Abstract
The aetiology of sarcoidosis, an inflammatory granulomatous multi-system disorder, is unclear. It is thought to be the product of an unknown exogenous antigenic stimulus and an endogenous genetic susceptibility. Toll-like receptors (TLR) are signal molecules essential for the cellular response to bacterial cell wall components. Lipopolysaccharide (LPS), for example, binds to TLR 4. Two different polymorphisms for the TLR4 gene (Asp299Gly and Thr399Ile) have been described recently. This leads to a change in the extracellular matrix function of TLR4 and to impaired LPS signal transduction. We genotyped a total of 141 Caucasian patients with sarcoidosis and 141 healthy unrelated controls for the Asp299Gly and Thr399Ile polymorphisms in the TLR4 gene. The mutations were identified with polymerase chain reaction followed by restriction fragment length polymorphism (RFLP) analysis. Among sarcoidosis patients the prevalence for each Asp299Gly and Thr399Ile mutant allele was 15·6% (22/141). In the control group the prevalence was 5·67% (8/141) (P = 0·07). In the subgroup of patients with acute sarcoidosis there was no difference in the control group (P = 0·93), but there was a highly significant association between patients with a chronic course of sarcoidosis and TLR4 gene polymorphisms (P = 0·01).
Keywords: innate immunity, polymorphism, receptor, sarcoidosis, TLR4
Introduction
Sarcoidosis is an inflammatory granulomatous multi-system disorder, affecting primarily the lungs and lymph nodes. Multiple organs, however, can be affected. The disease is characterized by non-caseating granulomas and an exaggerated cellular immune response caused by increased inflammatory activity [1]. Overall, the course of most sarcoidosis patients is short and favourable. Chest X-ray is used to describe the different stages of sarcoidosis. Unfortunately, about 20% of patients have a chronic and prolonged course which needs to be treated with corticosteroids. Up to 5% of all patients have severe complications such as lung fibrosis. Although many attempts at understanding the pathogenesis of sarcoidosis have been made during recent years, the aetiology remains unclear; it is thought to be the product of an unknown exogenous antigenic stimulus and endogenous genetic susceptibility. In particular, earlier observations underline the importance of the interaction between unknown exogenous stimuli and cell surface receptors in sarcoidosis. In order to understand this supposed mechanism in sarcoidosis, we therefore focused our work on critical cell surface receptors which bind exogenous stimuli [2–4]. It is well established that immunity-related receptors and proinflammatory cytokines, which include tumour necrosis factor (TNF)-α [5], interleukin (IL)-1β [6], IL-2 [7], IL-6, IL-8 [8] and interferon (IFN)-γ [9], play a pivotal role in the formation of granulomas in sarcoidosis. The early phase in developing sarcoid granulomas is characterized by the accumulation and activation of T helper cells type 1 (Th1) lymphocytes, which lead to an immune response. Lymphocytic alveolitis develops after attraction of other immunocompetent cells by mediators leading to non-caseating granuloma formation.
There is evidence that the cytokine and cytokine receptor pattern in individual patients is able to differentiate a short, favourable course in sarcoidosis from a chronic course with ongoing inflammation [10].
Toll-like receptors (TLRs) have a crucial role in detecting the presence of infection. TLRs induce activation of the inflammatory and anti-microbial innate and adaptive immune responses [11,12]. It is known that TLRs are associated with a number of pathological conditions of different lung diseases (for review see [13]); at least 11 different types of TLRs have been described in mammals [14]. The best-characterized receptor is TLR4, which seems to be an important receptor of pathogen-associated molecular patterns (PAMPs) such as bacterial lipopolysaccharide (LPS) of Gram-negative organisms [15–17], and has been found to play a pivotal role in sepsis [18]. Furthermore, it has been demonstrated that specific mutations in the TLR4 coding region (Asp299Gly and Thr399Ile) are associated with diminished airway response to inhaled LPS in normal human volunteers [19]. According to these results, an increased risk for Gram-negative infections was found for people who carry these mutations [20].
The role of the TLR4 pathway in sarcoidosis, however, is poorly understood. As antigenic or even microbial factors seem to be involved in the pathogenesis of sarcoidosis, we examined 141 patients with sarcoidosis and 141 control subjects for TLR4 polymorphisms (Asp299Gly and Thr399Ile).
Material and methods
Patient population
The research was carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association, and the study was approved by the Ethics Committee of Bonn University School of Medicine. Written informed consent was obtained from each patient prior to their enrolment.
Patients with severe medical disorders, including pulmonary diseases such as chronic obstructive lung disease and asthma bronchiale or immunological disorders, were excluded from the study. All patients were at least 18 years old.
Sarcoidosis group
One hundred and forty-one Caucasian patients with sarcoidosis (76 females, 65 males, age 51·06 ± 12·90 years) were included in the present study. The patients were recruited from the Department of Internal Medicine, Medizinische Poliklinik, Rheinische Friedrich Wilhelms University, Bonn, Germany and the Department of Internal Medicine, Städtisches Klinikum St Georg, Leipzig, Germany.
The diagnosis of sarcoidosis was made when patients had biopsy evidence of non-caseating epitheloid cell granuloma in any organ and chest X-ray (posterior–anterior and lateral) abnormalities. The stages were defined as follows: stage I characterizes bilateral hilar lymphadenopathy; stage II additional parenchymal infiltrates; in stage III only pulmonary infiltrates can be seen; stage 0 describes an extrapulmonary manifestation; and stage IV lung fibrosis [21]. The diagnosis was completed by history data, physical examination, lung function tests, chest computed tomography and bronchoalveolar lavage (BAL) fluid analysis.
In the statistical analyses a chronic recurrent course was defined as all patients with a chronic course over at least 2 years or at least two episodes in a lifetime. Acute sarcoidosis was defined as only one episode of an acute sarcoidosis stage I, totally resolved at the date of examination.
Control group
One hundred and forty-one Caucasian healthy, unrelated volunteers served as control subjects (86 females, 55 males, age 38·32 ± 15·53 years). They were all residents of Germany and were selected in pre-employment physical examinations at the University of Bonn, Germany and the Städtisches Klinikum Leipzig, Germany. None had a history of lung disease or showed any symptoms of lung or other disease. All showed normal findings on chest X-ray and laboratory examination, which included complete blood counts, urine analyses, hepatic enzyme activities and blood, urea and nitrogen (BUN) levels.
Blood samples and DNA isolation
Peripheral venous blood samples (9 ml) were drawn from each patient by standard venous puncture. Each blood sample was collected in sterile tubes containing 15% K3 ethylenediamine tetraacetic acid (EDTA) solution. DNA was isolated by the salting-out procedure described by Miller et al. [22].
Determination of the genotype of the TLR4 gene
Polymerase chain reaction (PCR) was used to determine the genotypes of both TLR4 polymorphisms from genomic DNA. To determine the TLR4 genotypes of the samples with respect to residues 299 and 399 DNA was amplified with primers described first by Lorenz et al. (Table 1) [23].
Table 1.
Sequences of primers as used for polymerase chain reaction (PCR).
| Gene | Polymorphism | Primers | MgCl2 |
|---|---|---|---|
| TLR4 | Asp299Gly | F: 5′-GATTAGCATACTTAGACTACTACCTCCATG-3′ | 3 mM |
| R: 5′-GATCAACTTCTGAAAAAGCATTCCCAC-3′ | |||
| TLR4 | Thr399Ile | F: 5′-GGTTGCTGTTCTCAAAGTGATTTTGGGAGAA-3′ | 2·5 mM |
| R: 5′-ACCTGAAGACTGGAGAGTGAGTTAAATGCT-3′ |
PCR was carried out in a 25-µl reaction mixture containing 1 µg of genomic DNA, 1 µl of each 10 µM primer (MWG-Biotech, Ebersberg, Germany), 0·5 U Taq polymerase (Invitrogen, Karlsruhe, Germany), 1 µl of each 1·25 mM base (MWG-Biotech) and 2·5 µl 10 × PCR buffer (Invitrogen). The cycling condition consisted of an initial cycle 95°C for 6 min in a thermocycler followed by 31 cycles of denaturation with 95°C for 30 s, annealing with 61°C for 30 s and extension with 72°C for 30 s for all pairs of primers. A final synthesis step with 72°C for 10 min terminated the reaction. Genotypes were determined by electrophoresis on 2% SeaKem® agarose gel (Biozym, Hess Oldendorf, Germany) and staining with ethidium bromide.
Ten µl of the resulting PCR products were used for an overnight digest mixed with 0·2 µl of the appropriate restriction enzyme (10 U/µl; Asp299Gly: Nco I; Thr399Ile: Hinf I, Invitrogen), 1·5 µl 10 × restriction buffer and 1·3 µl water.
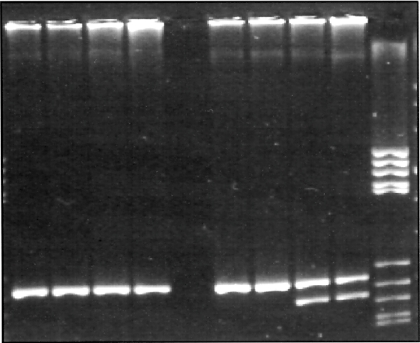
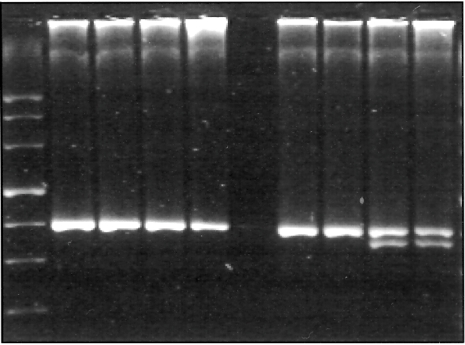
Gels were run on a 4% MetaPhor® agarose gel (Biozym) to determine the TLR4 alleles (Figs. 1 and 2, Table 2).
Fig. 1.
Toll-like receptor 4 Asp299Gly 3% MetaPhor® gel.
Fig. 2.
Toll-like receptor 4 Thr399Ile 3% MetaPhor® gel.
Table 2.
Restriction enzymes and length of restriction fragments.
| Gene | Polymorphism | Restriction enzyme | Restriction temp °C | Length of restriction fragments |
|---|---|---|---|---|
| TLR4 | Asp299Gly | Nco I | 37°C | Wild-type (allele A): 249 bp |
| Asp299Gly (allele G): 223 +26 bp | ||||
| TLR4 | Thr399Ile | Hinf I | 37°C | Wild-type (allele C): 407 bp |
| Thr399Ile (allele T): 378 +29 bp |
bp: Base pairs.
Statistical analysis
All statistical analyses were performed using a statistical software package (spss version 12·0; SPSS, Inc., Chicago, IL, USA). Demographic data of patients having different TLR4 gene polymorphisms were compared using a one-way analysis of variance. Differences in the frequencies of alleles and genotypes between patients and control subjects were tested by the χ2 test, as recommended and tested by independent researchers of the Institute of Biomathematics (IMBIE), University, Bonn.
Results
The sarcoidosis and the control groups are characterized in Table 3. Among 141 healthy German Caucasians tested, eight were heterozygous for Asp299Gly and eight were heterozygous for Thr399Ile (allele frequencies: 2·84%; 8/282) (Table 4). All healthy volunteers with mutations in Asp299Gly also showed a polymorphism in Thr399Ile. None of the patients in the control group showed a homozygous TLR4 polymorphism (Table 4). Among 141 patients with sarcoidosis in all stages, 22 had a heterozygous polymorphism for Asp299Gly and 22 were heterozygous for Thr399Ile (allele frequencies: 7·80%; 22/282) (Table 4). All polymorphisms occurred again in tandem. We found no homozygous mutations. Asp299Gly polymorphisms and Thr399Ile polymorphisms occurred more frequently, but not statistically, in patients with sarcoidosis compared to the healthy control group (P = 0·07, χ2 test). In addition, we compared 108 patients with a chronic recurrent course of sarcoidosis with healthy volunteers. It could be shown that 20 patients in the chronic sarcoidosis group showed an Asp299Gly polymorphism and 20 patients had a Thr399Ile polymorphism (allele frequencies: 9·26%; 20/216). The results were therefore even more prominent in patients with a chronic course of sarcoidosis (P = 0·01) (Table 5). Taken together there was an association, although not significant, with the TLR4 polymorphisms studied here and sarcoidosis. There was a significant correlation between a chronic course of sarcoidosis and the prevalence of TLR4 polymorphisms, whereas there seemed to be no association between the onset of acute sarcoidosis and the prevalence of TLR4 polymorphisms (P = 0·93).
Table 3.
Characterization of patients with sarcoidosis and healthy control subjects.
| No. | Age (years) | Female | Male | Stage at diagnosis | |
|---|---|---|---|---|---|
| Sarcoidosis | 141 | 51·06 ± 12·90 | 76 | 65 | I: 47 |
| All patients | II: 74 | ||||
| III: 17 | |||||
| 0: 3 | |||||
| Acute sarcoidosis | 33 | 48·09 ± 14·87 | 18 | 15 | |
| Chronic sarcoidosis | 108 | 51·96 ± 12·18 | 58 | 50 | |
| Control | 141 | 38·32 ± 15·53 | 86 | 55 |
Table 4.
Prevalence of the Toll-like receptor-4 mutant alleles (Asp299Gly, Thr399Ile) in individuals with sarcoidosis and the healthy control subjects.
| Total no. | Asp299Gly | Wild-type | Thr399Ile | Wild-type | |
|---|---|---|---|---|---|
| Sarcoidosis (total) | 141 | 22 | 119 | 22 | 119 |
| Sarcoidosis (acute/Löfgren's) | 33 | 2 | 31 | 2 | 31 |
| Sarcoidosis (chronic course) | 108 | 20 | 88 | 20 | 88 |
| Control | 141 | 8 | 133 | 8 | 133 |
Table 5.
Statistical analysis of data as obtained with Pearson's χ2-test (P < 0·05).
| Asp299Gly allele frequency (versus control) | Thr399Ile allele frequency (versus control) | |
|---|---|---|
| Sarcoidosis (total) | P = 0·07 | P = 0·07 |
| Sarcoidosis (acute) | P = 0·93 | P = 0·93 |
| Sarcoidosis (chronic course) | P = 0·01 | P = 0·01 |
Discussion
In this study we show that TLR4 polymorphisms are associated with a chronic course of sarcoidosis. Toll-like receptors have a crucial role in the detection of microbial infections. They are expressed on a variety of human cells, such as macrophages, endothelial cells, smooth muscle cells, T cells and dendritic cells (DC). The first and best-described of at least 11 different TLRs is TLR4. After detecting the presence of infection by recognizing conserved microbial products (PAMPs) such as LPS, activation of inflammatory innate immune responses is induced. A complex of LPS and the serum LPS-binding protein (LBP) initiates signals through either membrane-bound or soluble CD14 [24,25]. After binding to TLR4 the cytoplasmic adaptor protein MyD88 and nuclear factor kappa B (NF-κB) is activated, which induces the expression of inflammatory cytokines [26], but there seems to be a second MyD88 independent pathway leading to delayed activation of NF-κB [27]. Activation of the NF-κB signalling pathway has been connected to the pathogenesis of sarcoidosis [28]. There is evidence that in response to LPS stimulation, inhibitor kappa B-alpha (IκB-α) degrades and NF-κB is activated [29]. In sarcoidosis, IκB-α promoter polymorphisms in UK and Dutch sarcoidosis patients seem to be linked to the inflammatory process.
In adaptive immunity TLRs are expressed on antigen-presenting cells such as DC, and are thought to be involved in the maturation of DC and in the change of naive T cells to T helper cells [30,31]. Several investigations have provided evidence that signalling via TLR4-dependent pathways is crucial for the generation of antigen-specific Th1-mediated immune responses, but not Th2-type T cell responses [32–35]. In sarcoidosis a Th1-type T cell response is likely to favour the granulomatous response at sites of disease activity. It has been suggested that Th1 polarization is associated with the pathophysiology of sarcoidosis [36,37]. However, it is still unclear how this leading Th1 response is induced in the formation of granuloma. Furthermore, it is hypothesized that the DC may migrate into sarcoid-infected tissues, contributing to formation of the granulomas in sarcoidosis [38].
It has been shown previously that TLRs and particularly TLR4 are implicated in the regulation of a variety of inflammatory and immune disorders. For instance, TLR4 was attributed with the mediation of microbial recognition in the intestine, which ultimately triggers the development of chronic enterocolitis in mice [39]. Furthermore, it could be shown that the TLR4-activated MyD88 pathway may be involved in allograft transplantation rejection [40] as well as in the development of atherosclerosis [41].
Two specific mutations in the TLR4 coding region (Asp299Gly and Thr399Ile) are associated with diminished airway response to inhaled LPS in normal human volunteers. These mutations consist of single nucleotide polymorphisms (SNPs) which result in missense mutations. At Asp299Gly an A896G substitution leads to the replacement of an aspartic acid residue with glycine at amino acid 299, which leads probably to an altered extracellular domain of TLR4. At the Thr399Ile missense mutation an isoleucine also replaces a threonine at amino acid 399 in the extracellular matrix of TLR4. Both polymorphisms are frequent and occur at an allelic frequency of approximately 3–4% in accordance with a carriage rate of 6–8% in the Caucasian population [42–45]. Homozygous mutations occur very rarely. Therefore, the incidence of heterozygous polymorphisms could be of diagnostic value in the evaluation of genetic disease patterns. The overall prevalence of both TLR4 polymorphisms in the Caucasian population has been confirmed in our work and was found to be at 5·67%, although these results are slightly lower than the estimate.
TLR4 polymorphisms have been examined with regard to chronic inflammatory diseases; contradictory results have been shown. In 98 patients with ulcerative colitis, allele and carrier frequencies for the Thr399Ile mutation in TLR4 gene were increased significantly compared to 145 controls [46]. In 447 Belgian patients with Crohn's disease (CD) a significant association between the TLR4 Asp299Gly polymorphism was found. The same group examined 163 Belgian patients with ulcerative colitis and also found a significant association with the Asp299Gly polymorphism compared with the control population [47].
Asp299Gly and Thr399Ile polymorphisms showed no association with multiple sclerosis (MS) in Austrian patients [48]. These results could be confirmed in a second study: in 890 patients with MS and 350 healthy controls, no association with MS susceptibility or disease severity was found with regard to Asp299Gly polymorphism [49]. No association was observed between both TLR4 mutations and chronic periodontitis, which is regarded as an infection with Gram-negative bacteria. The prevalence of Asp299Gly and Thr399Ile mutant alleles was 4·1% and 4·5% among periodontitis patients, respectively, similar to the healthy volunteers [45]. In 527 Hungarian patients with CD compared to 200 healthy controls, no difference between wild-type and heterozygous TLR4 Asp299Gly polymorphisms could be found [50]. With respect to pulmonary disease patterns, Arbour and colleagues have demonstrated that individuals with the Asp299Gly mutation and the Thr399Ile mutation were hyporesponsive to inhaled LPS with regard to bronchial obstruction, although not every patient with a Asp299Gly polymorphism showed hyporesponsiveness and some of the tested people with hyporesponsiveness expressed wild-type TLR4 [19]. With regard to NF-κB, only Asp299Gly polymorphisms showed a significant reduction of activation in transfected human monocytic THP-1 cells. Erridge and colleagues have examined monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the TLR4 gene and found no deficits in lipopolysaccharide signalling [51]. These authors propose that there must be many more determinants of other genetic and acquired traits of individuals than of the TLR4 mutations. As it is apparent that both examined TLR4 mutations are co-segregated, it is possible that there is a coincidence with other polymorphisms in either TLR4 or in different genes, which cause a different reaction to either LPS or other cytokines. A difference has been shown in the responsiveness of inhaled LPS in vivo and incubated monocytes in vitro, so there could be more receptors binding to LPS than TLR4, which lead to bronchial obstruction or NF-κB activation. As TLR4 polymorphisms occur in an allele frequency that ranges from approximately 3% to 6% in the whole population, and the prevalence of sarcoidosis is approximately one case to 40 cases per 100 000 population (0·001–0·04%) other factors, endogenous or exogenous, innate or acquired, probably modulate the clinical course of sarcoidosis.
It could be shown that the human leucocyte antigens human leucocyte antigens (HLA)-DQB1*0201 and HLA-DR17 are genetic markers for a positive prognosis in sarcoidosis [52,53]. Furthermore, it has been demonstrated that the deletion allele of the angiotensin-converting enzyme (ACE) gene is associated with a prolonged clinical course in Finnish patients [54]. A polymorphism of TNF-β seems to be a marker of a prolonged clinical course in patients with sarcoidosis [55].
It has been demonstrated recently that sarcoidosis is associated with a truncating splice site mutation in the butyrophilin-like (BTNL) 2 gene, which leads to T cell activation after stimulation with proinflammatory cytokines such as IL-1β and LPS [56].
In conclusion, the functional effective mutations Asp299Gly and Thr399Ile of the Toll-like receptor 4 gene are much more prevalent in the sarcoidosis group than in healthy control subjects; among patients with acute sarcoidosis/Löfgren's syndrome the TLR4 mutations are found equally to healthy control subjects. However, there is a significant association between the chronic course of sarcoidosis and TLR4 mutations. Therefore, we propose that TLR4 polymorphisms, either via altered PAMP signalling or still-unknown ligands, may contribute to the chronic course of sarcoidosis.
Acknowledgments
The authors wish to thank all the patients in the present study for their participation. This study was supported by grants from the Deutsche Forschungsgemeinschaft and institutional grants from BONFOR. We would also like to thank B. Hecht for her participation in this study and Dr Rolf Fimmers of the Institute of Biomathematics (University of Bonn) for his help. S. Pabst and G. Baumgarten contributed equally to this work.
References
- 1.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 2.Lympany PA, du Bois RM. Diffuse lung disease: product of genetic susceptibility and environmental encounters. Thorax. 1997;52:92–4. doi: 10.1136/thx.52.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrath DS, Goh N, Foley PJ, du Bois RM. Sarcoidosis: genes and microbes − soil or seed? Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:149–64. [PubMed] [Google Scholar]
- 4.Müller-Quernheim J. Sarcoidosis: immunopathogenic concepts and their clinical application. Eur Respir J. 1998;12:716–38. doi: 10.1183/09031936.98.12030716. [DOI] [PubMed] [Google Scholar]
- 5.Bachwich PR, Lynch JP, III, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol. 1986;125:421–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Hunninghake GW. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984;129:569–72. [PubMed] [Google Scholar]
- 7.Müller-Quernheim J, Saltini C, Sondermeyer P, Crystal RG. Compartmentalized activation of the interleukin 2 gene by lung T lymphocytes in patients in active pulmonary sarcoidosis. J Immunol. 1986;137:3475–83. [PubMed] [Google Scholar]
- 8.Takizawa H, Satoh M, Okazaki H, et al. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: correlation with the clinical parameters. Clin Exp Immunol. 1997;107:175–81. doi: 10.1046/j.1365-2249.1997.d01-905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75:1488–95. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegenhagen N, Müller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med. 2003;253:18–30. doi: 10.1046/j.1365-2796.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 11.Akira A, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DR. Toll-like receptors and other links between innate and acquired alloimmunity. Curr Opin Immunol. 2004;16:538–44. doi: 10.1016/j.coi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–92. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 15.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyposensitive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 17.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophilia Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with Gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 19.Arbour NC, Lorenz E, Schutte B, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 20.Agnese DM, Calvano JE, Hahm SJ, et al. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of Gram-negative infections. J Infect Dis. 2002;186:1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 21.Costabel U, Hunninghake GW. Statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. [DOI] [PubMed] [Google Scholar]
- 22.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz Frees KL, Schwartz DA. Determination of the TLR4 genotype using allele-specific PCR. Biotechniques. 2001;31:22–4. doi: 10.2144/01311bm01. [DOI] [PubMed] [Google Scholar]
- 24.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 25.Akashi S, Ogata H, Kirikae F, et al. Regulatory roles for CD14 and phosphatidylinositol in the signaling via Toll-like receptor 4-MD-2. Biochem Biophys Res Commun. 2000;268:172–7. doi: 10.1006/bbrc.2000.2089. [DOI] [PubMed] [Google Scholar]
- 26.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of Myd88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 28.Abdallah A, Sato H, Grutters JC, et al. Inhibitor kappa B-alpha (IκB-α) promoter polymorphisms in UK and Dutch sarcoidosis. Genes Immun. 2003;4:450–4. doi: 10.1038/sj.gene.6364001. [DOI] [PubMed] [Google Scholar]
- 29.Mevedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 31.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnare M, Barton GM, Holt AC, Takeda K, Shizuo A, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nature Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 33.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–9. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 34.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10-/- setting. Immunity. 2002;16:429–39. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 35.Tesar BM, Zhang J, Li Q, Goldstein DR. Th1 immune response to fully MHC mismatched allografts are diminished in the absence of MyD88, a Toll-like receptor signal adaptor protein. Am J Transplant. 2004;4:1429–39. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 36.Wahlström J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–21. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 37.Moller DR, Forman JD, Liu MC, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–60. [PubMed] [Google Scholar]
- 38.Ota M, Amakawa R, Uehira K, et al. Involvement of dendritic cells in sarcoidosis. Thorax. 2004;59:408–13. doi: 10.1136/thx.2003.006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi M, Kweon M, Kuwata H, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent IL-12p40 production causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein DR, Tesar BM, Akira S, Lakkis F. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111:1571–8. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;357:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 43.Brand S, Staudinger T, Schnitzler F, et al. The role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn's disease. Inflamm Bowel Dis. 2005;11:645–52. doi: 10.1097/01.mib.0000168372.94907.d2. [DOI] [PubMed] [Google Scholar]
- 44.Gazouli M, Mantzaris G, Kotsinas A, et al. Association between polymorphisms in the Toll-like receptor 4, CD 14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J Gastroenterol. 2005;11:681–5. doi: 10.3748/wjg.v11.i5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folwaczny M, Glas J, Török HP, Limbersky O, Folwaczny C. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol. 2004;135:330–5. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torok HP, Glas J, Tonenchi L, Mussack T, Folwaczny C. Polymorphisms of the lipopolysaccharide-signaling complex in inflammatory bowel disease: association of a mutation in the Toll-like receptor 4 gene with ulcerative colitis. Clin Immunol. 2004;112:85–91. doi: 10.1016/j.clim.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Franchimont D, Vermeire S, El Housni H, et al. Deficient host–bacteria interactions in inflammatory bowel disease? The Toll-like receptor (TLR)-4 Asp299Gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut. 2004;53:987–92. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reindl M, Lutterotti A, Ingram J, et al. Mutations in the gene for Toll-like receptor 4 and multiple sclerosis. Tissue Antigens. 2003;61:85–8. doi: 10.1034/j.1399-0039.2003.610108.x. [DOI] [PubMed] [Google Scholar]
- 49.Kroner A, Vogel F, Kolb-Maurer A, et al. Impact of the Asp299Gly polymorphism in the Toll-like receptor 4 (TLR-4) gene on disease course of multiple sclerosis. J Neuroimmunol. 2005;165:161–5. doi: 10.1016/j.jneuroim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Lakatos PL, Lakatos L, Szalay F, et al. Hungarian IBD Study Group. Toll-like receptor 4 and NOD2/CARD15 mutations in Hungarian patients with Crohn's disease: phenotype–genotype correlations. World J Gastroenterol. 2005;11:1489–95. doi: 10.3748/wjg.v11.i10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erridge C, Stewart J, Poxton IR. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med. 2003;197:1787–91. doi: 10.1084/jem.20022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berlin M, Fogdell-Hahn A, Olerup O, Eklund A, Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 1997;156:1601–5. doi: 10.1164/ajrccm.156.5.9704069. [DOI] [PubMed] [Google Scholar]
- 53.Sato H, Grutters JC, Pantelidis P, et al. HLA-DQB1*0201: a marker for good prognosis in British and Dutch patients with sarcoidosis. Am J Respir Cell Mol Biol. 2002;27:406–12. doi: 10.1165/rcmb.4782. [DOI] [PubMed] [Google Scholar]
- 54.Pietinalho A, Furuya K, Yamagucchi E, Kawakami Y, Selroos O. The angiotensin-converting enzyme DD gene is associated with poor prognosis in Finnish sarcoidosis patients. Eur Respir J. 1999;13:723–6. doi: 10.1034/j.1399-3003.1999.13d04.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi E, Itoh A, Hizawa N, Kawakami Y. The gene polymorphism of tumor necrosis factor-beta, but not that of tumor necrosis factor-alpha, is associated with the prognosis of sarcoidosis. Chest. 2001;119:678–9. doi: 10.1378/chest.119.3.753. [DOI] [PubMed] [Google Scholar]
- 56.Valentonyte R, Hampe J, Hus K, et al. Sarcoidois is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357–64. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]