Abstract
Proteinase inhibitor 9 (PI-9) is an intracellular serpin expressed in lymphocytes and monocyte-derived cells. It is the only known endogenous natural antagonist of granzyme B (GrB), and its proposed function is protection of cells from misdirected GrB. We have studied the regulation of PI-9 in primary peripheral blood mononuclear cells (PBMCs) following ex-vivo stimulation, and in PBMCs from patients suffering from viral or bacterial infections. By intracellular flow cytometry, we found identical PI-9 expression in all lymphocyte subsets, lower levels in monocytes and none in granulocytes. PI-9 was stable for 48 h in the presence of cycloheximide, indicating slow protein turnover. Incubation of PBMCs with several stimuli including lipopolysaccharide (LPS) led to up-regulation in the monocyte, but not the lymphocyte fraction, within 48 h, inhibitable by the NF-κB inhibitor pyrrolidin dithiocarbamate (PTDC). Up-regulation of PI-9 was observed in lymphocytes and monocytes of patients with acute Epstein–Barr virus (EBV), but not bacterial infection. Preterm infants had similar PI-9 expression as adults in monocytes, but lower in lymphocytes, decreasing during bacterial infection. Taken together, our data indicate that PI-9 is rapidly up-regulated upon stimulation of monocytes, but not lymphocytes. By protecting monocytes and macrophages from misdirected GrB in the inflammatory process, PI-9 might be involved in the regulation of antigen presentation.
Keywords: cytotoxicity, granzyme B, lymphocytes, monocytes, PI-9
Introduction
Cytotoxic T and natural killer (NK) cells kill their targets by death-inducing ligands, such as CD95 ligand, and by cytolytic granules, which contain the pore-forming protein perforin, together with several proteolytic enzymes, especially granzyme B (GrB). GrB, when released into the cytosol, induces apoptosis by activation of several components of the caspase cascade. Proteinase inhibitor 9 (PI-9/SERPINB9) is a 42 kDa human serpin that inactivates GrB irreversibly [1–8]. It is the only known endogenous natural inhibitor of GrB, and PI-9 overexpressing cells acquire protection from GrB-dependent, but not CD95-dependent cytotoxicity [2]. PI-9 is expressed in the cytoplasm and nuclei of many cell types, especially cytotoxic lymphocytes (T and NK cells), antigen-presenting cells (dendritic cells, macrophages, B cells) and cells at immunoprivileged sites, such as the placenta, the testis, the ovary and the eye [6–11]. High expression of PI-9 has also been observed in Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines [2]. The present concept of the biological function of PI-9 is protection of cytotoxic or bystander cells at sites of inflammation from misdirected GrB [2,6,7,11]. Further, PI-9 may inhibit caspase 1, which mediates inflammation by activating the cytokines interleukin 1-beta (IL-1β) and IL-18 [12]. Therefore, PI-9 may be regarded as a protein down-regulating the cytotoxic immune response.
Several observations, however, indicate that the role of PI-9 may be more complex than thought previously. Elevated urine excretion of PI-9 RNA correlates with transplant rejection in kidney recipients, indicating that PI-9 may also be a marker of immune stimulation [13]. In hepatocytes, PI-9 up-regulation occurs during viral infection, induced by inflammatory cytokines such as interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), leading to protection of infected and non-infected hepatocytes in the inflammatory crossfire [14]. Furthermore, autologous GrB, leaking into the cytosol, mediates activation-induced cell death of NK cells. This suggests that PI-9 may be involved in the homeostasis of NK cells [15], and possibly in cytotoxic T lymphocytes (CTL) [16]. Further, PI-9 expressed in antigen-presenting cells may lead to enhanced T cell stimulation. Upon maturation of murine dendritic cells, up-regulation of the mouse PI-9 homologue serine protease inhibitor-6 (SPI-6) was observed [11]. In a DNA vaccine model T cellular responses could be potentiated by co-administration of DNA encoding SPI-6 with the vaccine [17].
Knowledge of the mechanisms regulating PI-9 expression on the cellular level has been growing in recent years. Nuclear factor-kappa B (NF-κB) and activating protein-1 (AP-1)-responsive elements have been identified in the promotor of PI-9 [12], and a unique downstream oestrogen-responsive element is involved [18]. However, most data on regulation and function of PI-9 have been obtained using transformed or transfected cell lines, and the observations of PI-9 involvement in different conditions of clinical disease are rare.
The purpose of this study was to analyse PI-9 expression and regulation in human leucocyte subsets upon different types of short-term stimulation and in clinical disorders. Our hypothesis was that PI-9 expression was regulated significantly upon stimulation of a cell, thus indicating a relevant biological role of PI-9 for the function of the leucocyte subset in inflammatory processes.
Materials and methods
Cells and culture conditions
For in-vitro cultivation, peripheral blood mononuclear cells (PBMC) were separated from fresh heparinzed blood of healthy adult donors by Biocoll (Biochrom, Berlin, Germany) density gradient centrifugation, followed by washing in phosphate-buffered saline (PBS, Biochrom). For ex-vivo incubation assays, cells were kept in RPMI-1640 medium (Life Technologies, Eggenstein, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Conco, Wiesbaden, Germany), 12·5 mM HEPES (Biochrom, Berlin, Germany), 100 U/ml penicillin/streptomycin solution (Life Technologies) and 2·0 mM l-glutamine solution (Biochrom); they were seeded at a density of 1 × 106 cells/ml in six-well plates with the given stimuli or inhibitory substances for the given times at 37°C, 5% CO2. Harvesting was performed by aspiration after detaching adherent cells with a cell scraper. For long-term assays, cell aliquots were frozen in FCS plus 10% dimethylsulphoxide (DMSO), and all specimens were analysed at the same time-point.
The following substances were used for in-vitro treatment: IL-2 (Sigma, Taufkirchen, Germany), dexamethasone (Merck, Darmstadt, Germany), phytohaemagglutinin (PHA, Sigma), cycloheximide (CHX, Sigma), lipopolysaccharide (LPS, Sigma), pyrrolidin dithiocarbamate (PDTC, Sigma) [19], IFN-γ (Boehringer/Roche, Mannheim, Germany) and phorbol-myristate-acetate (PMA, Sigma).
For analysis of patient-derived cells, surplus ethylenediamine tetraacetic acid (EDTA) blood specimens of adults and children who were treated in the hospital were used. In each case, written informed consent was given by the patients or, in the case of children, by the parents. The study was approved by the ethical committee of the University of Ulm, Germany (application number 201/2004).
‘Healthy’ was defined as absence of infectious or immunological disorder; bacterial infection was defined as clinical infectious disorder with C-reactive protein value > 50 mg/l. Acute EBV infection was defined by serological proof of the infection, acute graft-versus-host disease (GVHD) was defined by clinical criteria including all stages of the disorder.
Forty µl EDTA blood per probe was studied at the day of collection or the next day. Cells were washed once in PBS, then stained as detailed below.
Flow cytometry
For detection of intracellular PI-9, the PI-9 specific monoclonal mouse IgG1 antibody 2E7 [3] or the mouse IgG1 isotype control (BD Biosciences, Erembodegem, Belgium) were applied on cells fixed and permeabilized using the IntraPrep™ kit (Immunotech, Marseille, France) according to the manufacturer's instructions, with a fluorescein isothiocyanate (FITC)-labelled goat anti-mouse IgG (Serotec, Oxford, UK) as secondary antibody (incubation 30 min each, followed by washing twice in PBS). Ten µl per pellet of about 200 µl were added in each case. Analysis was performed using a FACScan™ flow cytometer (Becton-Dickinson, San Jose, CA, USA) according to the manufacturer's instructions. Mean fluorescence intensity (MFI) ratio was determined as the ratio between MFI of 2E7-stained and MFI of control-stained cells.
For two-colour flow cytometry, cells were first surface-stained by a phycoerythrin (PE)-coupled IgG2a or IgG2b antibody (10 µl per pellet), followed by the fixation and permeabilization procedure. As secondary antibody for intracellular staining, a FITC-labelled goat anti-mouse IgG1 (Serotec) was used. The surface antibodies used were: CD4 IgG2b-PE (Santa Cruz Biotechnology, San Diego, CA, USA), CD8 IgG2a-PE (Santa Cruz), CD3 IgG2a-PE (BD Biosciences), CD20 IgG2b-PE (BD Biosciences), CD14 IgG2a-PE (Dako, Glostrup, Denmark) and CD1a IgG1-PE (BD Biosciences) (10 µl per pellet).
For isolation of lymphocyte subsets, microbead-coupled antibodies and the Mini-MACS™ (Milteny, Bergisch-Gladbach, Germany) device were used, following the manufacturer's recommendations. The antibodies used were CD19 microbeads (Milteny), CD3 Microbeads (Milteny) and CD56 Microbeads (Milteny).
Calculation of means, standard deviations and P-values were carried out using MS-Excel™ and Origin™ (Microcal Software Inc., Northampton, MA, USA) software. Patient-derived data were visualized as box charts (the horizontal lines in the box denote the 25th, 50th and 75th percentile values. The error bars denote the 5th and 95th percentile values. The two symbols below the 5th percentile error bar denote the 0th and 1st percentile values. The two symbols above the 95th percentile error bar denote the 99th and 100th percentiles. The square symbol in the box denotes the mean of the column of data).
Results
PI-9 is present in all lymphocyte subsets and monocytes but is not detectable in granulocytes
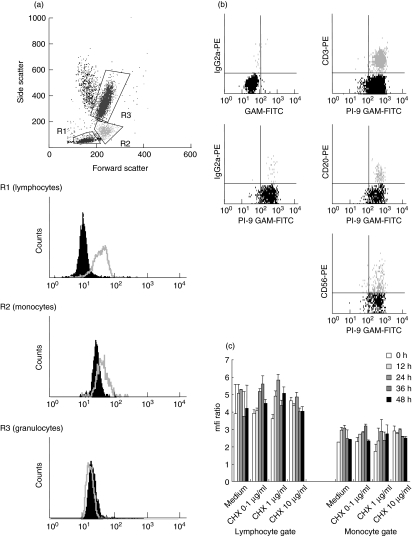
In fresh EDTA blood probes of healthy adults, the different leucocyte subpopulations could be identified easily by gating in the forward–side-scatter mode of the flow cytometer (Fig. 1a). After intracellular staining with the specific antibody 2E7 or the isotype control, PI-9 expression was analysed by calculation of the MFI ratio. As shown in Fig. 1a the ratio was highest in lymphocytes, while in the monocyte gate MFI ratio was lower, and in the granulocyte fraction no PI-9 expression was detectable by flow cytometry. Complete negativity of granulocytes is in contrast to data by Hirst et al. [6], who found low levels of PI-9 in granulocytes. This difference is due probably to methodology [6].
Fig. 1.
Expression of proteinase inhibitor 9 (PI-9) in normal peripheral blood mononuclear cells (PBMC) in fresh blood samples from three healthy adults were analysed by flow cytometry. The different leucocyte subpopulations were identified by gating in the forward–side-scatter mode, and PI-9 expression was analysed by intracellular staining with the specific antibody 2E7 compared to the isotype control, followed by calculation of the mean fluorescence intensity (MFI) ratio. (a) PI-9 expression is highest in lymphocytes (R1) and lower in monocytes (R2). No PI-9 expression was detectable in the granulocyte fraction (R3). The non-gated population represent dead cells. One typical example of three separate experiments is shown. (b) To analyse lymphocyte subsets, cells were co-stained with CD3, CD20 or CD56 specific mouse IgG2a or IgG2b marker, followed by fluorescein isothiocyanate (FITC)-coupled IgG1-specific goat-anti-mouse antibodies. No significant differences in PI-9 expression were evident among the lymphocyte subsets. One typical example of three experiments is shown. (c) To assess the stability of PI-9, we monitored PI-9 levels in the presence of the protein synthesis inhibitor cycloheximide (0·1 µg/ml; 1·0 µg/ml; 10 µg/ml) for 48 h. Based on gating in the forward–side-scatter, lymphocytes and monocytes were analysed separately. Mean and standard deviations of triplicate experiments are given.
To analyse the different lymphocyte subsets separately, cells were co-stained with CD3, CD20 or CD56 specific mouse IgG2a or IgG2b antibodies, followed by intracellular 2E7 staining with the use of FITC coupled IgG1-specific goat-anti-mouse antibodies. There were no significant differences in PI-9 expression among the lymphocyte subsets (Fig. 1b).
PI-9 within cells is very stable
The stability and turnover of PI-9 within cells is unknown. To determine whether it resembles an early response gene (high turnover) or a housekeeping or late response gene (lower turnover), we studied PI-9 expression upon incubation in the presence of the protein synthesis inhibitor cycloheximide (0·1 µg/ml; 1·0 µg/ml; 10 µg/ml) for 48 h. Based on gating by the forward–side-scatter mode, lymphocytes and monocytes were analysed separately. We found that within 48 h no significant decrease in PI-9 expression was visible, indicating that PI-9 has a slow turnover in these cells (Fig. 1c).
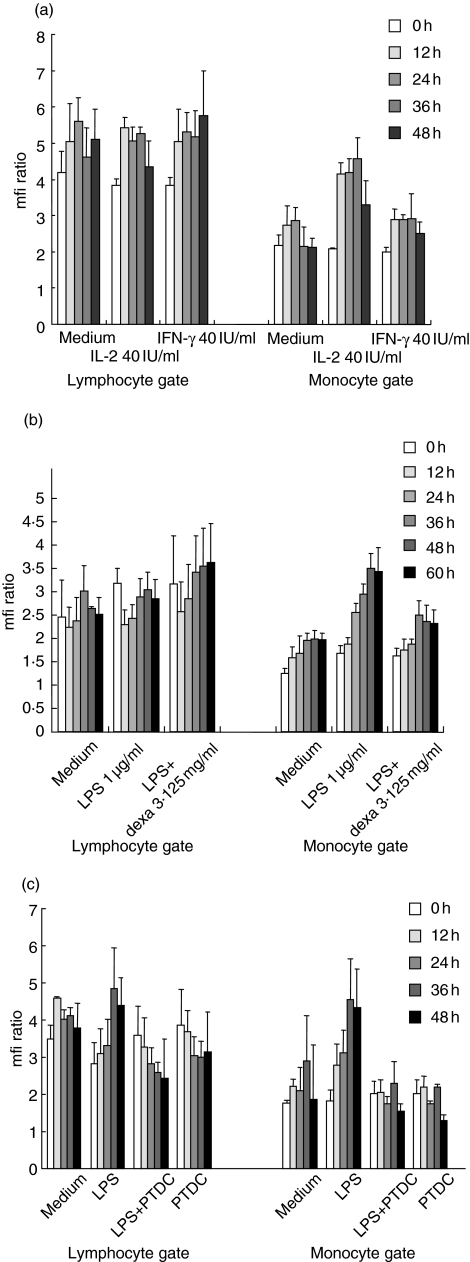
PI-9 is up-regulated by IL-2 and LPS in monocytes but not lymphocytes; up-regulation is inhibited by PDTC and dexamethasone
As a model of inflammation, PBMC were treated in vitro with IL-2 (40 IU/ml), IFN-γ (40 IU/ml) or LPS (1 µg/ml); and PI-9 expression was assayed for 48 h separately in lymphocytes and monocytes (Fig. 2a). Besides an initial small and probably unspecific increase which occurred in all cells, no alteration of PI-9 expression was induced in the lymphocytes. However, in monocytes IL-2 and, more profoundly, LPS induced a marked up-regulation of PI-9 (twofold: P = 0·00238 and 2·5-fold: P = 0·01251, respectively, increase at 36 h) (Fig. 2a,c). This effect was inhibited by dexamethasone (3·125 mg/ml) (Fig. 2b) and by the NF-κB inhibitor pyrrolidin dithiocarbamate (PDTC, 0·3 µM) [19] (1·2-fold up-regulation of median at 36 h; P = 0·23811, thus no significant change) (Fig. 2c). This indicates a NF-κB-dependent regulation of PI-9 in monocytes. Dexamethasone alone induced no change in PI-9 expression (data not shown).
Fig. 2.
Expression of proteinase inhibitor 9 (PI-9) in peripheral blood mononuclear cells (PBMC) stimulated in vitro. PBMC were treated in vitro with interleukin (IL)-2 (40 IU/ml), interferon (IFN)-γ (40 IU/ml) or lipopolysaccharide (LPS) (1 µg/ml); and PI-9 expression was assessed by flow cytometry for 48 h in lymphocytes and monocytes separately. Mean and standard deviations of triplicate experiments are given. (a) No significant alteration of PI-9 expression was induced in the lymphocytes. In the monocytes, IL-2 led to a marked up-regulation of PI-9. IFN-γ induced a mild up-regulation. (b) LPS led to a marked up-regulation of PI-9 in monocytes, which could be inhibited by dexamethasone (3·125 mg/ml). No effect was seen in lymphocytes. (c) In the presence of the nuclear factor-kappa B (NF-κB) inhibitor pyrrolidin dithiocarbamate (PTDC) (0·3 µM), the up-regulation of PI-9 by LPS in monocytes was completely inhibited.
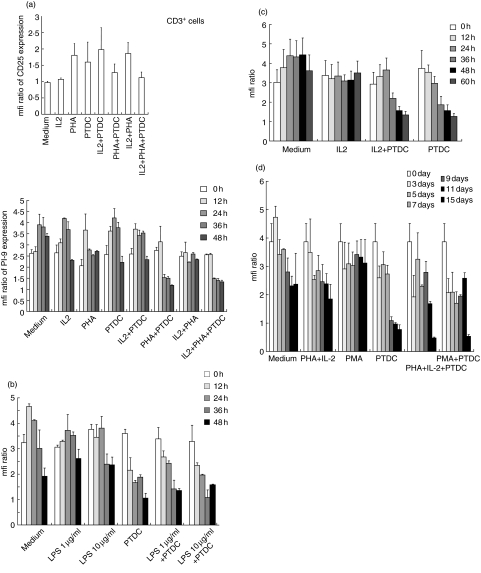
PI-9 is not up-regulated by IL-2, PHA or LPS in lymphocyte subsets
To study PI-9 regulation in lymphocytes more specifically, lymphocyte subfractions were isolated by magnetic sorting, leading to a >90% pure suspension of cells that were CD3+ (T cells), CD20+ (B cells) or CD56+ (NK cells) (Fig. 3a–d). Purity was confirmed by flow cytometry (data not shown). To stimulate cells, stimuli were selected depending on what was regarded as the strongest activator of the specific cell subset.
Fig. 3.
Proteinase inhibitor 9 (PI-9) expression in lymphocyte subsets following in vitro stimulation. Lymphocyte subsets were isolated by magnetic sorting, leading to a >90% pure suspension of cells that were CD3+ (T cells), CD20+ (B cells) or CD56+ [natural killer (NK) cells]. Purity was confirmed by flow cytometry (data not shown). Mean and standard deviation of triplicate experiments are given. (a) CD3 positive cells were analysed. First, stimulation was assessed by CD25 staining. Up-regulation of CD25 within 48 h was seen upon stimulation with phytohaemagglutinin (PHA) (20 µg/ml) alone or in combination with interleukin (IL)-2 (200 U/ml). Secondly, PI-9 expression was analysed in the cells. However, no stimulus-dependent up-regulation of PI-9 was seen. Pyrrolidin dithiocarbamate (PDTC) (0·3 µM) had inconsistent effects on stimulation; however, in isolated T lymphocytes, it led to a marked down-regulation of PI-9. (b) To stimulate CD20+ cells (B cells) in vitro, lipopolysaccharide (LPS) (1 µg/ml and 10 µg/ml) was applied. No up-regulation of PI-9 was evident. PDTC (0·3 µM) led to a marked down-regulation of PI-9, both in the presence or absence of lipopolysaccharide (LPS). (c) CD56+ cells (NK cells) were stimulated with high-dose IL-2 (200 U/ml) to induce rapid activation of NK cells. This did not up-regulate PI-9. PDTC (0·3 µM) led to a marked down-regulation of PI-9 both in the presence or absence of IL-2. (d) Non-adherent peripheral blood mononuclear cells (PBMC) (lymphocytes) were stimulated over 15 days upon incubation with PHA/IL-2 (PHA 20 µg/ml; IL-2200 U/ml) or phorbol-myristate-acetate (PMA) (20 ng/ml), and in the presence of PDTC (0·3 µM). Cells were collected at the given time-points and frozen until they were analysed. We saw no up-regulation of PI-9 as shown by flow cytometry. Once more, PDTC (0·3 µM) led to a marked down-regulation of PI-9 both in the presence or absence of stimuli.
First, CD3 positive cells were analysed, and stimulation was assessed by CD25 staining. Up-regulation of CD25 within 48 h was seen by stimulation with PHA (20 µg/ml) alone or in combination with IL-2 (200 U/ml). However, no up-regulation of PI-9 was seen in these cells. PDTC (0·3 µM) had inconsistent effects on stimulation – in cells treated with IL-2, it led to up-regulation of CD25, while in those treated with PHA or PHA plus IL-2, CD25 declined; however, in isolated CD3+ cells it led to a marked down-regulation of PI-9 (Fig. 3a). This cannot be explained as impaired production, as treatment with the protein synthesis inhibitor cycloheximide did not induce any down-regulation of PI-9 within 48 h (Fig. 1c). This may be due to the toxic effects of PTDC; however, no alterations of the cells were visible by microscopic examination or flow cytometry.
To stimulate CD20+ cells (B cells) in vitro LPS (1 µg/ml and 10 µg/ml), which is known to induce rapid activation of B cells [12], was applied. However, we did not observe any up-regulation of PI-9. Again, PDTC (0·3 µM) led to a marked down-regulation of PI-9, both in the presence or absence of LPS (Fig. 3b).
In vitro stimulation of CD56+ cells (NK cells) with high-dose IL-2 (200 U/ml), which is known to induce rapid activation of NK cells, did not induce any up-regulation of PI-9 as shown by flow cytometry. Once more, PDTC (0·3 µM) led to a marked down-regulation of PI-9 both in the presence or absence of IL-2 (Fig. 3c).
Finally, we studied non-adherent PBMC over a period of 15 days upon incubation with PHA/IL-2 (PHA 20 µg/ml; IL-2200 U/ml) or PMA (20 ng/ml) and in the presence of PDTC (0·3 µM). Besides an unspecific initial up-regulation and down-regulation in the presence of PDTC, we found no significant alterations in the PI-9 expression (Fig. 3d).
Taken together, these data show that while up-regulation of PI-9 correlates with stimulation of monocytes, this does not occur in lymphocytes, either in specific subsets or upon long-term stimulation. The fact that PDTC is sufficient to down-regulate PI-9 expression in isolated lymphocyte subsets (Fig. 3) but not in lymphocytes co-incubated with monocytes (Fig. 2c) might indicate protection from a direct toxic effect of PDTC by a monocyte-derived factor. It is a relatively common finding in lymphocyte stimulation assays that the presence of monocytes has a large impact on lymphocyte reactivity. The possible interaction mechanisms −via cytokines or direct cell–cell contact – are too numerous to be analysed completely in this work although, of course, they are certainly of biological significance.
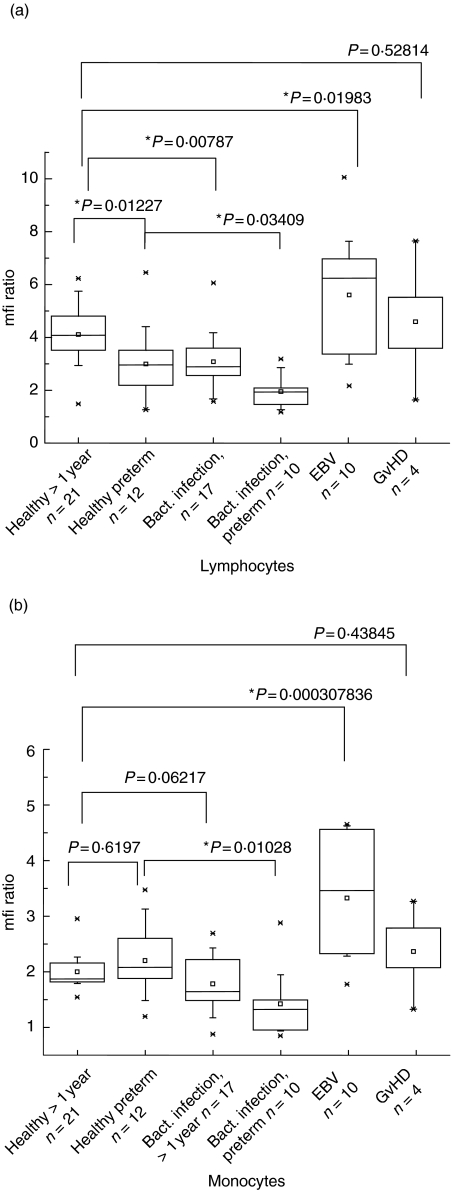
Regulation of leucocyte PI-9 during acute EBV infection and bacterial infection
Using EDTA blood specimens of healthy or sick adults and children, PI-9 expression in the lymphocyte and monocyte fraction under different clinical conditions were analysed (Fig. 4a,b). In monocytes, no differences were detected for healthy adults compared with preterm infants (< 30 weeks of gestation), while in lymphocytes, preterm infants had lower PI-9. In bacterial infections in older children or adults, PI-9 was unchanged in monocytes, but decreased in lymphocytes. In preterm infants, a marked down-regulation was observed upon infection, both in lymphocytes and in monocytes. Acute EBV infection, proved by positive VCA-IgG and -IgM and positive EA-antibodies, however, was associated with PI-9 up-regulation, which was stronger in the monocyte than in the lymphocyte fraction. Higher PI-9 expression was also observed in GVHD, although only a limited number of patients could be included in the study. High expression of PI-9 in EBV was not restricted to a specific subset of lymphocytes, but involved all subsets in a similar way (data not shown).
Fig. 4.
Proteinase inhibitor 9 (PI-9) expression in patient-derived lymphocytes and monocytes. PI-9 expression in the lymphocyte and monocyte fractions from the blood of healthy or sick adults and children was analysed. Each specimen was tested in duplicate, and statistical analysis was performed using the Origin™ software. In the box charts, the horizontal lines in the box denote the 25th, 50th and 75th percentile values. The error bars denote the 5th and 95th percentile values. The two symbols below the 5th percentile error bar denote the 0th and 1st percentile values. The two symbols above the 95th percentile error bar denote the 99th and 100th percentiles. The square symbol in the box denotes the mean of the column of data. (a) In lymphocytes no differences were detected for healthy adults compared with preterm infants (< 30 weeks of gestation). In bacterial infections in older children or adults, PI-9 was unchanged; however, it was down-regulated in preterm infants. Graft-versus-host disease and Epstein–Barr virus (EBV) infection were associated with PI-9 up-regulation. (b) In monocytes no differences were detected for healthy adults compared with preterm infants (< 30 weeks of gestation). In contrast PI-9 in bacterial infections, while unchanged in older children or adults, was down-regulated in preterm infants. EBV infection and graft-versus-host disease were associated with marked PI-9 up-regulation.
Discussion
Because PI-9 may influence homeostasis of NK cells [15], CTLs [16] and antigen-presenting cells [17], knowledge of PI-9 regulation will probably improve our understanding of a number of autoimmune or infectious diseases. Although PI-9 has been analysed in a number of model systems in vitro [2,4,11,20,21], its expression and regulation in clinical conditions such as infections or immune disorders is largely unknown to date.
Here, we first demonstrate the lack of PI-9 protein up-regulation upon activation of lymphocytes. In concordance with this, in a recent study [21] constant expression of PI-9 protein – despite up-regulation of PI-9 RNA, which was not analysed in our study – was shown upon stimulation in a CD8-positive T cell clone. Because in NK cells activation-induced death has been shown to be mediated by self-destructive GrB leaking into the cytosol of the cell [15], here, and also possibly in CTL [16], constant PI-9 protein expression despite stimulation may have a function in homeostasis regulation, leading to the long-term survival of inactive but short-term survival of activated cytotoxic cells. Interestingly, LPS, which is sufficient to activate B lymphocytes similarly to monocytes, is unable to induce PI-9 up-regulation in B cells. Thus, these cells behave similarly to T and NK cells.
In contrast, PI-9 is up-regulated rapidly in activated monocytes, which do not express GrB but are exposed to GrB crossfire upon inflammation or antigen presentation. Here, PI-9 may provide a mechanism for homeostasis contrary to that in cytotoxic lymphocytes: survival of stimulated but suppression of unstimulated cells.
Secondly, we found that PI-9 regulation is probably mediated by NF-κB, as up-regulation in monocytes can be inhibited by PDTC [19]. Thus, PI-9 regulation follows the pattern of several other activation-related proteins in these cells, e.g. TNF-α[19].
To understand fully the role of PI-9 in vivo, we searched for a correlation between in vitro findings and some specific clinical conditions. As both bacterial liposaccharides and immune-activating cytokines such as IL-2 are relevant in the pathogenesis of severe bacterial infections, it was surprising that no up-regulation of PI-9 was found in patients suffering from severe bacterial infection. This may be due in part to high cell turnover in the course of bacterial infection, leading to a loss of stimulated cells. Our findings in bacterial infection were in contrast to a marked effect seen in acute EBV infection, where PI-9 expression increased significantly. In acute GVHD, a trend for high PI-9 expression was seen. These findings indicate that PI-9 up-regulation is not an epiphenomenon of inflammation in general, but it is regulated heterogeneously in different types of disease.
In acute EBV infection, the virus predominantly enters CD21/EBV receptor-positive B cells, transforming them into lymphoblastoid cells expressing a number of latent virus proteins, especially latent membrane protein 1 (LMP-1), that alter the cellular lifespan and function. As LMP-1 has a well-known NF-κB activating effect [22,23], this might be one reason for the high expression of PI-9 in EBV-transformed lymphoblastoid cell lines [2]. Concerning up-regulation of PI-9 in peripheral blood specimens which we found in all cellular subsets, including T cells and monocytes, besides LMP-1, the state of cellular immune activation which is characteristic for EBV infection is certainly the main cause. Further studies are required to analyse the underlying mechanism more precisely.
As in EBV infection, in GVHD a number of alterations involving the interplay of T lymphocytes and the monocyte–macrophage system are described, e.g. up-regulation of cytokines such as IL-2 and TNF-α[23,24]. This may account for the relatively high PI-9 in GVHD.
Several authors have studied maturation of immune functions in preterm and term infants, showing a delay in immunocompetence especially in monocyte, but also lymphocyte function [25,26]. Down-regulation of PI-9 in preterm infants with sepsis may therefore represent a consequence of immaturity upon conditions of inflammation.
In summary, our data suggest a complex regulation pattern for the GrB inhibitor PI-9 in the monocyte/macrophage system and in the different lymphocyte subsets, with rapid alterations in the monocytes and slow alterations in lymphocytes. This in turn has a strong impact on the regulatory loop between antigen presentation and lymphocyte stimulation. If T cells undergo a relatively rapid activation-induced cell death after being stimulated by antigen-presenting cells and co-stimulation, continuation of the immune response depends largely on the presence of antigen-presenting cells recruiting new T cells. In the context of inflammation, where much GrzB is present, protection of antigen-presenting cells by PI-9 may have a relevant impact on the duration and thus the intensity of an immune response. Specific mechanisms, such as direct involvement of EBV-derived proteins, may alter this process further. Future work should be aimed at means to manipulate PI-function for therapeutic purposes.
References
- 1.Eyre HJ, Sun J, Sutherland GR, Bird P. Chromosomal mapping of the gene (PI9) encoding the intracellular serpin proteinase inhibitor 9 to 6p25 by fluorescence in situ hybridization. Genomics. 1996;37:406–8. doi: 10.1006/geno.1996.0580. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Bird CH, Sutton V, et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271:27802–9. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 3.Bird CH, Sutton VR, Sun J, et al. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol. 1998;18:6387–98. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000;191:1535–44. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanamori H, Krieg S, Mao C, et al. Proteinase inhibitor 9, an inhibitor of granzyme B-mediated apoptosis, is a primary estrogen-inducible gene in human liver cells. J Biol Chem. 2000;275:5867–73. doi: 10.1074/jbc.275.8.5867. [DOI] [PubMed] [Google Scholar]
- 6.Hirst CE, Buzza MS, Bird CH, et al. The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol. 2003;170:805–15. doi: 10.4049/jimmunol.170.2.805. [DOI] [PubMed] [Google Scholar]
- 7.Bladergroen BA, Strik MC, Bovenschen N, et al. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J Immunol. 2001;166:3218–25. doi: 10.4049/jimmunol.166.5.3218. [DOI] [PubMed] [Google Scholar]
- 8.Hirst CE, Buzza MS, Sutton VR, Trapani JA, Lovelan KL, Bird PI. Perforin-independent expression of granzyme B and proteinase inhibitor 9 in human testis and placenta suggests a role for granzyme B-mediated proteolysis in reproduction. Mol Hum Reprod. 2001;7:1133–42. doi: 10.1093/molehr/7.12.1133. [DOI] [PubMed] [Google Scholar]
- 9.Buzza MS, Hirst CE, Bird CH, Hosking P, McKendrick J, Bird PI. The granzyme B inhibitor, PI-9, is present in endothelial and mesothelial cells, suggesting that it protects bystander cells during immune responses. Cell Immunol. 2001;210:21–9. doi: 10.1006/cimm.2001.1806. [DOI] [PubMed] [Google Scholar]
- 10.Bird CH, Blink EJ, Hirst CE, et al. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medema JP, Schuurhuis DH, Rea D, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–67. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannan-Thulasiraman P, Shapiro DJ. Modulators of inflammation use nuclear factor-kappa B and activator protein-1 sites to induce the caspase-1 and granzyme B inhibitor, proteinase inhibitor 9. J Biol Chem. 2002;277:41230–9. doi: 10.1074/jbc.M200379200. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumar T, Ding R, Dadhania D, et al. Serine proteinase inhibitor-9, an endogenous blocker of granzyme B/perforin lytic pathway, is hyperexpressed during acute rejection of renal allografts. Transplantation. 2003;75:1565–70. doi: 10.1097/01.TP.0000058230.91518.2F. [DOI] [PubMed] [Google Scholar]
- 14.Barrie MB, Stout HW, Abougergi MS, Miller BC, Thiele DL. Antiviral cytokines induce hepatic expression of the granzyme B inhibitors, proteinase inhibitor 9 and serine proteinase inhibitor 6. J Immunol. 2004;172:6453–9. doi: 10.4049/jimmunol.172.10.6453. [DOI] [PubMed] [Google Scholar]
- 15.Ida H, Nakashima T, Kedersha NL, et al. Granzyme B leakage-induced cell death: a new type of activation-induced natural killer cell death. Eur J Immunol. 2003;33:3284–92. doi: 10.1002/eji.200324376. [DOI] [PubMed] [Google Scholar]
- 16.Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J Immunol. 2004;173:3801–9. doi: 10.4049/jimmunol.173.6.3801. [DOI] [PubMed] [Google Scholar]
- 17.Kim TW, Hung CF, Boyd DA, et al. Enhancement of DNA vaccine potency by coadministration of a tumor antigen gene and DNA encoding serine protease inhibitor-6. Cancer Res. 2004;64:400–5. doi: 10.1158/0008-5472.can-03-1475. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AJ, Krieg SA, Ahn BS, Shapiro DJ. Interplay between estrogen response element sequence and ligands controls in vivo binding of estrogen receptor to regulated genes. J Biol Chem. 2003;279:5025–34. doi: 10.1074/jbc.M307076200. [DOI] [PubMed] [Google Scholar]
- 19.Liu SF, Ye X, Malik AB. Inhibition of NF-kB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–7. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 20.Medema JP, de Jong J, Peltenburg LT, et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci USA. 2001;98:11515–20. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie O, Saigo K, Murayama T, Ryo R. Differential expression of proteinase inhibitor-9 and granzyme B mRNAs in activated immunocompetent cells. Tohoku J Exp Med. 2005;205:103–13. doi: 10.1620/tjem.205.103. [DOI] [PubMed] [Google Scholar]
- 22.Luftig M, Prinarakis E, Yasui T, et al. Epstein–Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc Natl Acad Sci USA. 2003;100:15595–600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler DH, Foley J, Whit-Shan Hou J, et al. Clinical ‘cytokine storm’ as revealed by monocyte intracellular flow cytometry: correlation of tumor necrosis factor alpha with severe gut graft-versus-host disease. Clin Gastroenterol Hepatol. 2004;2:237–45. doi: 10.1016/s1542-3565(04)00011-4. [DOI] [PubMed] [Google Scholar]
- 24.Jaksch M, Remberger M, Mattsson J. Increased immune transcript levels are correlated with acute graft-versus-host disease and cytomegalovirus response after allogeneic stem cell transplantation. Transplantation. 2004;77:195–200. doi: 10.1097/01.TP.0000100465.83529.42. [DOI] [PubMed] [Google Scholar]
- 25.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–7. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 26.Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–6. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]