [PubMed] [Google Scholar]
- 25.Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm. 2000;59:5981–9. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- 26.Koedel U, Paul R, Winkler F, Kastenbauer S, Huang PL, Pfister HW. Lack of endothelial nitric oxide synthase aggravates murine pneumococcal meningitis. J Neuropathol Exp Neurol. 2001;60:1041–50. doi: 10.1093/jnen/60.11.1041. [DOI] [PubMed] [Google Scholar]
- 27.Leib SL, Kim YS, Black SM, Tureen JH, Tauber MG. Inducible nitric oxide synthetase and the effect of aminoguanidine in experimental neonatal meningitis. J Infect Dis. 1998;177:692–700. doi: 10.1086/514226. [DOI] [PubMed] [Google Scholar]
- 28.Benveniste EN. Cytokines: influence on glial cells gene expression and function. In: Blalock JE, editor. Neuroimmunoendocrinology. 3. Basel: S. Karger; 1997. pp. 31–75. [DOI] [PubMed] [Google Scholar]
- 29.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanimirovic D, Satch K. Inflammatory mediators of cerebral endothelium and its role in ischemic brain inflammation. Brain Pathol. 2000;10:113–26. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 32.Yang GY, Mao Y, Zhou LF, Ye W, Liu XH, Gong C, Lorris Betz A. Attenuation of temporary focal cerebral ischemic injury in the mouse following transfection with interleukin-1 receptor antagonist. Mol Brain Res. 1999;72:129–37. doi: 10.1016/s0169-328x(99)00205-3. [DOI] [PubMed] [Google Scholar]
- 33.Kolitz JE, Mertelsman R. The immunotherapy of human cancer with interleukin-2: present status and future directions. Cancer Invest. 1991;9:529–42. doi: 10.3109/07357909109018951. [DOI] [PubMed] [Google Scholar]
- 34.Tham EL, Shrikant P, Mescher MF. Activation-induced nonresponsiveness. a Th-dependent regulatory checkpoint in the CTL response. J Immunol. 2002;168:1190–7. doi: 10.4049/jimmunol.168.3.1190. [DOI] [PubMed] [Google Scholar]
- 35.Ampel NM, Ahmann DM, Delgado KL, Galgiani JN, Cloud GA. Tumor necrosis factor-alpha and interleukin-1 beta in cerebrospinal fluid of patients with coccidioidal meningitis during therapy with fluconazole. J Infect Dis. 1995;171:1675–8. doi: 10.1093/infdis/171.6.1675. [DOI] [PubMed] [Google Scholar]
- 36.Kumar K. Proinflammatory cytokines in cerebrovascular ischemia. Curr Opin Invest Drugs. 2001;2:1748–50. [PubMed] [Google Scholar]
- 37.Beaman L. Effects of recombinant gamma interferon and tumor necrosis factor on in vitro interactions of human mononuclear phagocytes with Coccidioides immitis. Infect Immun. 1991;59:4227–9. doi: 10.1128/iai.59.11.4227-4229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Häusler KG, Prinz M, Nolte C, Weber JR, Schumann RR, Kettenmann H, Hanisch UK. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide and pneumococcal cell wall-stimulated mouse microglia and macrophages. European J Neurosci. 2002;16:2113–22. doi: 10.1046/j.1460-9568.2002.02287.x. [DOI] [PubMed] [Google Scholar]
- 39.Popko B, Corbin JG, Baerwald KD, Dupree J, Garcia AM. The effects of interferon ã on the central nervous system. Mol Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–9. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 41.Ashcroft GS. Bidirectional regulation of macrophage and function by TGF-β. Microbe Infect. 1999;1:1275–82. doi: 10.1016/s1286-4579(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 42.Lodge PA, Sriram S. Regulation of microglia activation by TGF-β, IL-10 and CSF-1. J Leukoc Biol. 1996;60:502–8. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- 43.Weyand CM, Wanger AD, Bjornsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–9. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]