Abstract
Osteomyelitis is a bone infection caused mostly by Staphylococcus aureus but also by Gram-negative bacteria. Toll-like receptors (TLRs), after recognizing microbial products, induce a signal in neutrophils, leading to NF-κB activation and transcription of pro-inflammatory genes. Polymorphisms in TLR2 (Arg753Gln) and TLR4 (Asp299Gly, Thr399Ile) genes are associated with bacterial infections, we therefore studied these polymorphisms in osteomyelitis patients. Homozygotes for the TLR4 (Asp299Gly) polymorphism were significantly more frequent among the 80 osteomyelitis patients than in the 155 healthy controls (3/80, 3·8% versus 0/155, 0%; P = 0·038). Carriers of one or two G alleles of this tlr4 polymorphism were more likely to have Gram-negative, haematogenous and/or chronic osteomyelitis than those without this mutation (P < 0·031). Patients with the TLR4 (Thr399Ile) mutant, which cosegregates with the TLR4 (Asp299Gly), were also carriers of this second polymorphism. No differences for the TLR2 (Arg753Gln) genotypes were found between patients and controls. Neutrophils of patients homozygous for the TLR4 (Asp299Gly) polymorphism showed lower LPS-induced apoptosis reduction, phosphorylation of the inhibitor of NF-κB, and lower IL-6 and TNF-α levels (P < 0·05). We report here for the first time an association between this TLR4 polymorphism and susceptibility to Gram-negative bacteria and haematogenous osteomyelitis.
Keywords: neutrophils, toll-like receptors, polymorphisms, osteomyelitis, Gram-negative bacteria
Introduction
Osteomyelitis is a bone infection characterized by its progressive inflammatory destruction, and the induction of new bone apposition at the site of infection. In adults, osteomyelitis is usually a complication of open wounds involving the bone, from fractures or surgery (metallic or prosthetic devises), or after bacteraemia in a noninjured bone, mostly in prepubertal children and in elderly patients. It is reported that 0·4–7% of trauma and orthopaedic operations are complicated by osteomyelitis [1,2]. Staphylococcus aureus is the microorganism most frequently isolated in post-traumatic and haematogenous osteomyelitis, although Gram-negative bacteria are also frequently detected. Despite appropriate combined medical and surgical therapies, up to 30% of osteomyelitis cases become chronic, causing major economic losses, morbidity and mortality [3]. Much attention has been devoted to improving the surgical and medical treatment of osteomyelitis, but little progress has been made regarding its pathogenesis. This bone infection is multifactorial and influenced mainly by local factors related to the bone lesion and microorganisms inoculated in the bone. However, hereditary and immunity factors may also contribute to its development [4–6].
The Toll-like receptors (TLRs), members of the IL-1R superfamily, are transmembrane receptors with extracellular leucine-rich repeats and an intracellular signalling domain and are found in monocytes, macrophages and neutrophils. TLRs recognize microbial products (lipopolysaccharide (LPS), lipoproteins and peptidoglycans) and induce a signal in the affected cell, through the p38 mitogen-activated protein kinase (MAPK) and NF-κB [7–9]. The transcription factor NF-κB is located in the cytosol in an inactive state and is complexed with IκB. The signal induced by LPS in TLRs causes the phosphorylation of the IκB protein, resulting in the release and nuclear translocation of active NF-κB. Subsequently NF-κB regulates the activation of several pro-inflammatory genes [10].
Polymorphisms in TLR2 (Arg753Gln) and TLR4 (Asp299Gly, Thr399Ile) genes have been linked to variations in responses to Staphylococcal[11] and Gram-negative bacterial infections and to septic shock [12,13] and could modify the inflammatory response of carriers of these polymorphic alleles to these microorganisms. Neutrophils have a short half-life and die by apoptosis [14]. Activation of TLR2 and TLR4 by either LPS or lipoteichoic acid (LTA), derived from Gram-negative and -positive bacteria, respectively, delays the apoptosis of neutrophils through the production of IL-1β, IL-8, TNF-α and G-CSF [15–17]. This delay may contribute to the chronicity of the bone infection [5]. Finally, NF-κB enhances the activity of osteoclasts [18] and modifications of NF-κB transcription, as occurs in patients with TLR mutations, could modify bone metabolism and lead to predisposition to the development of osteomyelitis or to its chronification.
Here we examined the frequency of the TLR2 (Arg753Gln) and TLR4 (Asp299Gly and Thr399Ile) polymorphisms in osteomyelitis patients and in healthy controls, and the possible association of these mutations with the isolation of a specific type of microorganism or with a determined pathogenic mechanism as cause of the bone infection. In addition, we studied the lifespan of neutrophils, the levels of phosphorylated IκB-α, and the production of cytokines after addition of LPS, in carriers and noncarriers of the TLR4 polymorphic alleles.
Patients and methods
Patients
Eighty patients (54 men, 26 women; mean age 52·3 ± 18·3 years, range 16–89 years) admitted to the Hospital Central de Asturias (Spain) between January 1998 and June 2004 were studied. Patients with acute (24 cases) and chronic (56 cases) osteomyelitis were included in the study and followed for one year. Osteomyelitis was diagnosed by clinical, roentgenographic, computerized tomographic (CT), magnetic resonance imaging (MRI) and isotopic bone imaging criteria. The demonstration of bone sequestra and/or sinus tract in bone X-ray, CT or MRI, a positive Ga67 uptake bone scan and a positive culture of the sequestra or sinus tract were considered diagnostic features of osteomyelitis [1,3]. Osteomyelitis was considered chronic when present for more than three months, and cured when patients did not relapse during a year of follow-up. Surgical and sinus tract pus samples were cultured in all the osteomyelitis patients. We diagnosed 13 patients with haematogenous and 67 with post-traumatic osteomyelitis. Overall, the infection in 23 patients was caused by Gram-negative bacteria (8 by Pseudomonas aeruginosa, 4 by Proteus mirabilis and the rest by several Gram-negative rods) and in 57 by Gram-positive bacteria (47 by S. aureus). Thirty-eight patients had predisposing factors for osteomyelitis (12 had paraplegia, 10 diabetes, 6 peripheral vascular disease, 4 cavus foot and 6 other different factors). All except two of the patients with predisposing factors for osteomyeltis developed chronic bone infection. In addition, a group of 155 Blood Bank donors, matched for age and sex with the patients, were used as controls. Patients and controls were members of a homogeneous population, all Caucasians and residents of the same region (Asturias, Northern Spain) and were in Hardy–Weinberg equilibrium. Each participant gave informed consent for the study, which was approved by the Research Committee of the Hospital Central de Asturias.
Neutrophil isolation
We simultaneously collected 10 ml of peripheral blood for each assay from one or more osteomyelitis patients, and from one or two healthy donors, in glass tubes containing potassium-EDTA. Neutrophils were separated by the following consecutive steps:
sedimentation in 3% dextran T-500 (Pharmacia, Uppsala, Sweden) in 0·9% NaCl;
standard Ficoll-Hypaque (Lymphoprep, Nicomed Pharma, Norway) gradient centrifugation;
hypotonic lysis of the remaining red blood cells by resuspension in 0·2% NaCl;
resuspension in Ham's medium (Biochrom KG, Germany) [5].
The cells were counted in a Coulter autoanalyser (Coulter, Izasa, Spain), adjusted to 0·5 × 107/ml and kept on ice (4 °C) until used. Cells collected from the gradient interface were > 95% neutrophils by Coulter identification and > 95% viable by Trypan blue exclusion.
Genotypic analysis
Other 10 ml of blood from each patient were simultaneously collected in a glass tube containing potassium-EDTA. Genomic DNA was extracted from peripheral blood leucocytes.
TLR2 Arg753Gln genotyping
To analyse the TLR2 Arg753Gln polymorphism, we used the primers described in Table 1, as previously reported [11]. The polymerase chain reaction (PCR) was performed in a final volume of 30 µl, containing 100 ng of genomic DNA. This reaction consisted of an initial denaturation at 94 °C for 5 min, followed by 32 cycles of 30 s at 94 °C, 1 min at 62 °C and 1 min at 72 °C and a final extension of 3 min at 72 °C. The G to A nucleotide change at position 2251 produces a site for the restriction enzyme Pst I. PCR products were separated by electrophoresis on a 3% agarose gel and visualized after ethidium bromide staining.
Table 1.
Oligonucleotide primer sequences, PCR conditions and restriction enzymes used for genotyping and sequencing of the three polymorphisms.
| Gene | Polymorphism | Primers | PCR pr. length (bp) | Annealing temp (°C) | Restriction enzyme |
|---|---|---|---|---|---|
| TLR2 | Arg753Gln | F: 5′-GAGTGGTGCAAGTATGAACTGGA-3′ | 260 | 62 | Pst I |
| R: 5′-TCCCAACTAGACAAAGACTGGTCT-3′ | |||||
| TLR4 | Asp299Gly | F: 5′-GATTAGCATACTTAGACTACTACCTCCATG-3′ | 263 | 56 | Nco I |
| R: 5′-GATCAACTTCTGAAAAAGCATTCCCAC-3′ | |||||
| TLR4 | Thr399Ile | F: 5′-TGGCAACATTTAGAATTAGTTAAC-3′ | 227 | 52 | Msp I |
| R: 5′-CTCAGATCTAAATACTTTAGGCCG-3′ |
The underlined bases in the primers differ from the original sequences and served to introduce a restriction site or to disrupt a natural restriction site within the primer sequence.
TLR4 Asp299Gly genotyping
The A to G change at position 896 produces a site for the restriction enzyme Nco I. A sequence of 263 bp was amplified by PCR with the primers described in Table 1, as previously reported [19]. The underlined bases in the primers differed from the original sequences and served to introduce a restriction site or to disrupt a natural restriction site within the primer sequence. The PCR consisted of an initial denaturation at 95 °C for 1 min, followed by 32 cycles of 30 s at 95 °C, 1 min at 58 °C and 1 min at 72 °C, and a final extension of 5 min at 72 °C. The PCR products were digested overnight with the appropriate restriction enzyme (New England Biolabs, Beverly, USA). The fragments were analysed by electrophoresis on 3% agarose gel stained with ethidium bromide. The lengths of restriction fragments for the distinct alleles are also given in Table 1.
The results of the restriction analysis were confirmed by sequencing representative samples for each genotype. These samples served as standards for the Restriction Fragment Length Polymorphism (RFLP) analysis. PCR products were electrophoresed on a 2% low-melting agarose gel, and the fragments were then excised from the gel, purified with spin columns (DNA gel extraction Kit; Millipore, Billerica, MA, USA), and the fragments were directly sequenced on an ABI Prism 310 Genetic Analyser (Applied Biosystems, Foster City, CA, USA).
TLR4 Thr399Ile genotyping
To determine this polymorphism, we amplified a sequence of 227 bp with the primers described in Table 1. Like the primers used before, the underlined bases in the primers differed from the original sequences and served to introduce a restriction site or to disrupt a natural restriction site within the primer sequence; in this case for the enzyme Msp I.
The PCR consisted of an initial denaturation at 95 °C for 1 min, followed by 32 cycles of 30 s at 95 °C, 30 s at 52 °C and 1 min at 72 °C, and a final extension of 5 min at 72 °C. The PCR products were digested overnight with the appropriate restriction enzyme, Msp I (New England Biolabs). The fragments were analysed by electrophoresis on 3% agarose gel stained with ethidium bromide. The lengths of restriction fragments for the distinct alleles are also given in Table 1.
Collection of sera, culture conditions of polymorphonuclear neutrophils and apoptosis assay
Sera collection was done as previously described [5]. For culture assays, 0·5 × 107 neutrophils in 200 µl of fresh autologous serum were incubated at 37 °C for 12 h with/out LPS and apoptosis was then measured. Lyophilized LPS from Escherichia coli serotype 0111:B4 (Sigma, St. Louis, MO, USA) was used at a final concentration of 10 µg/ml. Apoptosis was assessed by flow cytometry using propidium iodine staining of 5 × 106 neutrophils, as previously described [5,20] and by DNA isolation and gel eletrophoresis using 0·5–1 × 107 neutrophils, as previously reported [5]. All the reagents in the latter assay were purchased from Sigma.
Western blot analysis of the phosphorylated inhibitor of κB (P-IκB-α)
Five × 106 neutrophils were collected and washed in PBS. These cells were then resuspended in 50 µl of lysis buffer (50 mM Tris-HCl, pH 7·5, 150 mM NaCl, 1% Triton-X100, 2 mM EDTA, 8 mM EGTA, 1 mM AEBSF), incubated for 30 min on ice and centrifuged at 16000 × g at 4 °C for 20 min. Protein concentration was measured from the supernatant using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Samples were resuspended in a loading buffer (200 mM Tris, pH 6·8, 8% SDS, 0·4% bromophenol blue, 40% glycerol, 400 mM dithiotreitol), and heated for 5 min at 90 °C. Denatured proteins (80 µg/sample) were separated on 12% denaturing polyacrylamide gels (SDS-PAGE) and transferred to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences, Little Chalfont, UK). Membranes were blocked for 1 h with a 5% (w/v) nonfat dry milk solution containing 10 mM Tris-HCl, pH 7·5, 140 mM NaCl, and 0·1% Tween 20 (TBS-T) before incubation overnight at 4 °C with a primary antibody anti P-IκB-α (Cell Signalling Technology, Danvers, MA, USA), diluted 1/1000 with 5% BSA in TBS-T. After washing, the membranes were incubated for 1 h with a horseradish peroxidase-labelled secondary antibody at a dilution of 1 : 4000 in 1% nonfat dry milk/TBS-T, and the labelled proteins were detected using enhanced chemiluminescence (ECL) reagents, as described by the manufacturer (Amersham Biosciences). To simplify their graphical representation, optical density values of the Western blot assays were transformed to arbritary units using the software Quantity One in a densitometer GS-800 (both from Bio-Rad, Hercules, CA, USA).
Cytokines
IL-6 and TNF-α were measured in supernatant cultures stored at −70 °C using ELISA kits (Amersham Biosciences), following the manufacturer's instructions.
Statistical analysis
The statistical analysis was performed with the SPSS package (Version 11·0, Chicago, IL, USA). Statistical analysis of the data was performed by the χ2 test, the Fisher's exact test or the Mann–Whitney U-test where apropriate. The level of significance was P < 0·05.
Results
Frequency of TLR4 (Asp299Gly and Thr399Ile) and TLR2 (Arg753Gln) polymorphisms in osteomyelitis
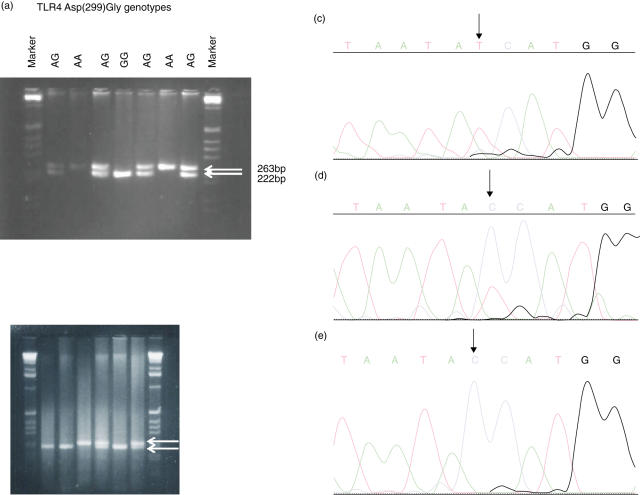
To determine the 896 A/G polymorphism in the TLR4 gene, genomic DNA from osteomyelitis patients was amplified and PCR products were subsequently digested with the enzyme NcoI. RFLP of the 299 section of the gene was then performed. Homozygotes for the TLR4 (Asp299Gly) polymorphism (GG genotype) were significantly more frequent among the 80 osteomyelitis patients than in the 155 healthy controls (χ2 = 5·86, P = 0·038 by the Fisher's exact test) (Table 2, Fig. 1a). However, although carriers of the G allele were more frequent among the former, the difference between groups was not significant (P = 0·08). Patients with the TLR4 (Asp299Gly) polymorphism, which cosegregates with the TLR4 (Thr399Ile), were also carriers of this second polymorphism (Table 2, Fig. 1b). The frequency of heterozygous CT or the homozygous CC for this TLR4 (Thr399Ile) polymorphism did not differ between controls and osteomyelitis patients (Table 2).
Table 2.
Polymorphisms of Toll-like receptors (TLR) 2 and 4 genes in osteomyelitis (OM) patients and controls.
| Gene | Genotype frequencies | OM | Controls | Pearson χ2 | Odds ratio (95% CI) | P-value | Allele frequencies | OM | Controls | Pearson χ2 | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TLR4 Asp(299)Gly | ||||||||||||
| No. of patients. (%) | 80 (100·0) | 155 (100·0) | ||||||||||
| GG | 3 (3·8) | 0 (0·0) | 5·86 | NA | 0·038 | A | 18 (0·11) | 20 (0·07) | 3·11 | 1·81 (0·88–3·71) | 0·08 | |
| AG | 12 (15·0) | 20 (12·9) | ||||||||||
| AA | 65 (81·2) | 135 (87·1) | G | 142 (0·89) | 290 (0·93) | |||||||
| TLR4 Thr(399)Ile | ||||||||||||
| No. of patients (%) | 80 (100·0) | 155 (100·0) | ||||||||||
| TT | 3 (3·5) | 0 (0·0) | 5·86 | NA | 0·038 | T | 16 (0·1) | 22 (0·08) | 1·19 | 1·45 (0·7–2·99) | 0·27 | |
| CT | 10 (12·04) | 22 (14·45) | ||||||||||
| CC | 67 (84·3) | 133 (85·44) | C | 144 (0·9) | 288 (0·92) | |||||||
| TLR2 Arg(753)Gln | ||||||||||||
| No. of patients (%) | 80 (100·0) | 155 (100·0) | ||||||||||
| AA | 0 (0) | 0 (0) | NA | NA | NA | A | 2 (0·02) | 3 (0·01) | 0·08 | 1·3 (0·15–9·61) | 0·78 | |
| GA | 2 (2·4) | 3 (1·9) | ||||||||||
| GG | 78 (97·6) | 152 (98·1) | G | 158 (0·98) | 307 (0·99) |
NA, not applicable; OM, osteomyelitis; 95% CI, 95% confidence intervals.
Fig. 1.
(a) Detection of the 896 A/G polymorphism in the TLR4 gene. (Left) Genomic DNA of osteomyelitis (OM) patients was amplified and PCR products were digested with the enzyme NcoI. The agarose gel shows the RFLP of the 299 section of the TLR4 gene from wild-type, heterozygous, and homozygous OM patients. (c–e) Sequencing of the RFLP. Upon sequencing the sample in (c) was homozygous for alanine at position 896 (wild type), the sample in (d) was identified as heterozygous with both an alanine and a guanine at position 896 (heterozygous), and the sample in (e) was homozygous for a guanine at position 896 (homozygous with the mutation). (b) Detection of the Thr399Ile polymorphism in the TLR4 gene. Genomic DNA of OM patients was amplified and PCR products were digested with the enzyme MspI. The agarose gel shows the RFLP of the 399 section of the TLR4 gene from wild-type, heterozygous, and homozygous OM patients.
Regarding the TLR2 (Arg753Gln) polymorphism, no differences among the frequency of heterozygous GA or homozygous GG was found between controls and patients.
Effect of the TLR4 (Asp299Gly) polymorphism on the aetiology and pathogenesis of osteomyelitis
To establish whether the TLR polymorphisms are correlated with clinical presentation, we analysed several pathogenic, evolutive and microbiological parameters (Table 3). Carriers of one or two alleles of the TLR4 polymorphism were more likely to be infected with Gram-negative bacteria than noncarriers (60% patients with the TLR4 Asp 299Gly GG + AG genotypes versus 21·5% in patients with the AA genotype (χ2 = 7·02; OR(95%CI) = 5·46 (1·45 21·34); P = 0·0086 by the Fisher's exact test). Moreover, these carriers were more likely to have haematogenous osteomyelitis than noncarriers (40% patients with the TLR4 Asp 299Gly GG + AG genotypes versus 10·8% in patients with the AA genotype (χ2 = 5·65; OR (95%CI) = 5·52 (1·27,24·58); P = 0·013 by the Fisher's exact test). Furthermore, these carriers showed a greater probability of developing chronic osteomyelitis than noncarriers (93·3% patients with the TLR4 Asp 299Gly GG + AG genotypes versus 64·6% in patients with the AA genotype (χ2 = 3·52 OR(95%CI) = 0·13 (0·01–1·06); P = 0·031 by the Fisher's exact test) (Table 3). Finally, there were not significant differences in the predisposing factors to osteomyelitis among the carriers of the different TLR4 Asp(299)Gly genotypes (Table 4). Therefore, the predisposition to osteomyelitis was due to the carriage of the TLR4 polymorphism.
Table 3.
Clinical characteristics of the osteomyelitis (OM) patients, carriers of the different TLR4 Asp(299)Gly genotypes, and of the blood donor controls.
| TLR4 GG (n = 3) | TLR4 AG (n = 12) | TLR4 GG + AG (n = 15) | TLR4 AA (n = 65) | Controls (n = 155) | |
|---|---|---|---|---|---|
| Female sex/total cases (%) | 3/3 (100)* | 1/12 (8·3) | 4/15 (26·7) | 22/65 (33·8) | 55/155 (35·5) |
| Mean age (years) | 49·7 ± 15·6 | 59·8 ± 12·4 | 57·5 ± 13·2 | 52·5 ± 17·8 | 51·8 ± 20·1 |
| Acute OM (%) | 0/3 (0) | 1/12 (8·3) | 1/15 (6·7)† | 23/65 (35·4) | NA |
| Hematogenous source of infection (%) | 1/3 (33·3) | 5/12 (41·7)‡ | 6/15 (40·0)§ | 7/65 (10·8) | NA |
| Gram negative OM (%) | 2/3 (66·6) | 7/12 (63·6)¶ | 9/15 (60·0)** | 14/65 (21·5) | NA |
OM, osteomyelitis; NA, not applicable.
P = 0·045 by the Fisher's exact test while comparing the female frequency between OM patients carriers of the GG genotype versus AA genotype.
χ2 = 3·52; OR (95%CI) = 0·13 (0·01–1·06); P = 0·031 by the Fisher's exact test while comparing the frequency of acute OM between patients who were carriers of the GG + AG genotypes versus AA genotype.
χ2 = 5·19; OR (95%CI) = 5·92 (1·21–29·65); P = 0·017 by the Fisher's exact test while comparing the frequency of haematogenous OM between patients who were carriers of the AG genotype versus AA genotype.
χ2 = 5·65; OR (95%CI) = 5·52 (1·27–24·58); P = 0·013 by the Fisher's exact test while comparing the frequency of haematogenous OM between patients who were carriers of the GG + AG genotypes versus AA genotype.
χ2 = 5·18; OR (95%CI) = 5·1 (1·27–22·51); P = 0·025 by the Fisher's exact test while comparing the frequency of Gram negative OM between patients who were carriers of the AG genotype versus AA genotype.
χ2 = 7·02; OR (95%CI) = 5·46 (1·45–21·34); P = 0·0086 by the Fisher's exact test while comparing the frequency of Gram negative OM between patients who were carriers of the GG + AG genotypes versus AA genotype.
Table 4.
Predisposing factors for osteomyelitis (OM) in the patients, carriers of the different TLR4 Asp(299)Gly genotypes.
| Predisposing factors | TLR4 GG (n = 3) | TLR4 AG (n = 12) | TLR4 GG + AG (n = 15) | TLR4 AA (n = 65) | P-value |
|---|---|---|---|---|---|
| Paraplegia (%) | 0/3 (0) | 2/12 (16·7) | 2/15 (13·3) | 10/65 (15·4) | NS |
| Peripheral vascular disease (%) | 0/3 (0) | 1/12 (8·3) | 1/15 (6·7) | 5/65 (7·7) | NS |
| Cavus foot (%) | 1/3 (33·3) | 1/12 (8·3) | 2/15 (13·3) | 2/65 (3·1) | NS |
| Other factors (%)* | 0/3 (0) | 1/12 (8·3) | 1/15 (6·7) | 5/65 (7·7) | NS |
| Diabetes | 0/3 (0) | 1/12 (8·3) | 1/15 (6·7) | 9/65 (13·8) | NS |
| Total factors (%) | 1/3 (33·3) | 6/12 (50·0) | 7/15 (46·7) | 31/65 (47·7) | NS |
NS, not significant.
osteopetrosis, sensitive polineuropathy, hip dysplasia, Ewing sarcoma, prostate cancer, Munchausen syndrome.
Neutrophil apoptosis
To determine whether the TLR polymorphisms are associated with abnormal signal transduction, we studied several of the functional activities of neutrophils. Thus, we examined the apoptosis of neutrophils after LPS incubation in carriers and noncarriers of the TLR4 (Asp299Gly) G allele and in healthy donors. The apoptosis of neutrophils from patients was significantly decreased in relation to controls (P = 0·002), as we have previously reported [5]. After LPS treatment, apoptosis was significantly reduced in the neutrophils of healthy donors (65·1% to 36·9%; 43·3% reduction; P = 0·002). Among the patients with the distinct genotypes of the TLR4 (Asp299Gly) allele, a further, although less significant delay occurred in apoptosis after incubation of the neutrophils with LPS in patients in the AG group (22·9% to 18·4%; 19·7% reduction of apoptosis; P = 0·375) and even lower in patients in the GG group (8·9% to 7·6%; 14·6% reduction; P = 0·750) (Fig. 2a). Apoptosis reduction after LPS treatment in patients in the AA group (28·1% to 14·3%; 49·1% reduction; P = 0·189) was similar to that of the healthy controls. Interestingly, patients in the GG group had lower spontaneous and LPS-induced apoptosis rates than those in the AA and AG groups (P < 0·04) (Fig. 2a). These results were confirmed by DNA laddering using gel electrophoresis (Fig. 2b).
Fig. 2.
Decreased apoptosis in neutrophils of patients with the TLR4 (Asp299Gly) GG genotype. (a) apoptosis was measured by propidium iodide staining and FACS in 5 × 106 neutrophils incubated for 12 h in autologous serum with (▪) or without (□) LPS (10 µg/ml). Results represent the mean ± SD of at least three individuals. P = 0·034 while comparing the spontaneous apoptosis of OM patients with the TLR4 AA and GG genotypes. P = 0·038 for the LPS-induced apoptosis while comparing the TLR4 AA and AG versus GG genotypes. (b) apoptosis was determined by DNA laddering.
Phosphorylation levels of the IκB-α protein
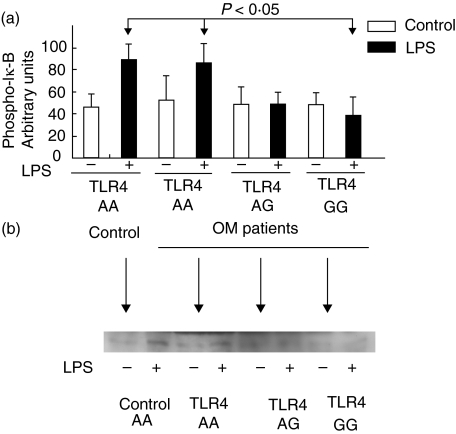
By testing the phosphorylation of Iκ-B, we determined the functional activity of the TLR4 genotypes on NF-κB. We studied the effect of LPS on the neutrophils of osteomyelitis patients by Western blotting. After LPS treatment, an antibody that specifically detects the phosphorylated form of the protein IκB showed a band in the neutrophils of controls (Fig. 3b). In the neutrophils of patients in the GG group the phosphorylation of Iκ-B was significantly decreased after LPS incubation compared with that found in the AA group (P < 0·05) (Fig. 3a). This finding indicates that the TLR4 mutation involves a decreased capacity to transmit signalling.
Fig. 3.
Decreased phospho-IκB production in neutrophils of patients with the TLR4 (Asp299Gly) GG genotype. 5 × 106 neutrophils were incubated under the same conditions as in Fig. 2 and the phospho-IκB was determined by Western blot analysis. Optical density values of the Western blot assay were transformed to arbitrary units to simplify their graphical representation using the software Quantity One in a densitometer GS-800. (a) results of the Western blot assay are shown. Results represent the mean ± SD of at least three individuals. P < 0·05 for the comparison of phospho-IκB production after LPS induction between individuals with the AA and GG genotypes. (b) shows the results of the phospho-IκB production assessed by Western blot in three OM patients with the distinct TLR4 genotypes and one control with the TLR4 AA genotype.
Cytokine secretion
Because LPS exposure induces an inflammatory response and cytokine secretion by the neutrophils, we measured the IL-6 and TNF-α levels in culture medium after 12 h of incubation with LPS. The neutrophils of patients released higher amounts of IL-6 to the culture media than the controls (Fig. 4a). After incubation with LPS, neutrophils from controls and also from patients with the AA phenotype increased the amount of IL-6 secreted. Interestingly, in neutrophils of carriers of one or the two G alleles of the TLR4 (Asp299Gly) polymorphism, the LPS treatment did not increase IL-6 secretion (P < 0·02) (Fig. 4a). In all the groups, the LPS treatment induced the release of TNF-α (Fig. 4b). However, this induction was lower in carriers of the G alleles of this TLR4 mutant although not at a significant level.
Fig. 4.
Production of pro-inflammatory cytokines by neutrophils of osteomyelitis patients with the distinct TLR4 (Asp299Gly) genotypes and healthy controls. 5 × 106 neutrophils were incubated for 12 h in autologous serum with (▪) or without (□) LPS (10 µg/ml) and cytokine levels, (a) IL-6 and (b) TNF-α, in the supernatant were measured by ELISA. Results represent the mean ± SD of at least three individuals. P = 0·02 while comparing the IL-6 LPS-induced of neutrophils of individuals with the AA and GG genotypes.
Discussion
Neutrophils are a main component of the innate immune system. Exposure to bacteria, or to bacterial products, such as LPS, activates these cells as part of the inflammatory response, thereby resulting in the clearance of pathogens and an increase in neutrophil survival [15,21]. However, inappropriate or excessive neutrophil activation or alterations in their lifespan can cause severe tissue damage, contributing to the pathology of a range of inflammatory and infectious diseases such as osteomyelitis [5,22,23]. TLR2 and 4, which are expressed on the cell surface of human neutrophils, play a major role in the detection of the microbial environment by regulating neutrophil activation and survival. Since the discovery of the TLRs as crucial receptors that recognize microbial components and alert the immune system, healthy and patient populations have been screened for polymorphisms in tlr2 and tlr4 to determine whether these mutations may be risk factors for bacterial infections. The results obtained to date are inconclusive, as only a few carriers of these polymorphisms have been identified in small populations, and functional assays of the patients' immunological response to bacterial stimuli were often not performed.
Although representing a small percentage of our osteomyelitis patients (3/80, 3·8%), homozygotes for the (Asp299Gly) polymorphism of tlr4 are more frequent among osteomyelitis patients than in the control population. In addition, we found that this polymorphism predisposes individuals to Gram-negative and to haematogenous osteomyelitis and perhaps also to the chronicity of the bone infection. The observation that osteomyelitis was associated with the GG genotype of the (Asp299Gly) polymorphism but not with the G allele frequency may indicate that this mutation has a dosage effect and that both alleles are required to produce a full pathogenic effect. We found no differences between osteomyelitis patients and controls in the frequency of the (Arg753Gln), a tlr2 polymorphism associated with S.aureus infections. Our results are consistent with those of Lorenz et al.[12] and Agnese et al.[24], who reported a predisposition of homozygous carriers of the TLR4 (Asp299Gly) polymorphism to Gram-negative bacterial infections and septic shock. However other authors could not find an association between carriage of this TLR4 polymorphism and meningococcal disease or sepsis [25,26].
The role of the tlr4 polymorphism in the pathogenesis of osteomyelitis is unclear. The hyporesponsiveness of the neutrophils of the carriers of the tlr4 polymorphic allele in response to LPS, a component of the Gram-negative wall, may contribute to the development of this infection. We found decreased levels of Iκ-B, and IL-6 after LPS treatment, and lower spontaneous and LPS-induced apoptosis of neutrophils in carriers of the GG or AG genotypes compared to noncarriers (AA genotype). Our results agree with those of Arbour et al. [27], who showed a decreased release of cytokines (IL-1β, IL-6, TNF-α) in addition to reduced NF-κB activity after LPS treatment of neutrophils from carriers of the tlr4 (Asp299Gly) G allele. Our results contrast with those from other studies that report no differences in cytokine secretion [28–31] or in MAPK activity [32] by blood mononuclear cells of carriers of the tlr4 (Asp299Gly) polymorphism after LPS challenge. These differences could be due to different stimulation techniques or to functional differences between the cell populations stimulated: blood monocytes versus neutrophils. Ayala et al. [22]. proposed that signalling through tlr4 is required to maximize neutrophil recruitment and/or migration to the damaged tissue but does not markedly affect priming for the cytokine response to sepsis. In addition, other microbial and host-derived products, such as the F-protein of respiratory syncytial virus, extra domain A of fibronectin, and both human and bacterial heat- shock proteins, activate cells via TLR4, and their signalling could be disrupted in carriers of the TLR4 polymorphism. However, this hypothesis, which could not be proved by van der Graaf et al.[31], was not assessed in this study. An additional point to explore is the finding that TLR4–deficient mice have reduced bone destruction following mixed anaerobic infections [33]. In addition, inhibition of NF-κB, which is produced by LPS, blocks osteoclastogenesis and decreases pro-inflammatory cytokine production and inflammatory bone loss in collagen-induced arthritic mice [18]. This observation may indicate that because patients with the TLR4 (Asp299Gly) polymorphism have a lower NF-κB activity than noncarriers in response to LPS challenge, by their neutrophils and perhaps by other cells, their osteoclast response to the bone damage caused by the infection may be impaired. The low frequency of homozygous carriers of this TLR4 polymorphism in the Caucasian population, which is below 1%[12,26,28,30,34], hinders the study of bone metabolism in osteomyelitis in larger series.
Further studies are required to confirm the association of the tlr4 (Asp299Gly) polymorphism and osteomyelitis and to determine the exact mechanism by which this polymorphism affects the pathogenesis of this bone infection. Examination of a more heterogenous population or another homogenous population distinct from the one we studied could help to determine the value of this polymorphism on osteomyelitis pathogenesis. It is plausible, as other authors have stated, that genetic contributions to an impaired immune response or susceptibility to infections, such as osteomyelitis, are caused by the cumulative effect of several mutations present in known and unknown candidate genes involved in the immune response [32], such as the IL-1α (−889) polymorphism reported recently by our group [4].
Acknowledgments
This study was supported by the research grants IR-00-519-59 and MB-02-519-1 from Oviedo University, Spain; FICYT grant PB02-019; and the Fondo de Investigaciones Sanitarias grant PI030282 (all given to Dr Victor Asensi). We thank Tanya Yates for editorial help.
References
- 1.Cierny GI, Mader JT. Adult chronic osteomyelitis. Orthopedics. 1984;7:1557–64. doi: 10.3928/0147-7447-19841001-07. [DOI] [PubMed] [Google Scholar]
- 2.Roesgen M, Hielholzer G, Hax PM. Post-traumatic osteomyelitis. Pathophysiology and management. Arch Orthop Trauma Surg. 1989;108:1–9. doi: 10.1007/BF00934149. [DOI] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–79. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Asensi V, Alvarez V, Valle E, et al. An IL-1α (-889) promoter polymorphism is a risk factor for osteomyelitis. Am J Med Genet. 2003;119A:132–6. doi: 10.1002/ajmg.a.20137. [DOI] [PubMed] [Google Scholar]
- 5.Asensi V, Valle E, Meana A, et al. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun. 2004;72:3823–8. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon KS, Fitzgerald RH, Jr, Sud S, Song Z, Wooley PH. Experimental acute hematogenous osteomyelitis in mice. II. Influence of Staphylococcus aureus infection on T-cell immunity. J Orthop Res. 1999;17:382–91. doi: 10.1002/jor.1100170313. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B. TLR4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–6. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 8.Sabroe ITJ, Jones EC, Usher LR, Whyte MKB, Dower SK. Toll-like receptor (TLR) 2 and TLR4 in human peripheral granulocytes: a critical role for monocytes in leukocyte polysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 9.Sabroe ITJ, Parker AG, Wilson AG, White MKB, Dower SK. Toll-like receptors, their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy. 2002;32:984–9. doi: 10.1046/j.1365-2745.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 10.Vancurova I, Miskolci V, Davidson D. NF-κB activation in tumor necrosis factor α-stimulated neutrophils is mediated by protein kinase Cδ. Correlation to nuclear IκBα. J Biol Chem. 2001;276:19746–52. doi: 10.1074/jbc.M100234200. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2002;68:6398–401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in the patients with Gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 13.Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infection disease. Lancet. 2005;5:156–64. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 14.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–22. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 15.Lotz S, Assa E, Wilde L, van Zandbergen G, Hartung T, Solbach W, Laskay T. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leuk Biol. 2004;75:467–77. doi: 10.1189/jlb.0803360. [DOI] [PubMed] [Google Scholar]
- 16.Power CP, Wang JH, Manning B, Kell MR, Aherne NF, Wu QD, Redmond HP. Bacterial lipoprotein delays apoptosis in human neutrophils through inhibition of caspase-3 activity. regulatory roles for CD14 and TLR-2. J Immunol. 2004;173:5229–37. doi: 10.4049/jimmunol.173.8.5229. [DOI] [PubMed] [Google Scholar]
- 17.Sabroe ITJ, Prince LR, Jones EC, et al. Selective roles for toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and lifespan. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 18.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature Med. 2004;10:617–24. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz E, Frees K, Schwartz DA. Determination of the TLR4 genotype using allele-specific PCR. Biotechniques. 2001;31:22–4. doi: 10.2144/01311bm01. [DOI] [PubMed] [Google Scholar]
- 20.Nicoletti L, Migliorati G, Oagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodine staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 21.Baran J, Guzik K, Hryniewicz W, Ernst M, Flad MHD, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;64:4242–8. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayala A, Chung CS, Lomas JL, et al. Shock-induced neutrophil mediated priming for acute lung injury in mice. Divergent effects of TLR-4 and TLR/FasL deficiency. Am J Pathol. 2002;161:2283–94. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez MF, Watson RW, Parodo J, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–71. doi: 10.1001/archsurg.1997.01430360009002. [DOI] [PubMed] [Google Scholar]
- 24.Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SM, Lowry SF. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of Gram-negative infections. J Infect Dis. 2002;186:1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 25.Read RC, Pullin J, Gregory S, et al. A functional polymorphism of Toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J Infect Dis. 2002;184:640–2. doi: 10.1086/322798. [DOI] [PubMed] [Google Scholar]
- 26.Feterowski C, Emmanuilidis K, Moethke T, et al. Effects of functional Toll-like receptor-4 mutations on the immune response to human and experimental sepsis. Immunology. 2003;109:426–31. doi: 10.1046/j.1365-2567.2003.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nature Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 28.Erridge CJ, Stewart J, Poxton LR. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med. 2003;197:1787–91. doi: 10.1084/jem.20022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heesen MB, Bloemeke B, Kunz D. The cytokine synthesis by heterozygous carriers of the Toll-like receptor 4 Asp299Gly polymorphism does not differ from that of wild type homozygotes. Eur Cytokine Network. 2003;14:234–7. [PubMed] [Google Scholar]
- 30.Von Aulock S, Schröder NWJ, Guenzius K, et al. Heterozygous toll-like receptor 4 polymorphism does not influence lipopolysaccharide–induced cytokine release in human whole blood. J Infect Dis. 2003;188:938–43. doi: 10.1086/378095. [DOI] [PubMed] [Google Scholar]
- 31.van der Graaf C, Kullberg BJ, Joosten L, Verver-Jansen T, Jacobs L, van der Meer JWM, Netea MG. Functional consequences of the Asp299Gly Toll-like receptor-4 polymorphism. Cytokine. 2005;30:264–8. doi: 10.1016/j.cyto.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Imahara SD, Jelacic S, Junker CE, O'Keefe GE. The TLR4+896 polymorphism is not associated with lipopolysaccharide hypo-responsiveness in leukocytes. Genes Immun. 2005;6:37–43. doi: 10.1038/sj.gene.6364147. [DOI] [PubMed] [Google Scholar]
- 33.Hou L, Sasaki H, Stashenko P. Toll-like receptor-4 deficient mice have reduced bone destruction following mixed anaerobic infections. Infect Immun. 2000;68:4681–487. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folwaczny M, Glas J, Török HP, Limbersky O, Folwaczny C. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol. 2004;135:330–5. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]