Abstract
Particular human leucocyte antigen (HLA) polymorphisms have been associated with a reduced risk of HIV transmission. However, protective alloimmune responses expected to result from such a genetic predisposition have not been demonstrated. To this end, we analysed and compared cellular and humoral alloimmune responses in a cohort of female sex workers who remained human immunodeficiency virus (HIV)-seronegative despite more than 3 years of high-risk sexual activity (ESN FSWs) with those of low-risk HIV-seronegative female blood donors in Abidjan, Côte d'Ivoire. ESN FSWs showed significantly lower allostimulated CD69 expression and secretion of interferon-γ, macrophage inflammatory protein (MIP)-1β and RANTES (regulated upon activation, normal T-cell expressed and secreted) by lymphocytes than controls. In contrast, ESN FSWs showed significantly higher mitogen-stimulated CD69 expression and secretion of tumour necrosis factor-α and MIP-1β than controls. Suppression of cellular alloimmune responses among ESN FSWs was associated with a higher self-reported frequency of unprotected sex. Levels of anti-HLA class I alloantibodies in plasma were not significantly different between ESN FSWs and controls. These findings indicate that frequent sexual exposure to multiple partners results in suppression rather than activation of cellular alloimmune responses. Our data support the hypothesis that suppressed cellular alloimmune responses may play a role in protection against HIV infection.
Keywords: activation, cytokines, HIV, MHC/HLA, disease susceptibility/resistance/polymorphisms
Introduction
The possibility that immune reactivity against allogeneic human leucocyte antigens (HLA) could contribute to protection against human immunodeficiency virus (HIV) infection has been proposed [1]. This suggestion is based on several observations [2–5]. Macaques were protected against a challenge with simian immunodeficiency virus (SIV) after vaccination with the uninfected human cells that were used to grow the SIV [2]. The sera from these macaques contained anti-HLA class I antibodies and their presence correlated with protection [3]. HIV acquires HLA class I and class II molecules from the host cell that outnumber viral gp120 [4]. In addition, gp120 and HLA share a degree of homology potentially leading to cross-reactive immune responses [5]. Together, these observations suggest that activated alloimmune responses could protect against sexual HIV transmission by two distinct mechanisms. Cellular alloimmune responses may directly reject the sexual partner's HIV-infected cells before virus production from these cells and transmission can occur. Anti-HLA antibodies may neutralize free virions by binding to HLA or gp120 on the viral envelope.
Some individuals remain HIV-seronegative despite frequent exposure to the virus and several mechanisms of HIV protection have been proposed (HIV-exposed seronegative or ESN, reviewed in [6]). Genetic studies have demonstrated the occurrence of particular HLA polymorphisms in these persons. ESN female sex workers (FSWs) in Nairobi displayed rare HLA alleles thus being capable of manifesting alloimmune responses against the largest proportion of the population [7], and possessed specific HLA subtypes that were associated with a reduced risk of HIV infection [8]. Similarly, a decreased risk of HIV-1 transmission from mother to child and in heterosexual couples was associated with HLA class I discordance and with particular HLA alleles [9–12]. However, the alloimmune responses that are expected to result from these particular HLA polymorphisms in ESN subjects have been more difficult to detect. Anti-HLA class I alloantibodies found in FSWs and in children born to HIV-infected mothers did not correlate with HIV protection [13,14]. To date, cellular alloimmune responses and their role in protection against HIV have not been studied in ESN subjects.
In the present study, cellular and humoral alloimmune responses were analysed in African FSWs who remained HIV-seronegative despite frequent high-risk sexual exposure for more than three years in comparison with seronegative female blood donors (FBDs) at lower risk for HIV infection. Surprisingly, we found decreased rather than increased cellular alloimmune responses in ESN FSWs compared with controls.
Materials and methods
Study population
Between June 1998 and July 1999, 20 ESN FSWs were enrolled in a confidential clinic in Abidjan, Côte d'Ivoire. The women were part of a multiple-centre trial testing the efficacy of the HIV microbicide nonoxynol-9 [15]. Blood samples and standard questionnaires with information on socio-demographics and sexual behaviour were collected. Blood samples were also collected from 18 HIV-seronegative female blood donors (FBDs) and from 24 HIV-seronegative male blood donors at the national blood transfusion centre in Abidjan. The study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention, Atlanta, GA, USA and the Ethical Committees of the Ministry of Health, Côte d'Ivoire and the Institute of Tropical Medicine, Antwerp, Belgium. Written informed consent was given by all study subjects before enrolment.
Laboratory methods
Whole blood was drawn from FSWs and blood donors in EDTA tubes (Becton Dickinson, San Jose, CA, USA). Within 4 h of collection, plasma was separated from whole blood by centrifugation, and stored at −70°C. The HIV status of all subjects was determined in plasma by using a combination of ELISAs and Western blots. The HIV-negative status of ESN FSWs was confirmed by HIV RT-PCR. Peripheral blood mononuclear cells (PBMC) were separated from whole blood by gradient centrifugation using lymphocyte separation medium (ICN Biomedicals, Aurora, OH, USA), resuspended in RPMI (Life Technologies, Paisly, UK) containing 50% FCS (Biochrom, Berlin, Germany) and 10% DMSO (Sigma-Aldrich, St. Louis, MO, USA), and stored in liquid nitrogen.
Set up of a one-way mixed leucocyte reaction (MLR) for the detection of alloimmune responses
Because irradiation could not be performed in the field, alternative methods for inactivation of stimulator cells were tested. PBMC from two controls were incubated in PBS containing 4% paraformaldehyde (PFA, Sigma-Aldrich) for 15 min at room temperature, with mitomycin C (MMC, Sigma-Aldrich) at 50 µg/ml in RPMI containing 10% FBS for 1 h at 37°C, and with actinomycin D (AMD, Sigma-Aldrich) at 10 µg/ml in RPMI containing 10% FBS for 1 h at 37°C, or left untreated (control). PBMC were washed three times with RPMI and surface stained with anti-CD3 mAb and 7-AAD to measure the degree of cell death, and with anti-CD3 and anti-HLA-DR or anti-MHC class I (clone W6/32, indirect staining with goat-anti-mouse antibody) mAbs to quantify HLA expression. To verify blocking of protein expression upon treatment, cells were stimulated with 0·02 µg/ml phorbol myristate acetate (PMA) and 1 µg/ml ionomycin in absence or presence of 10 µg/ml brefeldin A for 4 h at 37°C, and surface stained with anti-CD3 and anti-CD69 mAbs (absence of brefeldin A) or stained intracellularly with anti-CD3 and anti-IFN-γ or anti-IL-4 mAbs (presence of brefeldin A). Surface and intracellular staining protocols were as previously described [16], all mAbs were fluorochrome-labelled and obtained from Becton Dickinson (San Jose, CA, USA), and acquisition was done with a FACScan flow cytometer and CellQuest software (Becton Dickinson). Next, PBMC from 5 controls were inactivated with PFA or AMD like described above, pooled together, and incubated with PBMC from three different controls at a 1/1 ratio. Stimulation with medium alone and with 0·5 µg/ml phytohemagglutinin (PHA) served as negative and positive controls, respectively. At different time points up to 96 h of culture, cells were isolated and surface stained with anti-CD3 and anti-CD69 mAbs (for one control only), and analysed by flow cytometry like described above. After 7 days of incubation, supernatants were assessed with ELISA for interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4, IL-10, macrophage inflammatory protein (MIP)-1α, MIP-1β and RANTES (regulated upon activation, normal T-cell expressed and secreted) (for three controls, PFA-inactivated stimulator cells only). Antibody pairs for IFN-γ, TNF-α, IL-10, IL-4 and RANTES were obtained from Pharmingen (San Diego, CA, USA) and for MIP-1α and MIP-1β from R & D Systems (Minneapolis, MN, USA). Recombinant standards for IFN-γ, TNF-α, IL-4 and IL-10 were obtained from the National Institute for Biological Standards and Controls, UK, from Pharmingen for RANTES and from R & D Systems for MIP-1α and MIP-1β.
Cellular alloimmune responses
To mimic in vivo sexual alloantigen exposure to a wide range of prevalent HLA subtypes, allogeneic stimulator cells were prepared from 24 HIV-seronegative male donors from Abidjan. PBMC were separated from whole blood, labelled with 10 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) in PBS containing 1% BSA for 10 min at 37°C and 5% CO2, and washed three times in ice-cold RPMI containing 10% FBS. Cells were then inactivated with PFA like described above, and pooled together. To prepare autologous stimulator cells, freshly thawed PBMC were CFSE-labelled and PFA-fixed as described above. MLRs were set up as follows: 1 × 106 responder PBMC from FBDs and ESN FSWs were mixed together with 1 × 106 pooled allogeneic stimulator PBMC (allostimulation), 1 × 106 autologous stimulator PBMC (autostimulation) and 0·5 µg/ml PHA in RPMI containing 5% human AB serum (Irvine Scientific, Santa Ana, CA, USA) in duplicate 1-ml cultures in 48-well plates, and incubated at 37°C and in 5% CO2. To assess early responses, CD69 expression was measured after 40 h of culture. Cells were surface stained with fluorochrome-labelled anti-CD3, anti-CD69, anti-CD45RO (all from Becton Dickinson), and anti-CD4 (Dako, Copenhagen, Denmark) mAbs like described above, and analysed with a FACSCalibur flow cytometer (Becton Dickinson). CD69 expression was analysed within total, memory (CD45RO+) and naive (CD45RO–) CD4+ and CD8+ (as CD4–) T-cells of the responder cell fraction. CFSE-stained allogeneic and autologous stimulator cells showed a very bright signal in the FL-1 channel and could thus be excluded from the analysis. To assess late responses, supernatant levels of selected cytokines and β-chemokines were measured by ELISA after 7 days of culture like described above.
Anti-HLA class I antibodies
Anti-HLA class I antibodies were detected in plasma by the HLA panel reactive antibody (PRA) screening test (FlowPRA, One Lambda, Canoga Park, CA, USA). The method uses polystyrene microparticle beads coated with a wide range of HLA class I antigens. The test covered 100% of the prevalent HLA class I subtype A alleles, 81% of B alleles, and 92% of C alleles in Abidjan (among 31 blood donors, unpublished observation by A. Chahroudi, Centers for Disease Control, Atlanta, GA, USA). Testing was performed according to the manufacturer's instructions. In brief, HLA-coated beads were incubated with plasma for 30 min, washed twice and incubated with fluorochrome-labelled goat anti-human IgG antibodies for 30 min. Beads were washed again, resuspended in PBS containing 4% PFA, and analysed with a FACScan flow cytometer and CellQuest software (Becton Dickinson). For every experiment, positive and negative control samples were run together with the test samples. Percentages of HLA class I PRA were analysed in histogram plots with a marker set at the end of the negative control sample. The criteria for a positive result were:
a positive reaction with the positive control sample;
a percentage PRA of at least 10%;
a multiple peak pattern discriminating a negative and a positive bead population.
Statistical analysis
Mann–Whitney U-tests were used for comparing one variable among two groups, Wilcoxon signed rank tests were used for comparing two variables in the same group. Logistic regression was used for testing differences and associations between categorical variables. Linear and logistic regression was used for comparing continuous and categorical variables, respectively, among two groups while controlling for age. Normality of variables was tested with Kolmogorov-Smirnov tests. Correlations were analysed with Spearman rank correlation tests. For all analyses, the level of significance was set at P < 0·05.
Results
Characteristics of the study population
ESN FSWs were significantly older than FBDs (medians of 30 and 23 years; P = 0·008). All ESN FSWs included in the study had reported a duration of commercial sex work of ≥ 3 years (median 5·3 years; range 3·1–24 years). ESN FSWs reported a median number of 10 clients during the previous week (range 0–48). Eighty-five percent of ESN FSWs reported ‘always’ to use condoms with clients, 15% reported ‘often’. Forty-four percent of ESN FSWs confirmed having had sex with a regular partner during the previous week; 40% reported ‘never’ to use condoms with regular partners, 47% said ‘rarely’ and 13%‘always’. The HIV incidence among FSWs in Abidjan at the time of enrolment was 4%[15], and their HIV prevalence 32%[17]. HIV seropositivity among clients of FSWs in Abidjan was estimated at 14%[18].
Set up of a one-way MLR for the detection of alloimmune responses
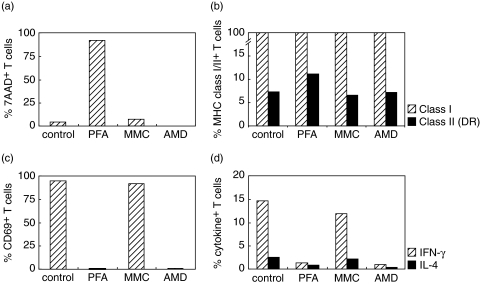
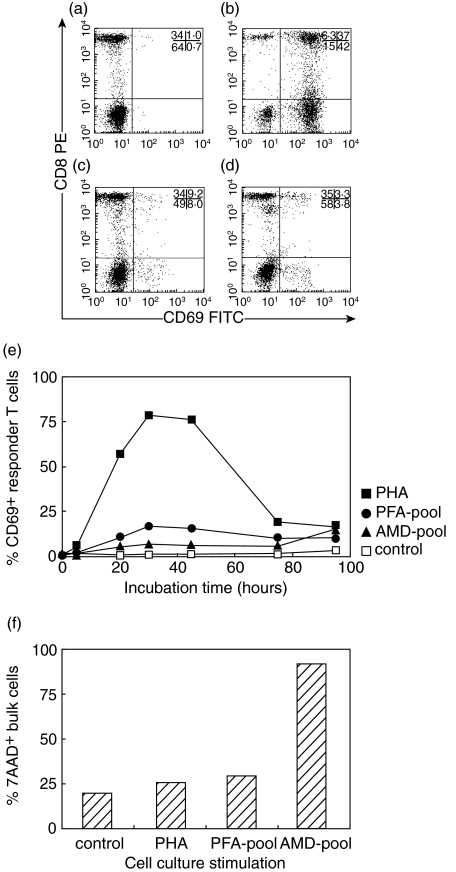
Because irradiation could not be performed in the field, treatment with MMC, AMD and PFA was tested for the inactivation of stimulator cells (Fig. 1). PFA treatment resulted in nearly 100% dead cells, MMC and AMD did not induce cell death (Fig. 1a). All methods left the expression of HLA class I and class II unaltered (Fig. 1b). PFA and AMD, but not MMC, blocked the expression of CD69, INF-γ and IL-4 upon PMA/ionomycin stimulation (Fig. 1c,d). PBMC from 5 controls were inactivated with PFA or AMD, pooled, and incubated with untreated PBMC from one other control. Expression of the early activation marker CD69 was measured as a well-established alternative for 3H-thymidine incorporation [19,20]. Dot plots showing the analysis of CD69 expression on gated CD3+ T-cells are shown in Fig. 2a–d. Higher levels of CD69 expression were induced by stimulator cells inactivated with PFA than with AMD, and a maximum was reached after 40 h of incubation (Fig. 2e). AMD-inactivated stimulator cells induced high levels of dead cells in the MLR after 96 h of incubation (Fig. 2f). PFA-inactivated pooled stimulator cells were then incubated with PBMC from three controls for 7 days as a reported optimal incubation period for allostimulated cytokine production [21,22]. IFN-γ, TNF-α, IL-10, MIP-1β and RANTES (but not MIP-1α or IL-4) were detected in supernatants and were thus selected for further study (data not shown).
Fig. 1.
Testing alternative inactivation methods for one-way MLR. (a) Percentage cell death (7-AAD staining) within T-cells after inactivation with paraformaldehyde (PFA), mitomycin C (MMC) and actinomycin D (AAD). (b) MHC class I (W6/32)( ) and class II (HLA-DR)(▪) expression on T-cells after inactivation with PFA, MMC and AMD. (c) CD69 membrane expression on T-cells after inactivation with PFA, MMC or AMD and stimulation with PMA/ionomycin (in the absence of brefeldin A). (d) Intracellular expression of IFN-γ (
) and class II (HLA-DR)(▪) expression on T-cells after inactivation with PFA, MMC and AMD. (c) CD69 membrane expression on T-cells after inactivation with PFA, MMC or AMD and stimulation with PMA/ionomycin (in the absence of brefeldin A). (d) Intracellular expression of IFN-γ ( ) and IL-4 (▪) in T-cells after inactivation with PFA, MMC or AMD and stimulation with PMA/ionomycin (in the presence of brefeldin A). Data are representative for PBMC from two controls.
) and IL-4 (▪) in T-cells after inactivation with PFA, MMC or AMD and stimulation with PMA/ionomycin (in the presence of brefeldin A). Data are representative for PBMC from two controls.
Fig. 2.
Kinetics of CD69 expression in a one-way MLR. Responder PBMC were derived from one control subject, pooled stimulator PBMC were derived from 5 different control subjects not related to the responder control. (a- d) Dot plots showing analysis of CD69 expression on gated CD3+ T-cells after 30 h of incubation in the presence of medium alone, PHA, paraformaldehyde-inactivated and actinomycin d-inactivated pooled stimulator cells, respectively. Percentages of gated events in the four quadrants are indicated. (e) CD69 expression on T-cells after incubation with medium alone (control, □), PHA (▪), paraformaldehyde-inactivated pooled stimulator cells (PFA pool, •), and actinomycin d-inactivated pooled stimulator cells (AMD pool, ▴) over a 96 h incubation period. (f) Percentage cell death (7-AAD staining) in bulk PBMC after 96 h of incubation.
Cellular CD69 expression in stimulated PBMC cultures from ESN and controls
PBMC from 20 ESN FSWs and 18 FBDs were stimulated with allogeneic and autologous stimulator cells, and PHA. To assess early responses, CD69 expression was measured on CD4+ and CD8+ T-cells by flow cytometry after 40 h of culture (Table 1). PHA stimulation induced higher CD69 expression than allo- or auto-stimulation (P< 0·001). Allo- and auto-stimulation induced similar levels of CD69 expression. ESN FSWs showed significantly lower allo- and auto-stimulated CD69 expression on memory subsets of CD4+ and CD8+ T-cells compared with FBDs, but tended to have higher PHA-stimulated CD69 expression. After adjusting for age, differences between ESN FSWs and FBDs became more pronounced for allo- and auto-stimulation and less pronounced for PHA-stimulation (Table 1). Among FBDs, PHA- and allo-stimulated CD69 expression correlated directly within memory CD4+ and CD8+ T-cells (r= 0·682, P= 0·002 and r = 0·639, P= 0·004, respectively). These correlations were lost in ESN FSWs (r= 0·380, P = 0·098 and r = 0·176, P = 0·458, respectively).
Table 1.
Percentages of CD69+ cells within T-cell subsets in allo-, auto- and PHA-stimulated PBMC cultures after 40 h of incubation.
| percentage CD69, median (interquartile range) | ||||
|---|---|---|---|---|
| T-cell subset | FBDs (n = 18) | ESN FSWs (n = 20) | P-value* | P-value† |
| Allostimulation | ||||
| CD4+ | 29·8 (18·9–39·5) | 23·4 (14·6–32·8) | 0·219 | 0·095 |
| CD4+ CD45RO– | 27·2 (18·0–35·4) | 22·3 (13·6–35·0) | 0·447 | 0·238 |
| CD4+ CD45RO+ | 32·2 (19·2–45·2) | 25·5 (15·0–33·3) | 0·136 | 0·030 |
| CD8+ | 25·0 (17·5–35·8) | 23·7 (16·4–29·9) | 0·388 | 0·050 |
| CD8+ CD45RO– | 24·6 (16·3–34·8) | 24·0 (16·3–29·8) | 0·640 | 0·104 |
| CD8+ CD45RO+ | 27·8 (23·3–40·5) | 21·8 (17·0–27·5) | 0·035 | 0·006 |
| Autostimulation | ||||
| CD4+ | 32·0 (11·5–42·5) | 24·9 (15·8–30·4) | 0·279 | 0·064 |
| CD4+ CD45RO– | 29·7 (10·9–40·5) | 22·0 (15·1–32·4) | 0·397 | 0·091 |
| CD4+ CD45RO+ | 32·9 (12·0–45·0) | 23·1 (17·0–33·1) | 0·267 | 0·044 |
| CD8+ | 24·1 (10·8–36·2) | 19·8 (15·6–24·5) | 0·397 | 0·032 |
| CD8+ CD45RO– | 22·2 (8·0–33·9) | 18·8 (12·4–23·7) | 0·520 | 0·062 |
| CD8+ CD45RO+ | 35·0 (21·3–42·1) | 24·9 (18·9–30·3) | 0·050 | 0·007 |
| PHA-stimulation | ||||
| CD4+ | 97·6 (96·9–98·4) | 98·1 (97·1–98·6) | 0·349 | 0·770 |
| CD4+ CD45RO– | 97·8 (96·2–98·7) | 98·4 (97·0–98·8) | 0·405 | 0·851 |
| CD4+ CD45RO+ | 98·0 (96·6–98·4) | 97·8 (96·8–98·5) | 0·907 | 0·923 |
| CD8+ | 91·1 (87·4–93·5) | 94·1 (93·1–96·4) | 0·019 | 0·167 |
| CD8+ CD45RO– | 90·3 (84·7–93·1) | 94·0 (91·5–96·1) | 0·018 | 0·128 |
| CD8+ CD45RO+ | 94·2 (91·3–95·7) | 95·3 (91·9–96·7) | 0·373 | 0·916 |
Mann–Whitney u-test.
Linear regression adjusting for age; all variables are normally distributed. P-values < 0·05 are in bold.
Cytokine and β-chemokine secretion in stimulated PBMC cultures from ESN and controls
To assess late cellular responses, levels of a selection of cytokines and β-chemokines were measured in the supernatants of allo-, auto- and PHA-stimulated cultures after 7 days of incubation (Table 2). PHA-stimulation induced higher production levels than allo- or auto-stimulation for all cytokines and β-chemokines (P< 0·001). Allostimulation revealed significantly higher levels of MIP-1β (P= 0·014 for FBDs, P = 0·001 for ESN FSWs) and RANTES (P< 0·001 for FBDs and ESN FSWs) than autostimulation. In line with CD69 expression, cytokine and β-chemokine levels in allo- and auto-stimulated PBMC cultures were significantly lower for ESN FSWs than for FBDs (significant for IFN-γ, MIP-1β and RANTES after allostimulation; for IFN-γ, TNF-α, IL-10, MIP-1β and RANTES after autostimulation). In contrast, ESN FSWs showed higher levels PHA-stimulated secretion than FBDs, reaching statistical significance for TNF-α and MIP-1β. Allostimulated RANTES levels and PHA-stimulated MIP-1β levels remained significantly different for ESN FSWs and FBDs after adjustment for age (Table 2).
Table 2.
Levels of cytokine and β-chemokine production in allo-, auto-, and PHA-stimulated PBMC cultures after 7 days of incubation.
| pg/ml, median (interquartile range) | ||||
|---|---|---|---|---|
| FBDs (n = 18) | ESN FSWs (n = 20) | P-value* | P-value† | |
| Allostimulation | ||||
| IFN-γ | 39·1 (6·6–549) | 10·4 (3·3–22·4) | 0·034 | N.A.‡ |
| TNF-α | 6·3 (0·8–289) | 6·3 (0·8–6·3) | 0·173 | N.A. |
| IL-10 | 19·1 (11·8–45·3) | 12·8 (6·1–23·6) | 0·299 | N.A. |
| MIP-1β | 440 (196–2531) | 194 (104–441) | 0·024 | N.A. |
| RANTES | 636 (425–1000) | 293 (262–446) | < 0·001 | < 0·001 |
| Autostimulation | ||||
| IFN-γ | 32·6 (12·2–529) | 3·3 (3·3–16·6) | 0·001 | N.A. |
| TNF-α | 6·3 (6·3–428) | 6·3 (0·8–6·3) | 0·023 | N.A. |
| IL-10 | 28·5 (12·5–46·9) | 16·5 (6·5–27·5) | 0·047 | 0·078 |
| MIP-1β | 306 (103·1–1852) | 66·1 (25·4–110) | 0·001 | N.A. |
| RANTES | 342 (187–606) | 122 (98·0–162) | < 0·001 | N.A. |
| PHA-stimulation | ||||
| IFN-γ | 2 210 (879–3902) | 2 505 (1793–3952) | 0·447 | 0·706 |
| TNF-α | 234 (130–427) | 406 (277–561) | 0·033 | 0·143 |
| IL-10 | 452 (275–739) | 525 (320–708) | 0·465 | 0·790 |
| MIP-1β | 12 607 (9 717–1 7661) | 22 438 (16 965–31 971) | 0·002 | 0·005 |
| RANTES | 3 846 (2 615–4 997) | 3 837 (3 001–6 054) | 0·569 | 0·372 |
Mann–Whitney U-test.
Linear regression adjusting for age.
Variable not normally distributed and linear regression not available (N.A). P-values < 0·05 are in bold.
Association between cellular alloimmune responses and sexual exposure among ESN FSWs
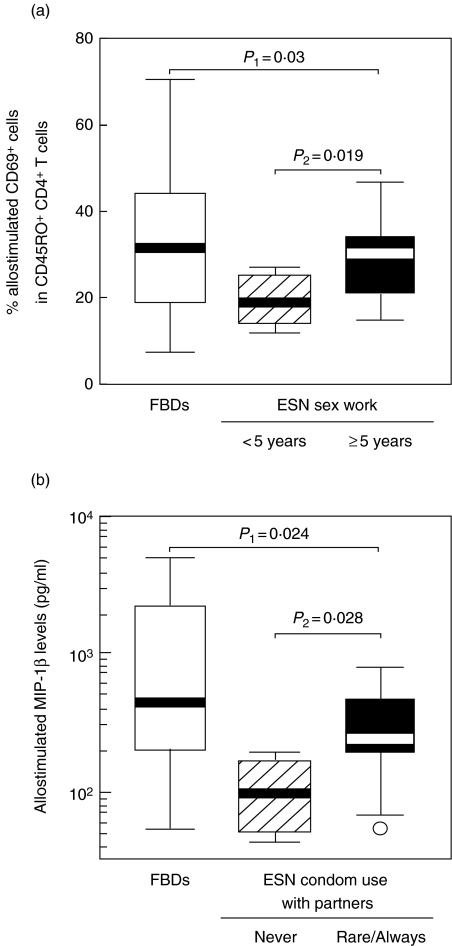
ESN FSWs with < 5 years of sex work showed lower levels of allostimulated CD69 expression on memory CD4+ and CD8+ T-cells than ESN FSWs with ≥ 5 years of sex work (Fig. 3a, data shown for CD4+ T cells). ESN FSWs reporting ‘never’ using condoms with regular partners showed lower levels of allostimulated MIP-1β than ESN FSWs reporting ‘rarely’ or ‘always’ using condoms (Fig. 3b). No association was found between cellular alloimmune responses and the numbers of clients of ESN FSWs. No correlation was detected between the duration of commercial sex work and the numbers of clients of ESN FSWs. ESN FSWs who stated using condoms ‘always’versus‘rarely’ with regular partners, and those that stated using condoms ‘always’versus‘often’ with clients showed similar allostimulated MIP-1β responses. The latter two associations likely lack statistical power due to low numbers of subjects.
Fig. 3.
Associations between cellular alloimmune responses and sexual exposure among ESN FSWs. (a) Association between allostimulated CD69 expression on memory CD4+ T-cells and the duration of commercial sex work among ESN FSWs. (b) Association between allostimulated MIP-1β secretion and the consistency in using condoms with regular partners. Allostimulated CD69 expression was tested with linear regression adjusting for age (variable normally distributed). Allostimulated MIP-1β secretion was tested with Mann–Whitney U-tests (variable not normally distributed). P1, comparison between FBDs and all ESN FSWs; P2, comparison within ESN subgroups.
Anti-HLA class I antibodies in ESN and controls
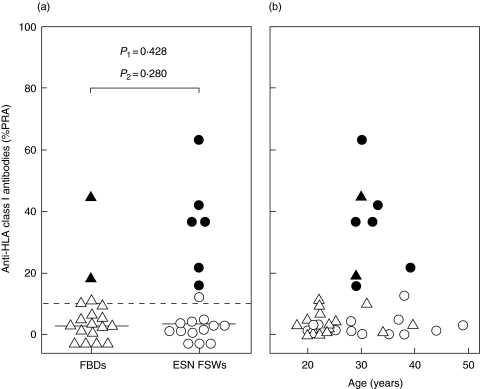
Anti-HLA class I antibodies were analysed in plasma from 19 ESN FSWs and 18 FBDs. Median antibody levels were similar among ESN FSWs and FBDs (Fig. 4a). By applying the criteria for a positive result, 6 of 19 ESN FSWs showed detectable anti-HLA class I antibodies compared to 2 of 18 FBDs (32%versus 11%; P = 0·146). The tendency towards a higher antibody prevalence among ESN FSWs disappeared after adjusting for age (P= 0·28, Fig. 4a). In fact, ESN FSWs and FBDs with detectable antibody levels were all between 29 and 39 years old (Fig. 4b). This age category was underrepresented among FBDs.
Fig. 4.
Plasma anti-HLA class I antibodies in ESN FSWs and FBDs. (a) Percentages of HLA class I panel reactive antibodies (% PRA) among ESN FSWs (○) and FBDs (▵). Filled symbols indicate percentages above 10% with a multiple-peak pattern in the histogram plot. Horizontal lines indicate median values. P1, Mann–Whitney U-test; P2, logistic regression adjusting for age. (b) Scatter plot of percentage HLA class I PRA against age among ESN FSWs (○) and FBDs (▵). Symbols as in (a).
Association between humoral alloimmune responses and sexual exposure among ESN FSWs
The 6 ESN FSWs with detectable anti-HLA class I antibodies did not show differences in duration of sex work, numbers of clients, sex with regular partners or consistency in using condoms compared with the 13 ESN FSWs without detectable antibodies. No correlations were noted between humoral and cellular alloimmune responses (data not shown).
Discussion
In the present study, we found that the ESN status of FSWs was not associated with enhanced cellular or humoral alloimmune responses in comparison with HIV-seronegative controls at lower risk for HIV infection. On the contrary, cellular alloimmune responses were decreased among ESN FSWs, suggesting that frequent unprotected sexual exposure results in suppression rather than activation of the immune system towards alloantigens. These data could support the hypothesis that suppressed cellular alloimmune responses play a role in protection against HIV infection.
Allostimulated T-cell activation and cytokine and β-chemokine secretion was significantly lower among ESN FSWs than among FBDs whereas PHA-stimulated responses were significantly higher. These opposite effects were confirmed by direct correlations between allo- and PHA-stimulated T-cell activation among FBDs but not among ESN FSWs. Cellular alloimmune responses were most down-regulated among ESN FSWs with the least frequent use of condoms with regular partners and the shortest duration of commercial sex work. In a previous study, we have reported that ESN FSWs in Abidjan with the shortest duration of commercial sex work have the highest numbers of clients per day [23]. This association was not detected in the present study, possibly because only ESN FSWs with > 3 years of sex work were selected. Nevertheless, together, these associations suggest a dose–response relation between the extent of sexual exposure and the suppression of cellular alloimmune responses.
Our observations are in line with previous reports suggesting peripheral alloimmune-tolerance after natural or artificial alloimmunization [24–32]. Prior pretransplant blood transfusions were reported to prolong renal allograft survival [24]. Successful pregnancy in mice was associated with repeated exposure to semen from a specific mate [25]. Normal pregnancy without symptoms of pre-eclampsia occurred more often among women with prior exposure to paternal alloantigens via unprotected sex or previous pregnancies [26,27]. Likewise, in women with recurrent spontaneous abortions, tolerance towards the fetus was achieved after alloimmunization with the male partner's PBMC [28,29]. Such alloimmune therapy resulted in decreased proliferation in maternal versus paternal MLR [30], decreased CD69 expression on T-cells [31], and a Th1 to Th2 cytokine shift [32]. The latter findings are in remarkable agreement with our results: ESN FSWs showed lower expression of CD69 and IFN-γ but not IL-10 after in vitro allostimulation.
In other studies, women with recurrent spontaneous abortions who were in vivo immunized with their partner's PBMC showed in vitro PHA-stimulated HIV inhibition, which was found to be associated with increased PHA-stimulated β-chemokine production [33,34]. Based on these observations, an HIV-protective effect of artificial alloimmunization was postulated. However, in vitro PHA-stimulation may not be an appropriate correlate of the in vivo alloimmune response. On the contrary, in our study we concurrently observed increased PHA-stimulated MIP-1β levels (in line with their data) and decreased allostimulated MIP-1β and RANTES levels. Unfortunately, in the former studies [33,34], in vitro alloimmune responses before and after alloimmunization were not analysed, hence a direct relationship between enhanced alloimmunity and HIV protection remains uncertain. A recent study in heterosexual couples practicing unprotected sexual intercourse reconfirmed the relationship between in vivo alloimmunization and in vitro PHA-stimulated HIV inhibition [35]. In addition, the authors reported increased in vitro alloimmune responses in these persons, which is at variance with our data. The latter conclusion may have been premature because of lack of an appropriate control group (i.e. heterosexual couples practicing protected sex). The contradictory findings may also be explained by differences in study population: frequent sexual exposure to multiple partners in our study and moderate sexual exposure to a single partner in theirs may trigger distinct immune reactions.
We have set up a well-designed new method for the detection of alloimmune responses without the need for radioactive pulsing or irradiation. Flow cytometry detection of CD69 in one way MLRs is a well-established alternative for 3H-thymidine incorporation [36]. As an alternative for irradiation, we have shown the superiority of PFA over MMC and AMD for the inactivation of stimulator cells. To mimic in vivo sexual alloantigen exposure to a wide range of HLA alleles as experienced by ESN FSWs, allogeneic stimulator cells were prepared from 24 male blood donors from Abidjan. CFSE-labelling of stimulator cells facilitated their discrimination from responder cells during flow cytometry analyses. Although the MLR has a heterogeneous cytokine profile and the kinetics of cytokine secretion are complex [22], 7 day-incubation was shown to be a good compromise for the cytokines and β-chemokines that were analysed [21,22]. However, stimulation with PFA-inactivated allogeneic cells initially resulted in similar levels of CD69 expression compared with PFA-inactivated autologous cells. The minimal differences between auto- and allo-stimulation may be explained by the capacity of PFA to modify both autologous and allogeneic HLA, thus increasing their antigenicity. Allostimulation eventually revealed higher levels of MIP-1β and RANTES than autostimulation, suggesting that our methodology provided physiologically meaningful results. Nevertheless, a comparison of the proposed methods with the gold standard MLR procedure using irradiated cells remains warranted.
ESN FSWs in this study reported a high frequency of condom use with their clients (85% reporting always using condoms). This is probably the result of the intensive counselling efforts in this population over the past years [17]. However, the question that was asked referred to the previous working days and may not reflect their behaviour over a longer period of time. As a result of the high numbers of clients per week (median of 10) and the long duration of commercial sex work (> 3 years), most ESN FSWs likely have experienced HIV exposure. This can also be witnessed by an HIV incidence rate of 4% among HIV-seronegative FSWs in Abidjan [15]. In addition, a much lower proportion of FSWs reported using condoms with their regular partners (13% reporting always using condoms). Regular partners of FSWs probably belong to an HIV risk group (due to partial overlap with FSWs' clients), and will thus contribute to the HIV exposure among ESN FSWs.
Several studies have shown a role for HLA polymorphism in protection against HIV transmission [7–12], but its mechanism of action remains uncertain. In addition to the induction of alloimmune responses, differential HLA-restricted epitope recognition by HIV-specific CTL in recipient and donor, and HLA–KIR interactions that regulate NK cell activity have been proposed [36,37]. The present data could at least suggest that the putative anti-HIV activity of HLA does not depend on activated alloimmune responses. However, our findings could suggest a role for suppressed cellular alloimmune responses in protection against HIV infection. In that respect, in vitro allogeneic stimulation rendered PBMC more susceptible to HIV infection and reactivated HIV replication in latently infected resting CD4+ T-cells [38,39]. Also, blood transfusion to HIV-infected persons increased plasma viral load levels [40]. Therefore, we could hypothesize that down-regulation of cellular alloimmune responses in ESN FSWs constitutes a mechanism to silence potential HIV enhancing effects. Indeed, multiparous women, thought to have acquired suppressed cellular alloimmune responses [27], were less prone to HIV infection [41] and less likely to transmit HIV to their babies [42].
Anti-HLA class I antibodies, detected by a novel and accurate flow cytometry method applying HLA-coated polystyrene beads [43], were not found to occur more frequently among ESN FSWs than among FBDs. These findings confirm previous studies in Nairobi that did not find higher frequencies of class I antibodies among ESN FSWs or HIV-negative children born to seropositive mothers [13,14]. However, they are at variance with higher class I antibody levels detected among ESN drug users and in HIV-discordant couples [44,45]. In fact, in our study, ESN FSWs and FBDs with detectable antibodies were all between 29 and 39 years old. This could suggest that other factors like pregnancy or (multi)parity are responsible for anti-HLA antibody induction. Unfortunately, such data were not available in this study.
In conclusion, our data support the hypothesis that suppressed rather than activated cellular alloimmune responses could play a role in protection against infection with HIV.
Acknowledgments
We thank the community of female sex workers in Abidjan for their cooperation. Mathieu Maran, Kabran N'Guessan and N'Depo Yenon for technical assistance, Emmanuel Abonga for providing blood donor samples, Jef Braem and Serge Blockmans for logistical support in the field, Guido Vanham and Ward Schrooten for critical remarks and discussion. This work was financially supported by the Belgian Fund for Scientific Research (FWO)-Vlaanderen (grant G.0396.99) and the Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.Shearer GM, Pinto LA, Clerici M. Alloimmunization for immune-based therapy and vaccine design against HIV/AIDS. Immunol Today. 1999;20:66–71. doi: 10.1016/s0167-5699(98)01392-9. [DOI] [PubMed] [Google Scholar]
- 2.Stott EJ. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 3.Chan WL, Rodgers A, Hancock RD, et al. Protection in simian immunodeficiency virus-vaccinated monkeys correlates with anti-HLA class I antibody response. J Exp Med. 1992;176:1203–7. doi: 10.1084/jem.176.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur LO, Bess JWJ, Sowder RC, et al. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–8. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 5.Habeshaw J, Hounsell E, Dalgleish A. Does the HIV envelope induce a chronic graft-versus-host-like disease? Immunol Today. 1992;13:207–10. doi: 10.1016/0167-5699(92)90155-Z. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni PS, Butera ST, Duerr AC. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003;5:87–103. [PubMed] [Google Scholar]
- 7.Fowke KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi. Kenya Lancet. 1996;348:1347–51. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald KS, Fowke KR, Kimani J, et al. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:1581–9. doi: 10.1086/315472. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald KS, Embree JE, Nagelkerke NJ, et al. The HLA A2/6802 supertype is associated with reduced risk of perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183:503–6. doi: 10.1086/318092. [DOI] [PubMed] [Google Scholar]
- 10.Polycarpou A, Ntais C, Korber BT, et al. Association between maternal and infant class I and II HLA alleles and of their concordance with the risk of perinatal HIV type 1 transmission. AIDS Res Hum Retroviruses. 2002;18:741–6. doi: 10.1089/08892220260139477. [DOI] [PubMed] [Google Scholar]
- 11.Lockett SF, Robertson JR, Brettle RP, Yap PL, Middleton D, Leigh Brown AJ. Mismatched human leukocyte antigen alleles protect against heterosexual HIV transmission. J Acquir Immune Defic Syndr. 2001;27:277–80. doi: 10.1097/00126334-200107010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Dorak MT, Tang J, Penman-Aguilar A, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363:2137–9. doi: 10.1016/S0140-6736(04)16505-7. [DOI] [PubMed] [Google Scholar]
- 13.Luscher MA, Choy G, Njagi E, et al. Naturally occurring IgG anti-HLA alloantibody does not correlate with HIV type 1 resistance in Nairobi prostitutes. AIDS Res Hum Retroviruses. 1998;14:109–15. doi: 10.1089/aid.1998.14.109. [DOI] [PubMed] [Google Scholar]
- 14.Luscher MA, Choy G, Embree JE, et al. Anti-HLA alloantibody is found in children but does not correlate with a lack of HIV type 1 transmission from infected mothers. AIDS Res Hum Retroviruses. 1998;14:99–107. doi: 10.1089/aid.1998.14.99. [DOI] [PubMed] [Google Scholar]
- 15.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 16.Jennes W, Sawadogo S, Koblavi-Deme S, et al. Positive association between beta-chemokine-producing T cells and HIV type 1 viral load in HIV-infected subjects in Abidjan, Cote d'Ivoire. AIDS Res Hum Retroviruses. 2002;18:171–7. doi: 10.1089/08892220252781220. [DOI] [PubMed] [Google Scholar]
- 17.Ghys PD, Diallo MO, Ettiegne-Traore V, et al. Increase in condom use and decline in HIV and sexually transmitted diseases among female sex workers in Abidjan, Cote d'Ivoire, 1991–98. AIDS. 2002;16:251–8. doi: 10.1097/00002030-200201250-00015. [DOI] [PubMed] [Google Scholar]
- 18.Vuylsteke BL, Ghys PD, Traore M, et al. HIV prevalence and risk behavior among clients of female sex workers in Abidjan, Cote d'Ivoire. AIDS. 2003;17:1691–4. doi: 10.1097/00002030-200307250-00014. [DOI] [PubMed] [Google Scholar]
- 19.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 20.Kalwak K, Turkiewicz D, Ussowicz M, et al. Clinical value of the flow cytometric method for measuring lymphocyte subset activation: spontaneous activation of T-cell subpopulations is associated with acute GvHD. Transplant Proc. 2003;35:1559–62. doi: 10.1016/s0041-1345(03)00512-8. [DOI] [PubMed] [Google Scholar]
- 21.Grene E, Pinto LA, Cohen SS, et al. Generation of alloantigen-stimulated anti-human immunodeficiency virus activity is associated with HLA-A*02 expression. J Infect Dis. 2001;183:409–16. doi: 10.1086/318085. [DOI] [PubMed] [Google Scholar]
- 22.Jordan WJ, Ritter MA. Optimal analysis of composite cytokine responses during alloreactivity. J Immunol Meth. 2002;260:1–14. doi: 10.1016/s0022-1759(01)00490-2. [DOI] [PubMed] [Google Scholar]
- 23.Jennes W, Vuylsteke B, Borget MY, et al. HIV-specific T helper responses and frequency of exposure among HIV-exposed seronegative female sex workers in Abidjan, Cote d'Ivoire. J Infect Dis. 2004;189:602–10. doi: 10.1086/381454. [DOI] [PubMed] [Google Scholar]
- 24.Opelz G, Vanrenterghem Y, Kirste G, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–7. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13:243–54. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 26.Dekker G. The partner's role in the etiology of preeclampsia. J Reprod Immunol. 2002;57:203–15. doi: 10.1016/s0165-0378(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 27.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 28.Mowbray JF, Underwood JL, Michel M, Forbes PB, Beard RW. Immunisation with paternal lymphocytes in women with recurrent miscarriage. Lancet. 1987;2:679–80. doi: 10.1016/s0140-6736(87)92457-3. [DOI] [PubMed] [Google Scholar]
- 29.Katano K, Aoki K, Ogasawara MS, Suzumori K. Adverse influence of numbers of previous miscarriages on results of paternal lymphocyte immunization in patients with recurrent spontaneous abortions. Am J Reprod Immunol. 2000;44:289–92. doi: 10.1111/j.8755-8920.2000.440507.x. [DOI] [PubMed] [Google Scholar]
- 30.Komlos L, Vardimon D, Notmann J, et al. Mixed maternal-paternal lymphocyte cultures before and after immunotherapy for recurrent spontaneous abortions. Am J Reprod Immunol. 1996;35:30–3. doi: 10.1111/j.1600-0897.1996.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramhorst R, Garcia V, Agriello E, et al. Intracellular expression of CD69 in endometrial and peripheral T cells represents a useful marker in women with recurrent miscarriage: modulation after allogeneic leukocyte immunotherapy. Am J Reprod Immunol. 2003;49:149–58. doi: 10.1034/j.1600-0897.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa S, Karasaki-Suzuki M, Itoh T, et al. Effects of paternal lymphocyte immunization on peripheral Th1/Th2 balance and TCR V beta and V gamma repertoire usage of patients with recurrent spontaneous abortions. Am J Reprod Immunol. 2000;43:107–15. doi: 10.1111/j.8755-8920.2000.430207.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Tao L, Mitchell E, et al. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat Med. 1999;5:1004–9. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Underwood J, Vaughan R, Harmer A, Doyle C, Lehner T. Allo-immunization elicits CCR5 antibodies, SDF-1 chemokines, and CD8-suppressor factors that inhibit transmission of R5 and X4 HIV-1 in women. Clin Exp Immunol. 2002;129:493–501. doi: 10.1046/j.1365-2249.2002.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters B, Whittall T, Babaahmady K, Gray K, Vaughan R, Lehner T. Effect of heterosexual intercourse on mucosal alloimmunisation and resistance to HIV-1 infection. Lancet. 2004;363:518–24. doi: 10.1016/S0140-6736(04)15538-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaslow RA, Dorak T, Tang JJ. Influence of host genetic variation on susceptibility to HIV type 1 infection. J Infect Dis. 2005;191:S68–S77. doi: 10.1086/425269. [DOI] [PubMed] [Google Scholar]
- 37.Stephens HA. HIV-1 diversity versus HLA class I polymorphism. Trends Immunol. 2005;26:41–7. doi: 10.1016/j.it.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Busch MP, Lee TH, Heitman J. Allogeneic leukocytes but not therapeutic blood elements induce reactivation and dissemination of latent human immunodeficiency virus type 1 infection: implications for transfusion support of infected patients. Blood. 1992;80:2128–35. [PubMed] [Google Scholar]
- 39.Moriuchi H, Moriuchi M, Fauci AS. Induction of HIV-1 replication by allogeneic stimulation. J Immunol. 1999;162:7543–8. [PubMed] [Google Scholar]
- 40.Mudido PM, Georges D, Dorazio D, et al. Human immunodeficiency virus type 1 activation after blood transfusion. Transfusion. 1996;36:860–5. doi: 10.1046/j.1537-2995.1996.361097017170.x. [DOI] [PubMed] [Google Scholar]
- 41.Celum CL, Coombs RW, Jones M, et al. Risk factors for repeatedly reactive HIV-1 EIA and indeterminate western blots. A population-based case-control study. Arch Intern Med. 1994;154:1129–37. [PubMed] [Google Scholar]
- 42.Kind C. Mother-to-child transmission of human immunodeficiency virus type 1: influence of parity and mode of delivery. Paediatric AIDS Group of Switzerland. Eur J Pediatr. 1995;154:542–5. doi: 10.1007/BF02074831. [DOI] [PubMed] [Google Scholar]
- 43.Worthington JE, Robson AJ, Sheldon S, Langton A, Martin S. A comparison of enzyme-linked immunoabsorbent assays and flow cytometry techniques for the detection of HLA specific antibodies. Hum Immunol. 2001;62:1178–84. doi: 10.1016/s0198-8859(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 44.Beretta A, Weiss SH, Rappocciolo G, et al. Human immunodeficiency virus type 1 (HIV-1)-seronegative injection drug users at risk for HIV exposure have antibodies to HLA class I antigens and T cells specific for HIV envelope. J Infect Dis. 1996;173:472–6. doi: 10.1093/infdis/173.2.472. [DOI] [PubMed] [Google Scholar]
- 45.Lopalco L, Pastori C, Cosma A, et al. Anti-cell antibodies in exposed seronegative individuals with HIV type 1-neutralizing activity. AIDS Res Hum Retroviruses. 2000;16:109–15. doi: 10.1089/088922200309458. [DOI] [PubMed] [Google Scholar]