Abstract
Whereas the involvement of Th1- and Th2-type cytokines in contact allergy to nickel (Ni) is well documented, the role of the regulatory cytokine IL-10 is less clear. We therefore investigated the impact of IL-10 on Ni-induced Th1- (IFN-γ) and Th2-type (IL-4 and IL-13) cytokine responses in human peripheral blood mononuclear cells (PBMC). PBMC from 15 blood donors with reactivity to Ni (Ni-PBMC) and 8 control donors devoid of reactivity (control PBMC) were stimulated with Ni and the frequency of cytokine-producing cells and the levels of secreted cytokines were analysed by ELISpot (IL-4, IL-13 and IFN-γ) and ELISA (IL-10, IL-13 and IFN-γ), respectively. The Ni-induced response was further assessed in the presence of recombinant IL-10 (rIL-10) or neutralizing antibody to IL-10 and the phenotype of the Ni-specific cytokine-producing cells regulated by IL-10 was determined by cell depletion experiments. Ni induced IL-10 production in Ni-PBMC (mean, (range); 33·1 pg/ml (0–93·4 pg/ml)) but not control PBMC (2·2 pg/ml (0–14·9 pg/ml)) (P = 0·002). Ni also induced significant production of IL-4, IL-13 and IFN-γ that correlated with the IL-10 response. Addition of rIL-10 down-regulated the Ni-induced production of all cytokines but with a more pronounced effect on IFN-γ. However, neutralization of Ni-induced IL-10 enhanced the levels of IFN-γ induced by Ni (P = 0·004) but did not affect the number of IFN-γ-producing cells or the production of other cytokines. Cell depletion experiments suggested that the Ni-specific IFN-γ (and Th2-type cytokine) producing cells were CD4+ T cells. The impact of IL-10 on Ni-induced IFN-γ responses by CD4+ T cells suggests that an important role of IL-10 in vivo is to counteract the allergic reactions mediated by Th1-type cytokines.
Keywords: contact allergy, IL-10, immunoregulation, nickel
Introduction
Allergic contact dermatitis (ACD) resulting from sensitization to Nickel (Ni) has been extensively reported [1–3]. The immunological (delayed-type hypersensitivity) response following re-exposure to Ni is mainly T-cell mediated [4–6]. Analyses of cytokine production by Ni-specific T cells have demonstrated a mixed Th1- and Th2-type cytokine profile in both T-cell clones and peripheral blood mononuclear cells (PBMC) [7–11]. Analyses of Ni-specific T-cell clones generated from PBMC and skin of allergic patients have suggested that both CD4+ and CD8+ T cells are involved in the immune response to Ni [12,13] whereas analysis of PBMC from Ni-allergic subjects have primarily identified Ni-specific CD4+ T cells [10,14,15]. We [16] and others [10] have reported production of significant levels of the immuno-regulatory cytokine IL-10 following Ni stimulation of PBMC from Ni-allergic subjects compared to healthy controls in vitro whereas another study [17] suggested Ni-induced IL-10 production primarily in PBMC from Ni tolerized subjects. Nevertheless, together these reports point to a possible role for IL-10 in the immunopathology of Ni-induced ACD, a question that has so far not been addressed.
Presently, the most widely accepted theory on the involvement of cytokine-producing T cells in ACD is that Th1-type responses mediate the allergic reaction whereas Th2-type responses are elicited to counterbalance the detrimental Th1-type responses [18]. IL-10, formerly described as a Th2-type cytokine [19] with regulatory effects on Th1-type cytokine production [20,21], has been shown to be produced in comparable amounts by other cell types than T cells including; monocytes, macrophages and dendritic cells [22,23]. IL-10 is thought to exert its immuno-modulatory effects by down-regulating the expression of costimulatory molecules required for appropriate antigen presentation [24]. Cavani et al.[25] demonstrated inhibition of dendritic cell-mediated stimulation of Ni-specific Th1-type T-cell clone responses by CD4+ T-regulatory cell clones derived from skin or blood by an IL-10 dependent mechanism. However, the effect of IL-10 on Ni-induced production of Th1- and Th2-type cytokines by ex vivo stimulated PBMC has not previously been assessed and may differ from mechanisms defined by clonally derived cells; a striking example of the difference between analyses of PBMC and clonal T cells is the fact that Ni-specific CD4+ T-cell clones can be derived from both allergic and nonallergic subjects whereas more recent studies of PBMC responses identified Ni-reactive CD4+ T cells in allergic subjects only [15].
In this study we aimed to investigate the impact of IL-10 on the induction of Th1- and Th2-type immune responses to Ni by assessment of in vitro cytokine responses of PBMC to Ni either by addition of exogenous human recombinant IL-10 (rIL-10) or a neutralizing monoclonal antibody (mAb) to human IL-10 (α-hIL-10 mAb). The phenotype of the Ni-specific cytokine producing cells was also investigated. Markedly reduced levels of Ni-induced Th1- (IFN-γ) and Th2-type (IL-4, IL-5, IL-13) cytokines were seen in the presence of rIL-10 and significantly enhanced detection of Ni-induced IFN-γ upon addition of α-hIL-10 mAb in PMBC cultures from donors with a reported history of ACD to Ni. This suggests an important down-regulatory effect on certain cytokines of IL-10 in Ni-induced ACD.
Materials and methods
Blood sample collection and processing
Buffy coats from regular blood donors were collected at the Karolinska University Hospital, Stockholm, Sweden. Each buffy coat was diluted four times in RPMI-1640 with 20 mM HEPES buffer from Gibco BRL (Life Technologies Ltd, Paisley, UK) and PBMC were isolated by Ficoll-paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. The PBMC were washed and resuspended in complete medium (RPMI-1640 with 20 mM HEPES, 2 mmol/l l-glutamine, 100 units/ml penicillin G and 100 µg/ml streptomycin sulphate) with 20% heat-inactivated fetal bovine serum (FBS) (all from Gibco BRL) and were frozen and stored as previously described [26]. Prior to testing, PBMC were thawed at 37°C, washed with medium twice and resuspended in complete medium with 10% heat-inactivated FBS. The viability of cells was confirmed by trypan blue exclusion (> 90% viable cells).
Definition of in vitro Ni-reactive and nonreactive PBMC
The intention of this study was to assess the impact of endogenous and exogenous IL-10 on the induction of other cytokines by Ni, using PBMC with or without in vitro reactivity to Ni. Prior to the experiments we therefore defined PBMC samples as in vitro reactive with Ni or not. To obtain sufficient amounts of PBMC for all experiments, buffy coats were used as a source of PBMC. The PBMC samples defined as Ni in vitro reactive (Ni-PBMC) were from blood donors with a reported history of ACD to Ni and displayed significant IL-4 and/or IL-13 responses to Ni measured in ELISpot using predefined cut off definitions [16]. Fifteen PBMC samples from 20 blood donors fulfilled these criteria and were used in the study. The PBMC samples defined as nonreactive with Ni in vitro (control PBMC) were selected from regular blood donors with unknown ACD status and the lack of any in vitro reactivity with Ni was confirmed as above. Eight of 10 PBMC samples fulfilled these criteria and were used for further analysis.
Enumeration of nickel-specific cytokine producing cells by cytokine ELISpot
ELISpot was performed essentially as described in [26], using polyvinylidene difluoride plates (ELIIP10SSP: Millipore Corp, Bedford, MA, USA) coated overnight with mAb 1-D1K (IFN-γ), 82·4 (IL-4) or IL13-I (IL-13) (Mabtech, Nacka Strand, Sweden). PBMC suspensions of 2·5 × 105 cells with or without 50 µM NiCl2 × 6H2O (Merck, Darmstadt, Germany) in 100 µl complete medium with 10% heat-inactivated FBS were added per well. As positive controls, 5 × 104 cells/well stimulated with phytohemagglutinin (PHA; 2 µg/ml; Orion Diagnostics, Trosa, Sweden) were used. Different concentrations of rIL-10 (0, 0·2, 1, 5, 25 ng/ml; R & D Systems, Minneapolis, MN, USA) or the neutralizing mAb 9D7 to human IL-10 (10 µg/ml; Mabtech) [27], were then added to the cultures. The plates were incubated at 37°C and 5% CO2 in humidified air for 44 h. After the incubation, the plates were washed in PBS and incubated with biotinylated detection mAbs (1 µg/ml in PBS/0·5%FBS); 7-B6-1 (IFN-γ), 12·1 (IL-4) and IL13-II (IL-13) (Mabtech) for 2 h at room temperature (RT). ELISpot assays were set up in triplicates. Detection of spots representing cytokine-producing cells was performed as described previously [26].
Assessment of levels of nickel-induced cytokine production by cytokine ELISA
In parallel with setting up the ELISpot, suspensions with the same corresponding cell fraction, stimuli and rIL-10 or α-hIL-10 mAb concentrations were set up in polystyrene round bottom tubes (BD, San Jose, CA, USA) at a volume of 2 ml. The cells were incubated at 37°C and 5% CO2 in humidified air for 44 h. Cell supernatants were collected by centrifugation and stored at −20°C prior to analysis by ELISA, performed as described previously [16]. MAb 9D7 (IL-10), IL13-I (IL-13) and 1-D1K (IFN-γ) were used for capture and biotinylated mAb 12G8 (IL-10), IL13-II (IL-13) and 7-B6-1 (IFN-γ) were used for detection (Mabtech). Standard curves were obtained using recombinant human IL-10, IL-13 (R & D Systems) or IFN-γ (Bender MedSystems GmbH, Vienna, Austria). The lowest detection limit in ELISA was 5 pg/ml.
Phenotyping of nickel-specific cytokine producing cells
Ni-PBMC were depleted of or enriched for CD3+, CD4+ or CD8+ cells and the frequency of IL-4-producing cells and the levels of IFN-γ production determined. Briefly, 2·0 × 107 PBMC in sterile 2% FBS/PBS were incubated with 4·0 × 107 antihuman CD3, CD4 or CD8 (Dynal Biotech, Oslo, Norway) mAb coated beads at +4°C for 30 min. The CD4+ and CD8+ enriched cell fractions (attached to anti-CD4 or CD8 beads) were washed five times with 3 ml sterile 2% FBS/PBS on a magnetic concentrator (Dynal Biotech). The washed CD4+ and CD8+ enriched as well as the CD3+, CD4+ and CD8+ depleted cell fractions were then resuspended in complete medium with 10% heat-inactivated FBS. The cell depletions were confirmed by flow cytometry using phycoerythrin conjugated antihuman CD3, CD4 or CD8 mAb (BD) staining as described previously but without saponin permeabilization [28]. Cytokine production was determined by ELISpot or ELISA using whole PBMC, CD3+, CD4+ or CD8+ depleted or CD4+ or CD8+ enriched cell fractions at 2·5 × 106 cells/ml with or without 50 µM NiCl2 × 6H2O (Merck) or 1 × 106 cells/ml with PHA.
Statistical analysis
The Mann–Whitney U-test was used to compare the cytokine responses between Ni-PBMC and control PBMC. The Wilcoxon matched pairs test was used to compare the Ni-induced responses for each PBMC sample in the absence and presence of different concentrations of rIL-10 or α-hIL-10 mAb. To evaluate the relationship between observed parameters, correlations were computed using the Spearman rank-order correlation coefficient rs. For comparison of Ni-specific responses, values with background (spontaneous production) subtracted were used. To evaluate the influence of rIL-10 or α-hIL-10 mAb on Ni-induced cytokine responses, the cytokine responses to Ni in the presence of a given concentration of either rIL-10 or α-hIL-10 mAb was subtracted from the matched spontaneous level of production in the presence of the same concentration of rIL-10 or α-hIL-10 mAbs (e.g. background +0·2 ng/ml rIL-10 was subtracted from Ni2++0·2 ng/ml rIL-10, etc.). For statistical purposes, all ELISA values below the detection limit were adjusted to 5 pg/ml. A P-value < 0·05 was considered to be statistically significant. All tests were performed using the software STATISTICA 5·1 (StatSoft, Tulsa, OK, USA).
Results
Ni induces production of IL-10 in vitro by Ni-PBMC
PBMC from donors with a stated history of ACD to Ni and in vitro-reactivity to Ni (Ni-PBMC; n = 15) and donors with a confirmed nonresponsiveness to Ni in vitro (control PBMC; n = 8) were used to quantify the levels of IL-10, IL-13 and IFN-γ and the IL-4-, IL-13- and IFN-γ-producing cells in response to Ni by ELISA and ELISpot, respectively. IL-10 ELISpot was not included since monocytes releasing low levels of IL-10 can interfere with the quantification of antigen-induced IL-10-producing T cells by ELISpot [29]. IL-4 ELISA was not used since consumption by cellular receptors upon secretion hampers IL-4 detection by ELISA whereas ELISpot, based on capture of IL-4 upon its release, works well [30].
Ni-PBMC showed significantly higher levels of endogenous IL-10 elicited by Ni as compared to the control PBMC; the mean increase in the level of IL-10 elicited by Ni, after subtraction of spontaneously produced IL-10, was 2·2 pg/ml and 33·1 pg/ml in the control and Ni-PBMC, respectively (P = 0·002); the level of IL-10 secreted spontaneously was similar in the two groups (Fig. 1). As expected, due to the fact that the PBMC samples had been preassessed for IL-4/IL-13 responses to Ni, the Ni-induced production of Th2-type, but also Th1-type, cytokines was significantly elevated in Ni-PBMC as compared to the control PBMC (data not shown). Importantly, the production of both Th1- and Th2-type cytokines by PBMC after Ni stimulation correlated positively with the levels of Ni-induced IL-10 (Fig. 2).
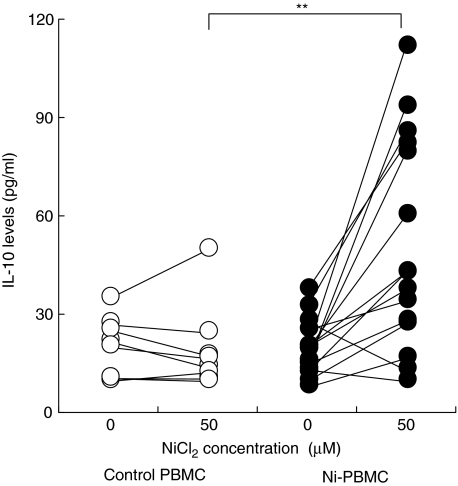
Fig. 1.
Levels of IL-10 secreted by PBMC incubated in the absence or presence of 50 µM NiCl2. The mean (range) of the spontaneous IL-10 levels were: for the control PBMC, 19 pg/ml (8·8–34 pg/ml) (n = 8; ○); Ni-PBMC, 19 (7·4–37 pg/ml) (n = 15; •). The statistical difference between the two groups following a Mann–Whitney U-test is depicted with asterisks (**P < 0·01) and is based on a comparison of the Ni-induced IL-10 levels minus the spontaneously produced IL-10 levels.
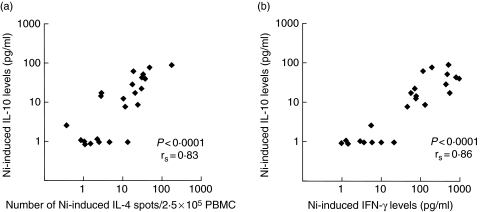
Fig. 2.
Correlated levels of Ni-induced IL-10 and the Th1- or Th2-type cytokine production by PBMC. Plots depict correlation between levels of IL-10 and (a) the number of IL-4-producing cells or (b) the levels of IFN-γ. Plots are based on data after subtraction of spontaneous background. Values between 0 and 1 were set to 1. All Ni-PBMC and control PBMC are included in the figures (n = 23). The Ni-induced production of IL-13 measured by ELISpot and ELISA and IFN-γ measured by ELISpot correlated with IL-10 in a similar manner.
Exogenous rIL-10 down regulates the Ni-induced Th1- and Th2-type responses
To define the regulatory role of IL-10 on the production of other Ni-induced cytokines, we incubated PBMC with Ni in the absence or presence of a concentration range of rIL-10 and measured the production of other cytokines by ELISA and/or ELISpot.
Addition of rIL-10 to Ni-PBMC reduced the levels of Ni-induced IL-13 and IFN-γ but had a more potent impact on the IFN-γ levels (Fig. 3a). For example, the mean levels of IFN-γ were reduced by 40% and 71% using 0·2 and 1 ng/ml of rIL-10, respectively, whereas the mean effect on IL-13 levels at these concentrations of rIL-10 was small. Due to the lack of any Ni-induced response in control PBMC, addition of rIL-10 had no significant effects (data not shown).
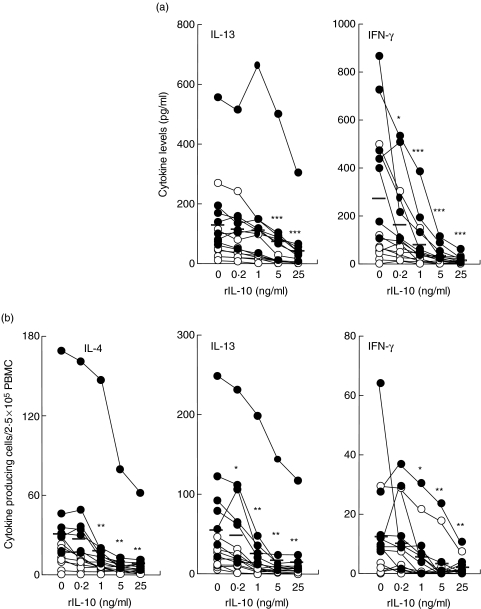
Fig. 3.
Down regulation of Ni-induced cytokine production in Ni-PBMC by recombinant IL-10. PBMC were incubated with 50 µM NiCl2 and a concentration range of rIL-10 followed by analysis of IL-13 and IFN-γ levels by (a) ELISA or (b) number of IL-4-, IL-13- and IFN-γ-producing cells by ELISpot. The effect of rIL-10 on the Ni-induced cytokine increment was calculated by subtracting the spontaneous cytokine production in the presence of rIL-10 from the Ni-stimulated cytokine production in the presence of the corresponding concentration of rIL-10. ○ (n = 8) and • (n = 7) represent Ni-reactive donors with levels of Ni-induced IL-10 < 30 pg/ml or > 30 pg/ml, respectively. Mean responses are indicated as horizontal bars. Statistical differences between the Ni-induced cytokine production in the absence of rIL-10 and after addition of different concentrations of rIL-10 following a Wilcoxon matched pairs test are depicted with asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
We further investigated the effect of rIL-10 on the frequency of cells producing IL-4, IL-13 and IFN-γ. In Ni-PBMC, the addition of rIL-10 caused a similar and significant dose-dependent reduction in the number of Ni-induced IL-4, IL-13 and IFN-γ-producing cells (Fig. 3b). This observed down-regulation was most prominent in PBMC that responded to Ni stimulation with high levels of endogenous IL-10 (i.e > 30 pg/ml; Fig. 3).
Neutralization of endogenous Ni-induced IL-10 enhances the Ni-induced IFN-γ levels
The down regulatory effect of IL-10 on Ni-induced production of both Th1- and Th2-type cytokines led us to speculate that Ni-induced cytokine responses could be enhanced by blocking the endogenous IL-10 elicited by Ni. PBMC were incubated with Ni in the presence or absence of 10 µg/ml of α-hIL-10 mAb and the levels of IL-13 and IFN-γ and the number of IL-4-, IL-13- and IFN-γ-producing cells were determined.
Measurement of Ni-induced production of cytokines other than IL-10 in the presence of mAb to IL-10 showed that the levels of production of IFN-γ, but not IL-13, were significantly affected (Fig. 4a). The addition of 10 µg/ml of mAb resulted in a 102% increase, on average, in the levels of Ni-induced IFN-γ produced by Ni-PBMC (P< 0·01) (Fig. 4a). Assuming that the effect of the IL-10 neutralizing antibody is dependent on the level of endogenous IL-10, one would expect a more positive effect in donors with higher IL-10 production. Indeed, a greater enhancing effect of α-hIL-10 mAb on IFN-γ production was observed in Ni-PBMC with an IL-10 response to Ni of at least 30 pg/ml (n = 7; mean Ni-induced IFN-γ increment of 468 pg/ml) than in Ni-PBMC with IL-10 responses below 30 pg/ml (n = 8; mean Ni-induced IFN-γ increment of 107 pg/ml) after addition of 10 µg/ml of IL-10 neutralizing mAb (Fig. 4a).
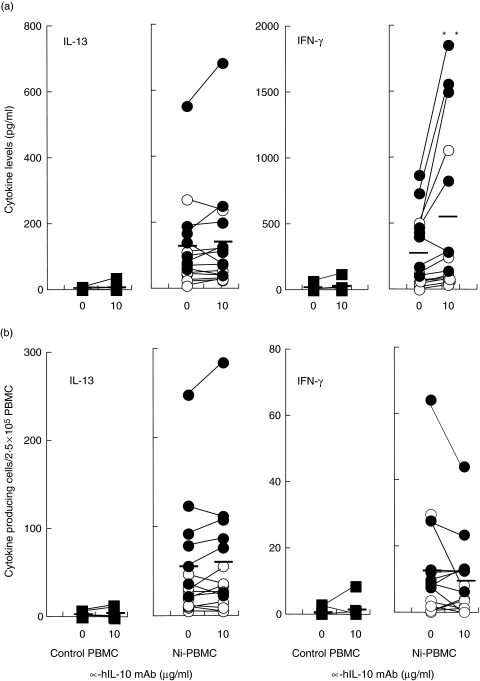
Fig. 4.
Increased IFN-γ levels in response to Ni by neutralization of endogenous Ni-induced IL-10. PBMC were incubated with 50 µM NiCl2 with or without α-hIL-10 mAb (10 µg/ml) and IL-13 and IFN-γ production were measured by (a) ELISA and (b) ELISpot. The Ni-induced cytokine increment was calculated by subtracting the spontaneous cytokine production in the absence or presence of α-hIL-10 mAb from the Ni-stimulated cytokine production at the same concentration of α-hIL-10 mAb. Control PBMC (n = 8) are shown in the left plot while Ni-PBMC (n = 15) are shown in the right plot; ○ (n = 8) and • (n = 7) represent Ni-PBMC with levels of Ni-induced IL-10 < 30 pg/ml or > 30 pg/ml, respectively. Mean responses at 0 or 10 µg/ml of α-hIL-10 mAb are indicated as horizontal bars. Statistical differences between the Ni-induced cytokine levels in the absence or presence of α-hIL-10 mAb following a Wilcoxon matched pairs test are indicated by asterisks (**P < 0·01).
Despite a significant increase in the Ni-induced IFN-γ levels after depletion of IL-10, there was no increase in the frequency of IFN-γ-producing cells following Ni stimulation (Fig. 4b). That is, IL-10 neutralization enhanced the IFN-γ-producing capacity of Ni-specific T cells without any effect on the IFN-γ production by Ni-nonspecific cells. Nor was the number of cells producing IL-13 in response to Ni affected by depletion of IL-10 (Fig. 4); analysis of IL-4 yielded similar results (data not shown). Addition of mAb to IL-10 did not affect the cytokine response to Ni by control PBMC (Fig. 4a,b).
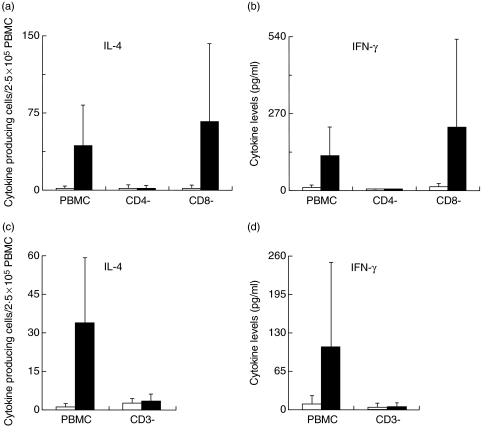
Ni-specific IL-4 and IFN-γ production is abrogated in CD4+ T-cell depleted PBMC
In order to determine the phenotype of cells producing the Th1- and Th2-type cytokines after Ni stimulation, hence the cells affected by the immuno-modulatory effects of IL-10, Ni-PBMC were enriched for CD4+ or CD8+ or depleted of CD3+, CD4+ or CD8+ cells. The frequency of Ni-induced IL-4-producing cells and the levels of IFN-γ production in response to Ni stimulation were determined in these different cell fractions. Gating within the lymphocyte population, between 89 and 92%, 95–99%, and 90–95% of CD3+, CD4+, CD8+ cells, respectively, were depleted using our protocol.
Depletion of the CD3+ and CD4+ cells completely abrogated both IL-4 and IFN-γ production after Ni stimulation (Fig. 5). On the other hand, depletion of CD8+ cells had a positive effect on the magnitude of the Ni-induced production of both cytokines (Fig. 5a,b). Noteworthy, was the observation of significant levels of Ni-induced production of both cytokines above background in the CD4+ enriched cell fraction albeit, lower than in whole PBMC (Ni-induced IL-4; 93 ± 110% (mean ± SD) and IFN- γ; 57 ± 55% of response in whole PBMC). There was no detectable Ni-induced production of either cytokine above background in the CD8+ enriched cell fraction. Depletion or enrichment of any of the cell fractions had no effect on the production of either cytokine in the absence of any stimulation (spontaneous background).
Fig. 5.
Phenotypic characterization of Ni-specific IL-4 and IFN-γ producing cells. Ni-PBMC were depleted of CD3+, CD4+ or CD8+ cells and (a, n = 6; c, n = 5) IL-4 and (b, n = 6; d, n = 4) IFN-γ production was determined by ELISpot (a, c) and ELISA (b, d). PBMC and depleted cell fractions were incubated at a concentration of 2·5 × 106 cells/ml in the absence or presence of 50 µM NiCl2. Cytokines produced spontaneously (□) and in response to Ni (▪) are depicted.
PHA induced significant cytokine production in PBMC fractions depleted of either CD4+ or CD8+ cells but the levels were however, lower than in whole PBMC. CD3+ cell depletion resulted in a complete loss of response to PHA.
Discussion
In the present study, we investigated the effect of the addition of rIL-10 or α-hIL-10 mAb on cytokine responses in Ni-stimulated PBMC. The addition of rIL-10 markedly reduced the detection of Ni-induced IL-4, IL-13 and IFN-γ production in PBMC cultures. In contrast, neutralization of endogenous, Ni-induced IL-10 enhanced the levels of IFN-γ produced in response to Ni but had no impact on the Th2-type cytokine responses. IL-10 exerted its down-regulatory effects, directly or indirectly, on CD4+ T cells in the PBMC since both the Th1- (IFN-γ) and Th2-type (IL-4) cytokines were produced by CD4+ T cells.
Ni-PBMC showed a mixed cytokine profile in response to Ni alone including elevated levels of IL-10 that correlated with the increase both of Th1- and Th2-type cytokines. The significant correlations between the Ni-induced IL-10 levels and the Th1- and Th2-type cytokines suggest that IL-10 is consequently induced to balance the induction of other cytokines known to be involved in the pathology of ACD in different ways. Our study demonstrates that IL-10 is of importance for regulating the immune reactivity to Ni and shows that also low levels of endogenously produced IL-10 induced by Ni suppress the Ni-specific Th1-type CD4+ T cells. Noteworthy, although Ni-PBMC displayed high Th1- and Th2-type cytokine responses to Ni, neutralization of IL-10 had a more prominent effect on the IFN-γ levels. In vivo a high IL-10 response may thus serve the purpose of suppressing an otherwise excessive detrimental Th1-type response.
A role for IL-10 in regulating atopic IgE mediated allergic immune responses has been demonstrated [31,32]. The regulatory mechanism may differ from ACD though since atopic allergy is mediated primarily by Th2-type responses. And whereas subjects with ACD to Ni, but not healthy controls, display cytokine responses to Ni, allergens causing atopic allergy elicit cytokine responses in both allergic and healthy controls [27,33]. However, subjects with atopic allergy display a predominating Th2-type response and the healthy controls primarily a T-regulatory response [33]. Interestingly, Akdis et al.[27] also showed that neutralization of endogenous IL-10 elicited by an atopic allergen in PBMC from healthy subjects led to an increase of both IFN- γ and IL-13 allergen-specific responses. We too found a pleiotropic down-regulatory effect on IL-4, IL-13 and IFN-γ responses to Ni after adding rIL-10 to the PBMC. Noteworthy, however, the IFN-γ response was affected more at the lowest concentration of rIL-10 (0·2 ng/ml), which better reflects the endogenous levels of IL-10 induced in response to Ni; consequently, neutralization of endogenous IL-10 only significantly affected the IFN-γ response.
IL-10 is thought to exert its effects by down-regulating the expression of costimulatory molecules required for appropriate antigen presentation [34,35]. At the molecular level, IL-10 has been shown to mediate direct T cell suppression by inhibiting CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding upon interaction with a CD28-associated IL-10 receptor [31,36]. De la Barrera et al.[35] demonstrated that neutralization of endogenous IL-10 induced strong CD4+ and CD8+ T cell-mediated lytic activity which correlated with an increased expression of costimulatory molecules on target cells from patients infected with Mycobacterium tuberculosis. A similar mechanism, i.e. an indirect activation by up-regulation of costimulatory molecules rather than direct effects on the T cells, is likely to also be the explanation for the enhanced Ni-induced IFN-γ production by CD4+ T cells upon neutralization of endogenous IL-10.
Studies involving long-term cultured T-cell lines or clones have attributed Th1- and Th2-type cytokine production in response to Ni to both CD4+ and CD8+ T cells [12,13]. Recently, however, Moed et al. [15] using cells directly isolated from PBMC, showed that only CD4+ CLA+ CD45RO+ and not CD8+ T cells proliferate and produce both Th1- (IFN-γ) and Th2-type (IL-5) cytokines in response to Ni. Therefore, in this study, we investigated the effect of depletion of the CD3+, CD4+ or CD8+ cell populations from PBMC, a different approach of confirming the role of these cells, on Ni-induced Th1- and Th2-type cytokine production. Our results suggest that CD4+ T cells are the cell population mainly responsible for production of both IL-4 and IFN-γ in response to Ni stimulation. The enhanced Ni-induced cytokine responses observed upon depletion of CD8+ T cells may be explained by the fact that depletion of CD8+ T cells results in enrichment for other cell populations including CD4+ T cells. Alternatively, Ni-specific memory CD8+ T cells, described by others [12,13] may instead have a suppressive effect on Ni-induced cytokine production by CD4+ T cells and their depletion abrogates this suppressive effect.
In parallel with the analyses of IFN-γ and IL-4 responses to Ni in CD3+, CD4+ and CD8+ cell depletion/enrichment experiments, we also attempted to define the cell fraction responsible for IL-10 production but no conclusive results could be obtained. Several studies have demonstrated IL-10 production in a variety of cell types, besides T cells, such as; monocytes, macrophages, dendritic cells, mast cells and keratinocytes [22,29,32]. However, the bulk of antigen-induced IL-10 production has been ascribed to CD4+CD25+ T regulatory cells [25,27,37]. Thus our inability to phenotype the Ni-specific IL-10-producing cells in our experiments may either reflect the heterogeneity of the cell populations capable of producing IL-10 or may be due to the generally low levels of IL-10 elicited by Ni in PBMC.
Taken together, our results suggest that IL-10 responses to Ni are found in PBMC from subjects with ACD to Ni and that IL-10 responses coincide with Th1- and Th2-type responses. Moreover, although IL-10 has an impact on both Th1- and Th2-type responses by CD4+ T cells, only Th1-type (IFN-γ) responses are down-regulated by the low endogenous IL-10 levels resulting from Ni stimulation, i.e. IL-10 concentrations that may reflect the local level in vivo where cells producing IL-10 are in close contact with IL-10 receptor expressing cells.
Acknowledgments
This investigation was supported by grants from the Swedish Council for Working Life and Social Research, the Vårdal foundation for Health Care Sciences and Allergy Research (Stockholm), The Asthma and Allergy Foundation and the Swedish Research Council.
References
- 1.Brasch J, Geier J. Patch test results in schoolchildren. Results from the Information Network of Departments of Dermatology (IVDK) and the German Contact Dermatitis Research Group (DKG) Contact Dermatitis. 1997;37:286–93. doi: 10.1111/j.1600-0536.1997.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 2.Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40 000 patients from a multicenter study (IVDK) Contact Dermatitis. 1997;37:200–9. doi: 10.1111/j.1600-0536.1997.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen NH, Linneberg A, Menne T, Madsen F, Frolund L, Dirksen A, Jorgensen T. Incidence of allergic contact sensitization in Danish adults between 1990 and 1998; the Copenhagen Allergy Study, Denmark. Br J Dermatol. 2002;147:487–92. doi: 10.1046/j.1365-2133.2002.04668.x. [DOI] [PubMed] [Google Scholar]
- 4.Silvennoinen-Kassinen S, Ikaheimo I, Karvonen J, Kauppinen M, Kallioinen M. Mononuclear cell subsets in the nickel-allergic reaction in vitro and in vivo. J Allergy Clin Immunol. 1992;89:794–800. doi: 10.1016/0091-6749(92)90433-3. [DOI] [PubMed] [Google Scholar]
- 5.Shah M, Lewis FM, Gawkrodger DJ. Nickel as an occupational allergen. A survey of 368 nickel-sensitive subjects. Arch Dermatol. 1998;134:1231–6. doi: 10.1001/archderm.134.10.1231. [DOI] [PubMed] [Google Scholar]
- 6.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 7.Probst P, Kuntzlin D, Fleischer B. TH2-type infiltrating T cells in nickel-induced contact dermatitis. Cell Immunol. 1995;165:134–40. doi: 10.1006/cimm.1995.1196. [DOI] [PubMed] [Google Scholar]
- 8.Falsafi-Amin H, Lundeberg L, Bakhiet M, Nordlind K. Early DNA synthesis and cytokine expression in the nickel activation of peripheral blood mononuclear cells in nickel-allergic subjects. Int Arch Allergy Immunol. 2000;123:170–6. doi: 10.1159/000024437. [DOI] [PubMed] [Google Scholar]
- 9.Jakobson E, Masjedi K, Ahlborg N, Lundeberg L, Karlberg AT, Scheynius A. Cytokine production in nickel-sensitized individuals analysed with enzyme-linked immunospot assay: possible implication for diagnosis. Br J Dermatol. 2002;147:442–9. doi: 10.1046/j.1365-2133.2002.04850.x. [DOI] [PubMed] [Google Scholar]
- 10.Cederbrant K, Anderson C, Andersson T, Marcusson-Stahl M, Hultman P. Cytokine production, lymphocyte proliferation and T-cell receptor Vbeta expression in primary peripheral blood mononuclear cell cultures from nickel-allergic individuals. Int Arch Allergy Immunol. 2003;132:373–9. doi: 10.1159/000074905. [DOI] [PubMed] [Google Scholar]
- 11.Lindemann M, Bohmer J, Zabel M, Grosse-Wilde H. ELISpot. a new tool for the detection of nickel sensitization. Clin Exp Allergy. 2003;33:992–8. doi: 10.1046/j.1365-2222.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- 12.Moulon C, Wild D, Dormoy A, Weltzien HU. MHC-dependent and – independent activation of human nickel-specific CD8+ cytotoxic T cells from allergic donors. J Invest Dermatol. 1998;111:360–6. doi: 10.1046/j.1523-1747.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 13.Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, Girolomoni G, Cavani A. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol. 2000;165:3058–64. doi: 10.4049/jimmunol.165.6.3058. [DOI] [PubMed] [Google Scholar]
- 14.Werfel T, Hentschel M, Kapp A, Renz H. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J Immunol. 1997;158:2500–5. [PubMed] [Google Scholar]
- 15.Moed H, Boorsma DM, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Gibbs S, Rustemeyer T. Nickel-responding T cells are CD4+CLA+CD45RO+ and express chemokine receptors CXCR3, CCR4 and CCR10. Br J Dermatol. 2004;151:32–41. doi: 10.1111/j.1365-2133.2004.05975.x. [DOI] [PubMed] [Google Scholar]
- 16.Minang JT, Troye-Blomberg M, Lundeberg L, Ahlborg N. Nickel elicits concomitant and correlated in vitro production of Th1-, Th2-type and regulatory cytokines in subjects with contact allergy to nickel. Scan J Immunol. 2005;62:289–96. doi: 10.1111/j.1365-3083.2005.01673.x. [DOI] [PubMed] [Google Scholar]
- 17.Rustemeyer T, von Blomberg BM, van Hoogstraten IM, Bruynzeel DP, Scheper RJ. Analysis of effector and regulatory immune reactivity to nickel. Clin Exp Allergy. 2004;34:1458–66. doi: 10.1111/j.1365-2222.2004.02045.x. [DOI] [PubMed] [Google Scholar]
- 18.Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, Nicolas JF. Allergic contact dermatitis. Eur J Dermatol. 2004;14:284–95. [PubMed] [Google Scholar]
- 19.Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bond MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–45. [PubMed] [Google Scholar]
- 20.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Elliott JF, Mosmann TR. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–78. [PubMed] [Google Scholar]
- 22.Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92–5. [PubMed] [Google Scholar]
- 23.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–6. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Invest Dermatol. 2000;114:295–302. doi: 10.1046/j.1523-1747.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- 26.Minang JT, Ahlborg N, Troye-Blomberg M. A Simplified ELISpot Assay Protocol Used for Detection of Human Interleukin-4, Interleukin-13 and Interferon-Production in Response to the Contact Allergen Nickel. Exog Dermatol. 2003;2:306–13. [Google Scholar]
- 27.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of Interleukin 10 in Specific Immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuber B, Levitsky V, Jönsson G, et al. Detection of human perforin by ELISpot and ELISA. Ex vivo identification of virus-specific cells. J Immunol Meth. 2005;302:13–25. doi: 10.1016/j.jim.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Guerkov REM, Targoni OS, Kreher CR, Boehm BO, Herrera MT, Tary-Lehmann M, Lehmann PV, Schwander SK. Detection of low-frequency antigen-specific IL-10-producing CD4(+) T cells via ELISPOT in PBMC. cognate vs. nonspecific production of the cytokine. J Immunol Meth. 2003;279:111–21. doi: 10.1016/s0022-1759(03)00240-0. [DOI] [PubMed] [Google Scholar]
- 30.Ewen C, Baca-Estrada ME. Evaluation of interleukin-4 concentration by ELISA is influenced by the consumption of IL-4 by cultured cells. J Interferon Cytokine Res. 2001;21:39–43. doi: 10.1089/107999001459141. [DOI] [PubMed] [Google Scholar]
- 31.Bellinghusen I, Brand U, Steinbrink K, Enk AH, Knop J, Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: differences from hydrocortisone-treated dendritic cells. J Allergy Clin Immunol. 2001;108:242–9. doi: 10.1067/mai.2001.117177. [DOI] [PubMed] [Google Scholar]
- 32.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001;31:694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 33.Akdis C, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De la Barrera S, Aleman M, Musella R, Schierloh P, Pasquinelli V, Garcia V, Abbate E, Sasiain Mdel C. IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosis-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin Exp Immunol. 2004;138:128–38. doi: 10.1111/j.1365-2249.2004.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–8. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 37.Sebastiani S, Allavena P, Albanesi C, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]