Abstract
Buruli disease (BU) is a progressive necrotic and ulcerative disease of the skin and subcutaneous tissue caused by Mycobacterium ulcerans. BU is considered the third most common mycobacterial disease after tuberculosis and leprosy. Three clinical stages of the cutaneous lesions have been described in BU: pre-ulcerative, ulcerative and healed lesions. In this study we used immunohistochemistry and automated morphometry to determine the percentage of macrophages and of CD4/CD8 lymphocytes and their expression of interferon (IFN)-γ, interleukin (IL)-10, tumour necrosis factor (TNF)-α and transforming growth factor (TGF)-β. Expression of these cytokines was correlated with the inflammatory response evaluated by histopathology. All the studied BU ulcerative cases showed extensive necrosis and chronic inflammation. The most important feature was the presence or absence of granulomas co-existing with a mixed pro-inflammatory/anti-inflammatory cytokine balance. When granulomas were present significantly higher expression of IFN-γ was seen, whereas in ulcerative lesions without granulomas there was increased expression of IL-10 and significantly higher bacillary counts. These features correlated with the chronicity of the lesions; longer-lasting lesions showed granulomas. Thus, granulomas were absent from relatively early ulcerative lesions, which contained more bacilli and little IFN-γ, suggesting that at this stage of the disease strong suppression of the protective cellular immune response facilitates proliferation of bacilli.
Keywords: Buruli disease, cytokines, immunopathology, Mycobacterium ulcerans
Introduction
Mycobacterium ulcerans is the cause of Buruli disease (BU), a progressive necrotic and ulcerative disease of the skin and subcutaneous tissue, considered to be the third most common mycobacterial disease after tuberculosis and leprosy [1]. M. ulcerans has been identified in stagnant or slowly moving water, such as swamps or lakes, in many tropical and temperate parts of the world, particularly in several West African countries and Australia [2]. The infection is acquired when contaminated water penetrates cutaneous injuries [3] or could possibly be introduced by the bites of aquatic insects (Naucoridae). M. ulcerans can invade the salivary glands of these species [4,5]. BU is primarily a skin disease that usually starts as a single subcutaneous nodule or plaque in the upper or lower limbs and enlarges over time. Eventually the skin that covers the nodule or plaque sloughs off and the subcutaneous tissues become necrotic, forming deep ulcers [2]. The patients usually have no systemic symptoms or ulcer pain. Spontaneous healing can occur, frequently producing a depressed scar that contracts and may produce severe deformities [2]. Laboratory diagnostic methods include culture, polymerase chain reaction (PCR) and histological examination with the Ziehl–Neelsen (ZN) stain [6].
The major virulence factor in M. ulcerans is a necrotizing toxin called mycolactone, a macrolide consisting of a polyketide side chain attached to a 12-membered core encoded by genes harboured in a large 174-kb plasmid [7]. This toxin has cytotoxic, analgesic and immunosuppressive activities [8–10]. Thus, many of the clinical and pathological manifestations of BU depend on this toxin [8,9].
In vitro studies have demonstrated that the immunosuppressive activities of mycolactone include inhibition of Th1 cytokine production [interleukin (IL)-12, interferon (IFN)-γ] and suppression of the production of tumour necrosis factor (TNF)-α by monocytes [10]. These effects of mycolactone on the immune system are important because mycobacterial infection is controlled essentially by Th1 cytokines and TNF-α, whereas Th2 cytokines (IL-4, IL-13) and anti-inflammatory cytokines, such as IL-10 and transforming growth factor (TGF)-β, have a detrimental effect on the control of bacterial proliferation [11–14]. Recent ex vivo studies using the reverse transcription–polymerase chain reaction (RT-PCR) to study skin biopsies and peripheral blood cells from patients with ulcerative BU lesions have demonstrated lower IFN-γ and higher IL-10 expression than in control Bacille Calmette–Guérin (CG)-vaccinated subjects or BU patients with nodular pre-ulcerative lesions [15]. However, to our knowledge, there are no published studies on the local, in vivo immunopathology in BU patients. Thus, the aim of the present work was to define the local expression of pro-inflammatory (IFN-γ, TNF-α) and anti-inflammatory cytokines (IL-10, TGF-β), as well as the percentage of macrophages and of CD4/CD8 lymphocytes, in skin biopsies from the ulcerative phase of BU. We used immunohistochemistry with automated morphometry to correlate the expression of these cytokines with the inflammatory response evaluated by histopathology.
Materials and methods
Histological material
Skin and subcutaneous tissue fragments from 11 patients with BU ulcers, fixed with formaldehyde and embedded in paraffin, were obtained from the Armed Forces Institute of Pathology in Washington, DC, USA. All the tissue fragments came from Benin, Africa, and were obtained as part of the treatment (surgical debridement). All the cases were confirmed by at least two positive tests, detection of acid-fast bacilli (AFB), by Ziehl–Neelsen staining (ZN) in vitro culture, IS-2404 PCR and histopathology [6]. The tissue sections were taken from the edge of the lesions, avoiding sampling from different parts of the same lesion.
Immunohistochemistry and automated morphometry
Sections of 5 µm were stained with haematoxylin/eosin and ZN for detection of acid-fast bacilli (AFB). For immunohistochemistry, 5-µm sections were placed on slides coated with poly l-lysine. Following deparaffination, the sections were immersed in an antigen retrieval solution (Dako, Glostrup, Denmark) for 40 min at 98°C. Endogenous peroxidase was blocked with 3% H2O2 in absolute methanol, followed by immersion in a universal blocking reagent (Powerblock, Biogenex, San Ramon, CA, USA) for 10 min. The sections were incubated overnight at room temperature. Mouse monoclonal antibodies against IFN-γ, TNF-α and IL10 (Santa Cruz Laboratory, Santa Cruz, CA, USA) were diluted 1/30, 1/200 and 1/100, respectively. Rabbit polyclonal antibodies against TGF-β1 (Santa Cruz Laboratory) were diluted 1/50 in phosphate-buffered saline (PBS), while polyclonal rabbit antibodies anti-CD4, CD8 and CD68 (a marker of macrophages) were diluted 1/100 (Dako, USA). Bound antibodies were detected with rabbit anti-mouse or goat anti-rabbit IgG labelled with peroxidase diluted 1/150 in PBS and diaminobenzidine. The slides were counterstained with haematoxylin [14]. The percentage of cells immunostained for each of the cytokines was determined by an automated image analyser (Q-Win Leica), after counting the positive and negative cells in five random fields of the inflammatory infiltrate located in the dermis or subcutaneous tissue, at 200× magnification [14]. The same procedure, but at 1000× magnification (total area per case 2763 µ2), was performed to determine the number of AFB, comparing numbers of bacteria inside and outside of inflammatory cells.
All the studied cases showed extensive necrosis and chronic inflammation. A major difference was the presence or absence of granulomas. Thus, we divided the lesions into two types: pure ulcerative without granulomas and ulcerative with granulomas. We compared the percentages of cytokine immunostained cells and numbers of bacilli in the two types of lesion. Comparison of cytokine expression was performed with the independent-samples t-test, as all variables were distributed normally. Significance was set at P < 0·05.
Results
Clinical and histopathological features
Table 1 shows the clinical and pathological profiles of the studied BU patients. Eleven cases were analysed, five men and six women. All the patients showed deep cutaneous ulcers located in the upper or lower limbs. Six patients also had osteomyelitis. The clinical diagnosis of osteomyelitis was confirmed by at least two positive results from direct smear examination for AFB, culture, IS2404 PCR and histopathology [6].
Table 1.
Clinical and histopathological features from the studied cases.
| Case | Gender | Age (years) | Patient delay (days) | Duration of hospitalization (days) | Clinical and pathological features |
|---|---|---|---|---|---|
| 1 | Female | 9 | 45 | 52 | Skin ulcer in inguinal area, severe oedematous form, without granulomas |
| 2 | Male | 10 | 30 | 11 | Ulcerated nodules in right thigh, no granulomas formation |
| 3 | Female | 16 | 90 | 84 | Severe ulcerated form in left hand, osteomyelitis, pregnant, without granulomas |
| 4 | Male | 30 | 30 | 9 | Early ulcerative nodule in left thigh without granulomas |
| 5 | Female | 4 | 45 | 37 | Ulcerated plaque in left leg, osteomyelitis, without granulomas |
| 6 | Female | 7 | 36 | 35 | Ulcer and nodules in left leg, without granulomas |
| 7 | Male | 20 | 180 | 14 | Ulcer in right leg, osteomyelitis, with granulomas |
| 8 | Male | 5 | 120 | 234 | Ulcer in left leg, osteomyelitis, with granulomas |
| 9 | Male | 5 | 120 | 234 | Disseminated for osteomyelitis with granulomas |
| 10 | Female | 10 | 150 | 180 | Ulcer in left arm, osteomyelitis, with granulomas |
| 11 | Female | 23 | 180 | 60 | Ulcer right leg, secondary infection with granulomas |
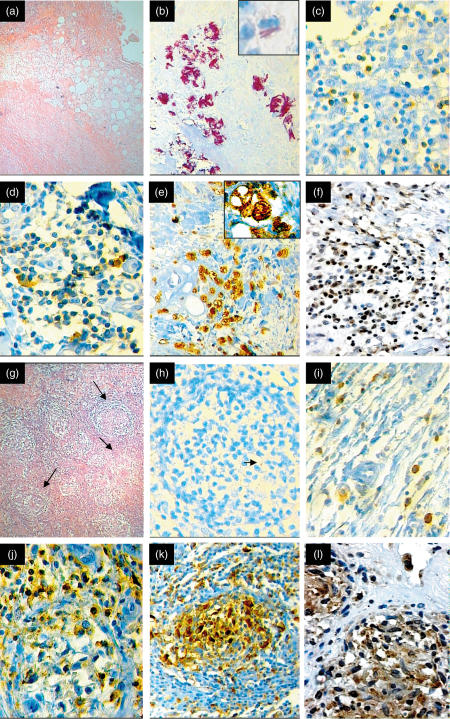
In all the studied cases, the histopathological analysis showed extensive coagulative necrosis of the skin and subcutaneous tissue with variable numbers of AFB. Variable amounts of inflammatory cells, essentially lymphocytes and macrophages, were seen around or within the necrotic tissue (Fig. 1). In five cases well-formed granulomas, constituted by lymphocytes, macrophages and giant cells, were observed in the subcutaneous tissues. The other six cases did not contain granulomas, but there were extensive areas of coagulative necrosis with numerous macrophages, some of them with cytoplasmic vacuoles (Fig. 1). Interestingly, a significantly higher number of extracellular and some intracellular AFB were found in the ulcerative non-granulomatous cases (Fig. 1b). In contrast, few AFB were seen in the granulomatous lesions, which also showed epidermal hyperplasia with formation of focal granulation tissue at the borders of the ulcers. These histopathological features correlated with the delay before medical assistance was sought and the duration of hospitalization (Table 1). Patients with granulomas had a median of 150 days of delay and 180 days of hospitalization, while patients with ulcers without granulomas had 45 and 36 days of delay and hospitalization time, respectively (P < 0·003). Thus, granulomas existed in very long-lasting lesions.
Fig. 1.
Representative histopathological and immunohistochemical features in Buruli disease ulcers (BU). (a) Low-power photomicrograph (× 40) from a representative ulcerative BU lesion without granulomas. Extensive coagulative necrosis in the subcutaneous tissue involving the adipose and muscular tissue. (b) This lesion has a massive number of extracellular and occasional intracellular acid-fast bacilli demonstrated by Ziehl–Neelsen staining (× 400). In the insert, intracellular bacilli are shown in a vacuolated macrophage (× 1000). (c) This kind of lesion has relatively few CD4 T lymphocytes (× 200). (d) More CD8 T lymphocytes are present in this ulcerative lesion without granulomas (× 200). (e) Numerous CD68-positive macrophages are present in this lesion (× 100). In the insert, strong positivity in a vacuolated macrophage (× 1000). (f) Some IFN-γ-positive cells are seen in this BU ulcerative lesion without granulomas (× 100). (g) Low power photomicrograph (× 40) from a representative BU ulcerative case with well-formed granulomas (arrows). (h) This granulomatous lesion shows scarce acid-fast bacilli (arrow) (× 200). (i) Like non-granulomatous ulcerative lesions, granulomatous lesions also show fewer CD4 cells, with numerous CD8 cells (j) and numerous macrophages (k). (i) These granulomas show more diffusely distributed IFN-γ immunostained cells than are seen in pure ulcerative lesions (compare with f) (× 400).
Immunohistochemical analysis of local cytokine expression and of CD4/CD8 lymphocytes in BU ulcers
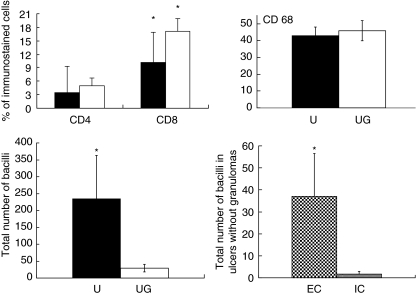
The percentage of CD4 immunostained cells was 5·0% ± 5 in the granulomatous cases and 3·4% ± 1·7 in non-granulomatous cases (P = 0·5) (Fig. 2). The percentage of CD8 immunostained cells was 17·2 ± 2·7 in granulomatous cases and 10·1 ± 6·7 in non-granulomatous cases (P = 0·038). Thus, a significantly higher percentage of CD8 lymphocytes was seen in BU ulcerative lesions (Figs 1 and 2). A high percentage of macrophages was observed in both types of ulcerative BU lesions (45–47%). Non-granulomatous lesions showed macrophages with vacuolated cytoplasm and some intracellular bacilli (Fig. 1).
Fig. 2.
Right and left upper panel show the percentages of CD4 cells, CD8 cells, and macrophages (CD68) determined by immunohistochemistry and automated morphometry in the subcutaneous inflammatory infiltrate from non-granulomatous ulcerative lesions (black bars) or ulcers with granulomas (white bars). Five randomly chosen fields at 200× magnification were studied in each of the 11 cases of Buruli disease. Left lower panel shows the number of acid fast bacilli in non-granulomatous ulcerative lesions (U, black bars) or ulcers with granulomas (UG, white bars), while the right lower panel shows the numbers of extracellular (EC) and intracellular (IC) bacilli in macrophages from ulcers without granulomatous lesions. Results are expressed as the mean and standard deviation. The asterisk means statistical significance.
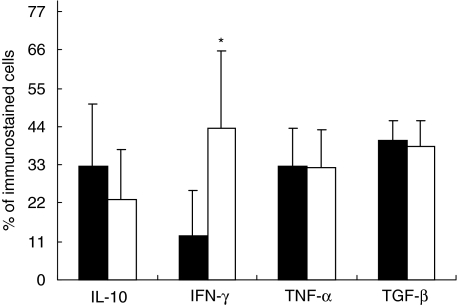
The percentage of immunostained cells, using specific antibodies to detect IFN-γ, IL-10, TNF-α and TGF-β, was compared in BU lesions with and without granulomas (Figs 1 and 3). The BU cases with granulomas showed numerous inflammatory IFN-γ-positive cells infiltrating the dermis (43·5% ± 22). Many of the positive cells were lymphocytes, but macrophages and some giant cells located in granulomas and/or inflammatory infiltrate were also positive. In contrast, the non-granulomatous cases showed a significantly lower percentage of IFN-γ immunostained cells (12·8% ± 12; P < 0·017). The analysis of IL-10 and TGF-β showed a higher percentage of immunostained cells in the non-granulomatous cases (32·5 ± 17 and 40·1 ± 6, respectively) than in the granulomatous cases (23·2 ± 14 and 38·2 ± 7, respectively). The positive cells were macrophages and lymphocytes infiltrating the dermis and subcutaneous tissue. The percentage of TNF-α immunostained cells was very similar between the two types of BU lesions. Thus, in terms of cytokine expression, we found significant differences between granulomatous and non-granulomatous BU ulcers. Although both types of lesion showed a mixed cytokine pattern, granulomatous lesions contained a significantly higher (threefold) percentage of IFN-γ immunostained cells than non-granulomatous lesions (Fig. 3). By contrast, BU ulcers without granulomas revealed an anti-inflammatory cytokine bias, showing 30% more IL-10 immunostained cells, with similar percentages of TGF-β and TNF-α immunostained cells. These patterns of cytokine expression correlated with the number of bacilli determined per surface area using ZN staining and automated morphometry. This method showed a significantly higher number of AFB in the ulcerative lesions without granulomas (Figs 1 and 2). This study also demonstrated a significantly higher concentration of extracellular than of intracellular bacilli. Some vacuolated macrophages were the infected cells (Fig. 3).
Fig. 3.
Percentages of cells immunostained for interleukin (IL)-10, interferon (IFN)-γ, tumour necrosis factor (TNF)-α and transforming growth factor (TGF)-β in the inflammatory infiltrate located in the subcutaneous tissue of ulcerative lesions without granulomas (black bars) or ulcers with granulomatous lesions (white bars). Five randomly chosen fields at 200× magnification were studied through automated image analysis in each of the 11 cases of Buruli disease. Results are expressed as the mean and standard deviation. The asterisk means statistical significance.
Discussion
BU is primarily an infection of skin and subcutaneous tissue. Three clinical stages of the cutaneous lesions have been described: pre-ulcerative (nodule, plaque or oedema), ulcerative and healed (scar) [2]. The clinical characteristics of these lesions are not specific, particularly during the pre-ulcerative stage. Due to its clinical non-specificity, and because patients usually seek medical assistance after ulcerative lesions have become well-established, it is difficult to obtain representative biopsies from pre-ulcerative and/or healed lesions [16]. Thus, in the present study only ulcerative lesions were analysed. This is the form of the disease subjected most often to histopathological study [16], and all our cases showed AFB confirming the diagnosis.
The histopathological features were similar to those described previously [16,17]. Epidermal hyperplasia was found at the lesion edges, as well as extensive coagulative necrosis affecting the subcutaneous tissue, with variable amounts of inflammatory cells with and without granulomas. We found that the most distinctive feature was the presence of granulomas, which permitted us to classify our cases as two types: ulcerative with granulomas and exclusively ulcerative. Interestingly, we found some differences in the local immune response of both types of ulcerative lesions.
The two types of ulcerative lesion were associated with similar TNF-α expression, but the presence of granulomas was associated with a significantly greater expression of IFN-γ. Ulcerative lesions without granulomas were associated with higher IL-10 and TGF-β expression, although this was not statistically significant. Thus, it seems that the presence of granulomas indicates better immune protection, as this was associated with more IFN-γ and lower bacillary counts.
The cytokine profile that we detected in active ulcerative BU was similar to that reported in active progressive tuberculosis, in which there can be some depression of Th1 cell function accompanied by enhanced Th2 activity, together with higher production of IL-10 and TGF-β[12,13,18]. A similar immune response has been observed in polar leprosy, in which a predominant Th1 cytokine profile with a resistant immune response predominates in tuberculoid leprosy, whereas Th2 cytokines and TGF-β are dominant in lepromatous leprosy patients with ineffective cellular immune responses [14,19]. Similarly, in vitro studies have demonstrated that subjects with M. ulcerans infection compared to exposed control subjects, have significantly lower production of IFN-γ and IL-12, with higher production of IL-4, IL-5, IL-6 and IL-10 in response to both M. ulcerans and BCG [20]. Similarly, RT-PCR analysis of cytokine gene expression in skin biopsies from patients with ulcerative BU, or analysis of the cytokine profile produced by their peripheral blood cells stimulated by specific immunodominant mycobacterial antigens, revealed lower IFN-γ production and higher IL-10 expression than in control BCG-vaccinated subjects or BU patients with nodular pre-ulcerative lesions [15]. Moreover, peripheral lymphocytes from patients before infection responded to in vitro stimulation with M. ulcerans by producing Th1 cytokines, but after ulcer development the response was shifted towards a predominantly Th2 cytokine profile [21]. Our results confirm and extend these in vitro observations, showing that in the local ulcerative lesion there is a mixed pro-inflammatory/anti-inflammatory cytokine pattern. Early ulcerative lesions had a predominantly immunosuppressive cytokine profile accompanied by high bacillary counts, whereas old lesions showed a mixed cytokine balance dominated by IFN-γ, together with low bacillary loads and distinctive granulomas. Thus it seems that granuloma formation represents a late event of the ulcerative stage, probably near to the phase of spontaneous healing. Moreover, granulomas in these older lesions co-existed with pseudo-epitheliomatous epidermal hyperplasia and granulation tissue, which are usually associated with chronic infection [16].
The major virulence determinant of M. ulcerans is the toxin mycolactone. There is considerable heterogeneity in the congeners produced by M. ulcerans strains from different geographical areas, which differ in potency, although the biological activity is conserved [22]. All our cases were from West Africa, where M. ulcerans strains share identical mycolactone profiles, produce the largest amount of the toxin and cause the most severe form of the disease [22]. Thus, we assume that the virulence of the strains involved in the patients we studied was similar.
In both types of ulcerative lesion we found masses of extracellular bacteria immersed in necrotic tissue. In the ulcerative non-granulomatous lesions it was common that bacteria were overlying cells, but in some peripheral areas distant to necrosis we found fewer and dispersed bacilli; in these areas some macrophages showed well-defined intracellular AFB, suggesting real phagocytosed mycobacteria. This observation is in agreement with recent in vitro studies which demonstrated that occasional macrophages can be infected by M. ulcerans[23], and with the concept that M. ulcerans has an intracellular phase that is necessary for the induction of a Th1 immune response [24,25]. It has been demonstrated recently that mycolactone is anti-phagocytic, but macrophages can phagocytose M. ulcerans during early experimental infection and transport the bacilli to the regional lymph nodes [26]. Mutant M. ulcerans unable to produce mycolactone is phagocytosed easily by macrophages and induced well-formed granulomas in experimental animal models [23,25,26]. Thus, the few bacilli that we detected in the cytoplasm of macrophages could correspond to dead bacilli or to bacteria which lacked toxin production, facilitating their phagocytosis.
In conclusion, we characterized the local immunopathology of ulcerative BU lesions in 11 cases from West Africa. Extensive subcutaneous necrosis and chronic inflammation were the most consistent histopathological features, accompanied by a mixed pro-inflammatory/anti-inflammatory cytokine balance. The oldest lesions showed well-formed granulomas with predominant IFN-γ expression, whereas the younger ulcerative lesions showed greater production of the immunosuppressive cytokine IL-10 with higher bacillary counts, and exclusively ulcerative lesions without granulomas.
Acknowledgments
This work was supported partially by the Mexican National Council of Science and Technology, CONACyT, grant no. G36923-M by the Damien Foundation (Brussels, Belgium) and by the American Registry of Pathology. A. E. Kiszewski received a scholarship from CAPES. Authors thank Professor G. A. W. Rook for reading the manuscript and making helpful suggestions.
References
- 1.Asiedu K, Sherpbier R, Raviglione MC. WHO Global Buruli Ulcer Initiative Report. Geneva, Switzerland: World Health Organization; 2000. Boruli ulcer Mycobacterium ulcerans infection. [Google Scholar]
- 2.van der Werf TS, van der Graff WTA, Tappero JW, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–18. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 3.Meyers WM, Shelly WM, Connor DH, Meyers EK. Human Mycobacterium ulcerans infections developing at sites of trauma to skin. Am J Trop Med Hyg. 1974;23:919–23. doi: 10.4269/ajtmh.1974.23.919. [DOI] [PubMed] [Google Scholar]
- 4.Marsollier I, Robert R, Aubry J, et al. Acquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol. 2002;68:4623–8. doi: 10.1128/AEM.68.9.4623-4628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsollier L, Aubry J, Coutanceau E, et al. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol. 2005;7:935–43. doi: 10.1111/j.1462-5822.2005.00521.x. [DOI] [PubMed] [Google Scholar]
- 6.Portaels F, Johnson P, Meyers WM. Buruli ulcer: diagnosis of Mycobacterium ulcerans disease. In: Portaels F, Johnson P, Meyers WM, editors. Buruli ulcer: a manual for health care providers. Geneva, Switzerland: WHO/CDS/GBUI; 2001. pp. 4–92. [Google Scholar]
- 7.Stinear T, Mve-Oblang A, Small PLC, et al. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc Natl Acad Sci. 2004;101:1345–9. doi: 10.1073/pnas.0305877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George KM, Chaterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–7. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 9.George KM, Pascopella L, Welty M, Small PL. A Mycobacterium ulcerans toxin mycolactone causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun. 2000;68:877–83. doi: 10.1128/iai.68.2.877-883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahlevan A, Wright DJ, Andrews C, George KM, Small PL, Foxwell BM. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J Immunol. 1999;163:3928–35. [PubMed] [Google Scholar]
- 11.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Lienhardt C, Azzurri A, Amedei A, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002;32:1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Tossi Z, Ellner J. The role of TGFβ in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol. 1998;87:107–14. doi: 10.1006/clin.1998.4528. [DOI] [PubMed] [Google Scholar]
- 14.Kiszewski AEC, Aguilar LD, Becerril E, Baquera J, Hernàndez Pando R. Expression of transforming growth factor beta isoforms and their receptors in lepromatous and tuberculoid leprosy. Scand J Immunol. 2003;128:279–85. doi: 10.1046/j.1365-3083.2003.01210.x. [DOI] [PubMed] [Google Scholar]
- 15.Prèvot G, Bourreau E, Pascalis H, Pradinaud R, Tanghe Am Huygen K, Launois P. Differential production of systemic and intralesional gamma interferon and interleukin 10 in nodular and ulcerative forms of Buruli disease. Infect Immun. 2004;72:958–65. doi: 10.1128/IAI.72.2.958-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarner J, Bartlett J, Spotts Whitney E, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651–6. doi: 10.3201/eid0906.020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayman J. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J Clin Pathol. 1993;46:5–9. doi: 10.1136/jcp.46.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Crevel R, Karyadi E, Preyers F, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis. 2000;181:1194–7. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 19.Yamamura M, Uyemura K, Deans R, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 20.Gooding TM, Johnson P, Campbell D, et al. Immune response to infection with Mycobacterium ulcerans. Infect Immun. 2001;69:1704–7. doi: 10.1128/IAI.69.3.1704-1707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooding T, Kemp A, Robins Browne M, Johnson P. Acquired T-helper 1 lymphocyte anergy following infection with Mycobacterium ulcerans. Clin Infect Dis. 2003;36:1076–7. doi: 10.1086/368315. [DOI] [PubMed] [Google Scholar]
- 22.Mve-Obiang A, Lee R, Portaels F, Small PLC. Heterogenity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect Immun. 2003;71:774–83. doi: 10.1128/IAI.71.2.774-783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adusumilli S, Mve-Oblang A, Sparer T, Meyers W, Hayman J, Small PL. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295–304. doi: 10.1111/j.1462-5822.2005.00557.x. [DOI] [PubMed] [Google Scholar]
- 24.Stienstra Y, van der Graff W, Meerman G, de Leij J. Susceptibility to development of Mycobacterium ulcerans disease; review of posible risk factors. Trop Med Int Health. 2002;7:554–2. doi: 10.1046/j.1365-3156.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira MS, Fraga AG, Torrado E, et al. Infection by Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect Immun. 2005;73:6229–310. doi: 10.1128/IAI.73.10.6299-6310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutanceau E, Marsollier L, Brosch R, et al. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell Microbiol. 2005;7:1187–96. doi: 10.1111/j.1462-5822.2005.00546.x. [DOI] [PubMed] [Google Scholar]