Abstract
The natural history of preclinical diabetes is partly characterized, but there is still limited information on the dynamics of the immune response to β-cell autoantigens during the course of preclinical disease. The aim of this work was to assess the maturation of the humoral immune response to the protein tyrosine phosphatase(PTP)-related proteins (IA-2 and IA-2β) in preclinical type I diabetes (TID). Forty-five children participating in the Finnish Type I Diabetes Prediction and Prevention (DIPP) Study who had seroconverted to IA-2 antibody positivity were analysed. Specific radiobinding assays were used to determine IA-2/IA-2β epitope-specific antibodies (the juxtamembrane (JM) region of IA-2, PTP-like domain and βPTP-like domain) and isotype-specific IA-2 antibodies. Individual areas under the curve (AUC) over the observation period were calculated for total IA-2 antibodies, each isotype and specific epitope responses. The children who progressed to TID tended to have an initial IA-2 JM epitope response more frequently (P = 0·06), and this response was more often dominant during the observation period (P < 0·05). The children who did not progress to TID had IgE-IA-2 more frequently (70%; versus progressors 27%; P < 0·05), and had higher integrated titres of IgE-IA-2 antibodies (P < 0·05). The occurrence of IgE-IA-2 antibodies was protective even when combined with positivity for IA-2 JM antibodies (P = 0·002). IgE-IA-2 antibody reactivity may be a marker of a regulatory immune response providing protection against or delaying progression to TID among IA-2 antibody-positive young children with HLA-conferred disease susceptibility.
Keywords: type I diabetes, IA-2 antibody, epitope and isotype
Introduction
Type I diabetes (TID) is a chronic autoimmune disease characterized by silent destruction of the β-cells in the pancreatic islets of Langerhans. During this asymptomatic destructive process, which usually lasts for years, various autoantibodies to islet cell autoantigens can be detected as a sign of an ongoing T-cell mediated process, e.g. the related protein tyrosine phosphatase (PTP)-like molecules, islet antigen (IA)-2 and IA-2β [1]. Antibodies to the intracellular portion of the IA-2/IA-2β molecule have been shown to be the most predictive ones in relation to TID [2–4]. Autoantibodies specific for the IA-2 JM (juxtamembrane) region, the IA-2 PTP-like domain, and the IA-2β PTP-like domain have been identified, as also autoantibodies that are cross-reactive between the IA-2 and IA-2β PTP-like domains [2–7].
The changes recognized in the isotype profile of antigen-specific autoantibodies may also mirror the maturation of that response, as the isotypes might indirectly reflect the T-helper 1/T-helper 2 (Th1/Th2) balance of β-cell autoimmunity [8–10]. It has been speculated that islet cell autoimmunity may start as a nonpathogenic Th2 response to the β-cells, but turn into a pathogenic and destructive Th1 response leading to TID [11–13]. The natural history of preclinical diabetes is partly characterized, but still we have limited information on the dynamics of the immune response to β-cell autoantigens during the course of preclinical disease. The aim of this work was to assess the maturation of the humoral immune response to IA-2 antibodies in preclinical TID by timing the emergence of various isotypes (IgG subclasses, IgM, IgA, IgE) of IA-2 antibodies, and observe possible signs of epitope spreading within the molecule after the initial appearance of such antibodies in genetically susceptible young children identified from the general population. This could provide more sensitive and specific markers for discriminating between early and later stages of preclinical TID.
Subjects and methods
Subjects
The series was derived from the Finnish Type I Diabetes Prediction and Prevention (DIPP) Study, which is a large ongoing population-based survey of genetically susceptible individuals aimed at studying the natural course of preclinical TID and assessing the predictive value of various immune and genetic risk markers in the general population [14]. According to the protocol, all newborn infants carrying HLA DQB1 genotypes conferring susceptibility to TID were observed from birth for the appearance of diabetes-associated autoantibodies. Islet cell antibodies (ICA) are used for primary screening of β-cell autoimmunity. If a child seroconverts to positivity for ICA, antibodies to insulin (IAA), glutamic acid decarboxylase 65 (GAD65Ab) and the protein tyrosine phosphatase-related IA-2 protein are analysed in all available samples from birth, and the children in all centres are monitored at 3-month intervals after ICA seroconversion. The protocol has been approved by the local Ethics Committees, and the parents of the children have given their written informed consent to participation.
Eighty-four (1·0%) children of a total of 8433 had tested positive for IA-2 antibodies on at least one occasion 7 years after the start of the DIPP study, and 16 of these had progressed to overt TID during the period of prospective observation. Fifteen of them had an IA-2 antibody-positive serum sample available from more than one point in time, and these were included as cases in the present study. Among the remaining 68 subjects, we identified 30 children who had remained nondiabetic (nonprogressors) and could be matched with the progressors for sex, HLA genotype, age at the end of observation and IA-2 antibody-positive observation time. IA-2 antibody subclasses and epitope specificities were analysed in the serum samples starting from the time at which IA-2 antibodies were first detected, or from the previous sample onwards if available (the mean age at seroconversation to IA-2 antibody positivity was 2·2 years; range 1·0–4·8 years).
Each child who progressed to clinical TID during the follow-up was observed up to diagnosis, and each nonprogressor up to the sample obtained at the corresponding age. The mean age of the 15 progressors at the time of the diagnosis of diabetes was 3·5 years (range 2·1–7·0 years), and the mean age of the 30 nonprogressors at the end of follow-up was 3·9 years (range 1·4–6·5 years, P = 0·34). A total of 465 samples were tested and analysed here, with a mean follow-up time of 2·0 years (range 0·4–4·5 years). The number of samples per subject varied from 3 to 16 (median 9) in the progressors and from 3 to 19 (median 8) in the nonprogressors (P = 0·32). There was no difference in the matched observation time between the progressors (mean 1·98 years, range 0·4–4·3 years) and nonprogressors (mean 1·99 years, range 0·6–4·5 years; P = 0·97).
Methods
Assays for IA-2 antibodies
The antibodies to the protein tyrosine phosphatase-related IA-2 protein were quantified with a specific radiobinding assay as described previously [15]. Antibody levels were expressed in relative units (RU) based on a standard curve from a pool of highly positive IA-2 antibody samples diluted in normal human serum (NHS). The limit for IA-2 antibody positivity was set at 0·43 RU, which represents the 99th percentile in 374 nondiabetic Finnish children and adolescents. The disease sensitivity of this assay was 62% and the disease specificity 100%, based on the 2002 CDC-sponsored Diabetes Autoantibody Standardization Programme (DASP) workshop. All samples with antibody levels between the 97·5th and 99·5th percentiles were retested to confirm the antibody status.
Epitope and isotype-specific IA-2 and IA-2β antibodies
Epitope-specific IA-2 and IA-2β antibodies were analysed according to a protocol identical with that used for IA-2 antibodies, but using IA-2 PTP687−979, IA-2β PTP741−1033, IA-2389−779 and IA-2601−682/IA-2β737−1033 (juxtamembrane region, JM) as radioligands. Isotype-specific IA-2 antibodies were analysed in an assay based on the same principles as that used for total IA-2 antibodies except that the protein A Sepharose precipitation was replaced by monoclonal subclass-specific antibodies linked to streptavidin agarose. The methods and constructs used have been described in detail previously [16]. All samples from the same individual were analysed in the same assay round. The intra-assay and interassay coefficients of variation were less than 16% and 19% in the epitope-specific assays and less than 15% and 20% when measuring isotype-specific IA-2 antibodies.
Assays for other diabetes-associated autoantibodies
Islet cell antibodies (ICA) were quantified by a standard indirect immunofluorescence method [17,18]. IAA were analysed with a radiobinding microassay [19], and GAD65Ab with a specific radiobinding assay as described previously [20].
Data handling and statistical analysis
The unpaired and paired Student's t-tests were used to compare mean ages and age differences. The Bonferroni correction for multiple comparisons was performed where appropriate. The distribution of IA-2 autoantibody positivities of epitopes and isotypes between the groups was evaluated by cross-tabulation and χ2–statistics or Fisher's exact test. Individual areas under the curve (AUC) over the observation period were calculated for total IA-2 antibodies, for each isotype and for specific epitope responses (IA-2 JM, IA-2 PTP, IA-2 βPTP) to avoid the problems associated with multiple data points [21]. The Mann–Whitney U-test was used to compare the specific antibody levels and AUC values between the two groups studied. Kaplan-Meier life-table survival analysis and log rank statistics were used to assess progression to clinical diabetes in relation to various epitope and isotype-specific IA-2 antibodies. A two-tailed P-value of 0·05 or less was considered to be statistically significant. All the statistical analyses were performed using the SPSS statistical software package, version 10·1 (SPSS, Chicago, IL, USA).
Results
Positivity for autoantibodies
As defined by the inclusion criteria, all 45 children were positive for IA-2 antibodies on inclusion in the series. Seroconversion to IA-2 antibody positivity occurred at a mean age of 1·9 years (range 1·0–4·7 years) among the progressors and at a mean age of 2·4 years (range 1·0–4·8 years; P = 0·17) among the nonprogressors, the median levels of initial IA-2 antibodies being 10·8 RU (range, 0·5–100·6 RU) and 7·1 RU (range 0·5–247·3 RU; P = 0·55) in the two groups, respectively.
Appearance of IA-2/IA-2β epitope reactivities and isotypes
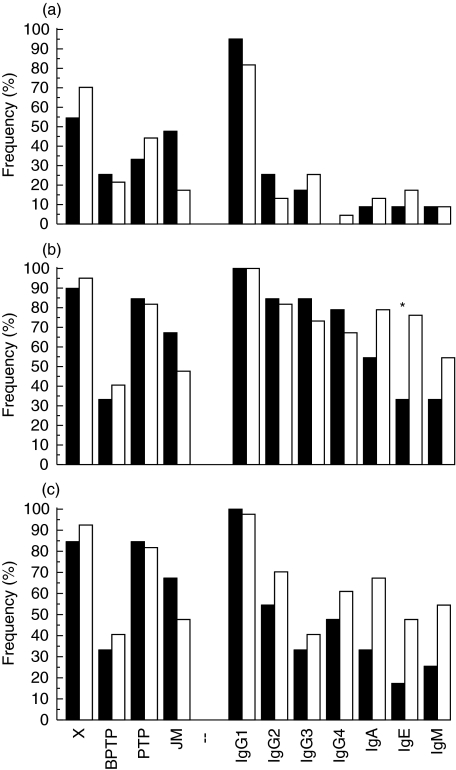
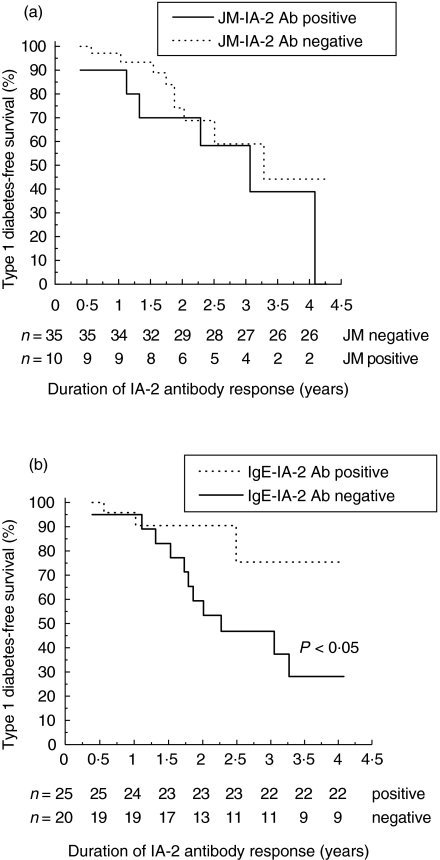
The progressors seroconverted to positivity for IgG1-IA-2 antibodies at the same time as total IA-2 antibodies appeared (at a mean age of 1·9 years), but cross-reactive IA-2 βPTP/PTP and IA-2 JM antibodies appeared very soon afterwards, whereas the other epitope and isotype-specific responses emerged later. The IgA, IgE and IgM-IA-2 class antibodies appeared as the last ones, and were detected at low frequencies (Table 1). The nonprogressors seroconverted to positivity for total IA-2 antibodies at a mean age of 2·4 years, and for IgG1 and specific IA-2 PTP antibodies at a mean age of 2·3 years. The next to appear were cross-reactive and IgG3-IA-2 antibodies. IA-2 JM antibodies emerged in a second phase, followed by IgE, IgA, IgG2, IgM and IgG4-IA-2 and finally antibodies specific to IA-2 βPTP (Table 1). The distribution of humoral IA-2 epitope and isotype responses in the initial sample did not differ between the groups, as shown in Fig. 1a. If a single response was seen, it was towards the IA-2 JM region among the progressors, and towards IA-2 βPTP/PTP antibodies among the nonprogressors. The children who progressed to TID tended to have an initial IA-2 JM epitope response more frequently (P = 0·06, versus nonprogressors), and their IA-2 JM epitope response tended to appear earlier (Table 1, P = 0·08, versus nonprogressors). In the life-table analysis, progression to clinical TID occurred at about the same rate among the children with or without IA-2 JM reactive antibodies (Fig. 2a).
Table 1.
Mean age (years; range) at the appearance of IA-2 antibodies and IA-2/IA-2β antibody epitope and isotype-specific responses in 15 progressors and 30 nonprogressors.
| Progressors (n = 15) | Non-progressors (n = 30) | P-value | |
|---|---|---|---|
| IA-2 Ab | 1·90 (1·0–4·7) (n = 15) | 2·37 (1·0–4·8) (n = 30) | 0·17 |
| sIA-2β PTP Ab | 1·60 (1·0–2·0) (n =4) | 3·00 (1·2–6·0) (n = 10) | 0·03 |
| IgG1-IA-2 Ab | 1·90 (1·0–4·5) (n = 15) | 2·34 (1·0–4·8) (n = 30) | 0·20 |
| crIA-2PTP/βPTP Ab | 1·93 (1·0–4·7) (n = 13) | 2·37 (1·0–4·8) (n = 28) | 0·21 |
| IA-2 JM Ab | 1·97 (1·0–4·0) (n =9) | 2·84 (1·0–4·5) (n = 12) | 0·08 |
| sIA-2 PTP Ab | 2·20 (1·3–4·7) (n = 12) | 2·30 (1·0–4·8) (n = 23) | 0·81 |
| IgG2-IA-2 Ab | 2·31 (1·3–5·0) (n = 12) | 3·02 (1·3–5·8) (n = 23) | 0·10 |
| IgG3-IA-2 Ab | 2·41 (1·3–5·7) (n = 11) | 2·58 (1·3–4·8) (n = 20) | 0·72 |
| IgG4-IA-2 Ab | 2·55 (1·2–4·7) (n = 11) | 3·17 (1·3–6·0) (n = 18) | 0·19 |
| IgA-IA-2 Ab | 2·84 (1·8–5·5) (n =7) | 2·97 (1·2–5·4) (n = 22) | 0·81 |
| IgE-IA-2 Ab | 2·94 (1·8–4·7) (n =4) | 2·95 (1·3–4·8) (n = 21) | 0·97 |
| IgM-IA-2 Ab | 2·95 (1·7–5·2) (n =4) | 3·02 (1·6–6·0) (n = 18) | 0·93 |
Fig. 1.
Frequency of various epitope and isotype-specific IA-2 antibodies in (a) the first positive sample, (b) the maximum response seen during follow-up (the total number of epitopes and isotypes detected) and (c) in the last sample taken at diagnosis in the 15 children who progressed to clinical type I diabetes during prospective observation (▪) and at the corresponding age in the 30 children who remained unaffected (□). X = βPTP/PTP cross-reactive antibodies, *P < 0·05 (χ2-statistics).
Fig. 2.
Probability of remaining nondiabetic among 45 IA-2 antibody-positive children. (a) IA-2 JM antibodies in the first IA-2 antibody-positive sample, and (b) IgE-class antibodies positive at least once versus never during the total observation period. The number of individuals remaining at each time point is indicated under the x-axis. Differences between groups were determined by Kaplan-Meier survival analysis (P < 0·05; log rank test).
Spreading of IA-2/IA-2β reactivity
All the progressors experienced seroconversion to positivity for additional epitopes and isotypes, and accordingly they all had detectable IgG1-IA-2 antibodies (Table 2). Cross-reactive IA-2 βPTP/PTP antibodies, IgG2 and IgG3-IA-2 antibodies were seen in 12 children. Most of them had IgG4-IA-2 and IA-2 JM antibody responses, and some tested positive for specific IA-2 βPTP, IgA, IgE and IgM-IA-2 antibodies during the observation period (Fig. 1b).
Table 2.
Sequence of the appearance of the various epitope and isotype-specific IA-2 antibodies: The data are given as months in relation to age at seroconversion to positivity for IA-2 antibodies.
| Appearance of IA-2/IA-2β antibody epitopes and IA-2 antibody isotypes related to age at first IA-2 A (months) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Sex | HLA-DQB1 genotype | Age at first IA-2 A sample (year) | CrβPTP/PTP | sβPTP | sPTP | JM | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | IgM |
| Progressors | ||||||||||||||
| 630 A | F | *02,*0302 | 4·74 | 0 | 0 | −3 | 3 | 12 | 0 | 9 | 0 | 6 | ||
| 2176 A | M | *02,*0302 | 1·32 | 3 | 18 | 3 | 15 | 3 | 15 | 15 | 18 | |||
| 3899 A | M | *02,*0302 | 1·02 | 9 | 9 | 9 | 0 | 3 | 3 | 3 | 9 | |||
| 6935 A | F | *02,*0302 | 1·02 | 0 | 0 | 9 | 0 | 0 | 27 | 27 | 27 | |||
| 7801 A | F | *0302/x | 4·02 | 0 | 0 | |||||||||
| 8069 A | F | *02,*0302 | 1·08 | 6 | 0 | 0 | 6 | 12 | ||||||
| 11619 A | F | *02,*0302 | 1·77 | 3 | 3 | 12 | 0 | 6 | 12 | 9 | ||||
| 12155 A | M | *0302/x | 2·53 | 0 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | |||
| 15709 A | M | *02,*0302 | 1·95 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 6 | 0 | |
| 31875 A | M | *0302/x | 1·47 | 0 | 0 | 0 | ||||||||
| 35160 A | F | *0302/x | 1·41 | 0 | 0 | 0 | 15 | 15 | 15 | |||||
| 35386 A | M | *0302/x | 1·02 | 3 | 9 | 3 | 0 | 0 | 12 | 3 | 9 | 9 | ||
| 36584 A | F | *0302/x | 1·58 | 3 | 3 | 0 | 0 | 9 | ||||||
| 38521 A | F | *0302/x | 1·90 | −3 | −3 | 3 | 0 | 0 | 6 | |||||
| 65067 A | M | *0302/x | 1·49 | 3 | 3 | 12 | 0 | 3 | 3 | 9 | 3 | |||
| Non-progressors | ||||||||||||||
| 272 A | M | *0302/x | 4·52 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 6 | −3 | −3 | |
| 579 A | F | *0302/x | 4·77 | 0 | 15 | 0 | 0 | 12 | 15 | 0 | 15 | |||
| 771 A | F | *02,*0302 | 2·27 | −3 | −3 | 6 | 6 | 18 | 6 | 24 | ||||
| 1427 A | F | *0302/x | 3·98 | 6 | 6 | 6 | ||||||||
| 3688 A | F | *02,*0302 | 1·04 | 0 | 0 | 0 | 6 | 6 | 6 | 6 | 12 | 12 | ||
| 3755 A | M | *0302/x | 1·58 | 3 | 3 | 12 | 0 | 18 | 39 | |||||
| 4233 A | M | *02,*0302 | 2·99 | 0 | 0 | 0 | 0 | 24 | 21 | 12 | 27 | 21 | 21 | |
| 5948 A | F | *02,*0302 | 3·04 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| 6753 A | M | *0302/x | 1·85 | 0 | 0 | 0 | 0 | 3 | ||||||
| 7998 A | M | *02,*0302 | 1·74 | 3 | 3 | 0 | 6 | 6 | 6 | 6 | 9 | |||
| 8955 A | F | *02,*0302 | 2·18 | 3 | 3 | −3 | 9 | 18 | ||||||
| 11417 A | M | *0302/x | 1·27 | 0 | 0 | 0 | 3 | 0 | 3 | 3 | 30 | 3 | ||
| 12047 A | F | *02,*0302 | 2·25 | 0 | 0 | 3 | 0 | 3 | 0 | 6 | 3 | 9 | 3 | |
| 12547 A | F | *0302/x | 2·19 | 6 | 6 | 12 | 0 | 9 | 9 | 12 | ||||
| 13262 A | M | *0302/x | 2·27 | 0 | 0 | −3 | 0 | 3 | ||||||
| 13594 A | M | *02,*0302 | 2·75 | 0 | 0 | 6 | 6 | 6 | 6 | |||||
| 15542 A | F | *0302/x | 1·53 | 0 | 0 | 0 | 9 | |||||||
| 30303 A | F | *0302/x | 2·60 | 3 | 0 | 9 | −3 | 9 | 3 | |||||
| 31537 A | F | *0302/x | 4·62 | 0 | −3 | 0 | 0 | 0 | 0 | |||||
| 31765 A | F | *0302/x | 4·19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 35006 A | F | *0302/x | 2·28 | 0 | 6 | 0 | 0 | 15 | 24 | 27 | 24 | 18 | ||
| 36809 A | M | *0302/x | 1·45 | 0 | 0 | 3 | 0 | 9 | 0 | 12 | 12 | 15 | 12 | |
| 39161 A | M | *02,*0302 | 2·03 | 0 | 0 | 0 | 0 | 6 | 3 | 6 | ||||
| 39233 A | M | *0302/x | 2·98 | 0 | 0 | 0 | −18 | 0 | 3 | 0 | 3 | |||
| 42305 A | F | *0302/x | 2·27 | 0 | 0 | 3 | 0 | 0 | 3 | −9 | 0 | |||
| 43655 A | F | *02,*0302 | 1·56 | 0 | 0 | 0 | 6 | |||||||
| 43704 A | F | *0302/x | 1·00 | 0 | 3 | 3 | 0 | 0 | 3 | 15 | 3 | 3 | 3 | 9 |
| 44550 A | M | *0302/x | 1·16 | 0 | 0 | 0 | 0 | 3 | 0 | |||||
| 62926 A | M | *0302/x | 1·30 | 0 | 0 | 9 | 0 | 9 | 0 | 3 | 9 | 3 | 9 | |
| 67949 A | M | *02,*0302 | 1·51 | 0 | 0 | 3 | 0 | 3 | 3 | 6 | 3 | 6 | ||
Cr or s in front of the epitope-specific antibodies indicates cross-reactive or specific epitope reactivities. Italic font marks samples that fluctuate, and non-bold font represents the occurrence of the response in a single sample. x ≠*02, *0602, *0603.
The appearance of additional IA-2/IA-2β reactivities was also observed during follow-up among the nonprogressors, in whom the increase in the number of positive antibodies was more conspicuous than among the progressors, with an increased frequency of all isotypes except for IgG1. The latter were seen in all of the 30 nonprogressors, and cross-reactive IA-2 βPTP/PTP and specific IA-2 PTP antibodies in 77–90% of the 30 nonprogressors. The frequency of IgE-IA-2 antibodies was higher (70%) than that seen among the progressors (27%; P < 0·05; Fig. 1b), and a significantly higher proportion of the nonprogressors who were positive for IA-2 JM antibodies also had IgE-IA-2 antibodies (83%versus 11%; P = 0·002). The frequencies of IgA and IgM-IA-2 antibodies tended to be increased among the nonprogressors (P = 0·08 for IgA; and P = 0·06 for IgM; Fig. 1b). Life-table analysis revealed a significantly lower risk of progression to clinical TID among those with an IgE-IA-2 response (P < 0·05, Fig. 2b), while no other differences were seen (data not shown). The integrated IgE-specific IA-2 antibody response was significantly higher among the nonprogressors than among the progressors (P < 0·05; data not shown), but the titres were low in both groups. The nonprogressors had a higher maximal number of epitope and isotype responses during the follow-up (median 4 and 5) than the progressors (median 3 and 4), and higher maximal titres of total IA-2 antibodies, but the differences remained nonsignificant. According to the life-table analysis, a multiple epitope or isotype response during the follow-up was not related to an increased risk of progression to clinical TID (data not shown).
Inverse seroconversions occurred less frequently among the nonprogressors than among the progressors, but the isotype samples fluctuated more. The IgG3 and IgE-IA-2 antibody responses disappeared most often, in 10 (33%) and nine (30%) cases, respectively, while the decrease in the frequency of the other isotypes varied from 3 to 13% (Fig. 1c). The only difference between the two groups in the epitope and isotype profiles in the last analysed sample that tended to be significant was related to IgA-IA-2 antibodies, which were detected in 18 nonprogressors and four progressors (P = 0·06; Fig. 1c). IgE- and IgM-IA-2 antibodies also tended to be more frequent among the nonprogressors, 40% and 47% of whom had such responses compared with 13% and 20% among the progressors (P = 0·09 for IgE and P = 0·11 for IgM). The nonprogressors had a higher number of isotype responses (median 4·5 versus 2 isotypes; P < 0·05) in the last sample.
Discussion
Cell-mediated immunity is thought to play a major role in the destructive islet inflammation that leads to selective β-cell damage. An autoimmune process of variable duration precedes clinical TID, and even though the autoantibodies specific to islet antigens such as the related PTP–like autoantigens IA-2 and IA-2β do not play any pathogenic role, they have been confirmed as markers of an ongoing autoimmune process and as being useful for assessing the risk of developing clinical TID [1,15].
Despite increasing knowledge of specific autoantibodies and genetic disease susceptibility, it is not possible to predict accurately whether a given child will develop TID, or when the clinical manifestation will occur. There is a need to identify additional markers facilitating disease prediction on an individual basis among subjects with an increased risk for progression to diabetes, and this study was performed to analyse epitope spreading and isotype and IgG subclass distribution as a sign of the maturation of the humoral immune response to IA-2. As far as we are aware, this is the first time these parameters have been analysed in parallel in the same risk subjects, and the first time that the appearance of such antibodies has been reported in detail based on frequent sequential samples taken from the time of seroconversion in young children with increased HLA-conferred TID susceptibility identified from the general population.
The subjects studied here were grouped into progressors and non-progressors according to whether they presented with TID or not during the observation period. Admittedly this classification does not exclude the possibility that some, or maybe most, of those classified as ‘non-progressors’ may later present with TID, since they all already had a susceptible HLA genotype and multiple TID-associated autoantibodies, including IA-2 antibodies, at a very young age, these being well-known risk factors for TID. IA-2 antibody titres are related to progression to clinical disease, too [1,15], and accordingly individuals at increased risk tend to have higher IA-2 antibody levels, which must be taken into account when comparing the numbers and levels of various epitope and isotype-specific IA-2 responses.
We observed that the response was primarily directed towards the JM epitope and secondarily towards the cross-reactive βPTP/PTP – like domains of the IA-2 molecule among the progressors, whereas the order was the opposite among the nonprogressors. The number of isotypes increased consistently as a function of the duration of the response among the nonprogressors, and accordingly it was significantly broader among the nonprogressors than among the progressors at the time of diagnosis of clinical TID. Especially the frequencies of those isotypes implicated as reflecting Th 2-like immunity, i.e. IgA and IgE antibodies, were higher. A considerable loss in the number of isotype responses was seen during the follow-up, particularly among the progressors, and one might speculate that the inverse seroconversions and decreasing isotype titres are related to progression to overt T1D, since it is well established that a strong Th1 response suppresses antibody production [9].
There was some heterogeneity in the isotype-specific IA-2 antibody response, although the IgG1-IA-2 antibodies dominated over the other isotypes in terms of both frequency and levels in every sample collected. The IgG1-IA-2 antibodies were the first to appear even before the first epitope-specific responses, and in a few cases before the detection of total IA-2 antibodies, and the IgG1-IA-2 response was also the most stable. In contrast to the BABYDIAB study [22], in which the IgG1-IAA-dominated peak response occurred concurrently with presentation of clinical diabetes, we could not find any correlation between any specific IgG subclass and progression to TID. However, the IgG3-IA-2 antibodies, defined rather as Th1-associated antibodies, appeared earlier than autoantibodies to IgG2-IA-2 among the nonprogressors, which suggests that Th1–type immunity is also prevalent in the nonprogressors at the time of IA-2 antibody seroconversion. Regulatory and protective responses may appear later, however, since IgE, IgA, and IgM IA-2 antibodies emerged in subsequent samples among the nonprogressors.
IgG4-IA-2 subclass antibodies were detected only in a small proportion of the first IA-2 antibody-positive samples and appeared usually as the last antibodies during the follow-up, but their frequency increased at similar rate in both groups. Neither the frequency nor the integrated levels of IgG4-IA-2 antibodies differed between the progressors and nonprogressors, as reported recently by Seissler et al. [23] in 32 IA-2 antibody-positive siblings of children with TID, where the presence of IgG4-IA-2 antibodies was interpreted as being associated with protection from TID, since IgG4-IA-2 antibodies resulted in a reduced progression rate to clinical diabetes in a life-table analysis. According to life-table analyses, the IgG4-IA-2 response showed no association with a reduced risk of TID in the present series, although the IgG4-IA-2-negative children tended to present with overt diabetes somewhat earlier than the IgG4-IA-2-positive ones. Our recent findings in IA-2 antibody-positive siblings of children with TID recruited from the DiMe study [16], with a cohort quite similar to that of Seissler et al. [23] were more in line with the German results, since the nonprogressing siblings with a mean age of 10 years tended to have a higher frequency of IgG4-IA-2 antibodies. It is possible that the frequency of IgG4-IA-2 antibodies increases among older children who remain diabetes-free for a longer time. The IgG4-subclass of GAD65 antibodies turned out to be associated with protection from clinical diabetes even at young age among GAD65Ab-positive DIPP children (Ronkainen et al. unpublished observations), whereas IgG4-IAA were unable to discriminate progressors from nonprogressors in either the DIPP study [24] or the BABYDIAB study [22].
The most interesting finding made here was based on the co-occurrence of IA-2 JM and IgE-IA-2 antibodies. In accordance with previous reports, the presence of IA-2 JM antibodies was associated with an increased risk of progression to clinical TID, since such antibodies tended to appear more frequently and earlier among the progressors than among the nonprogressors, and were present at higher levels than most other epitope-specific antibodies during the whole follow-up period until the diagnosis of TID in the progressors. Likewise, when comparing the integrated levels (AUC), dominance of IA-2 JM-specific antibodies was significantly more common among the progressors than among the nonprogressors. The nonprogressors who were positive for IA-2 JM antibodies usually also had IgE-class IA-2 antibodies, which according to our data might be linked to relative protection from overt disease, since these were present more than twice as often among the nonprogressors than among the progressors during the follow-up, and integrated IgE antibody levels were significantly higher among the nonprogressors than among the progressors. Our observation among the progressors that the children positive for IgE-class IA-2 antibodies were somewhat older than those who were negative for IgE antibodies at the time of diagnosis of clinical disease (4·2 years versus 3·5 years) supports the hypothesis that an IgE antibody response to IA-2 may be a sign of protective or regulatory antigen-specific autoimmunity in young children. The protective sign of IgE-subclass seems to be specific for the IA-2 antigen, since we could not detect such a response among GAD65Ab-positive siblings [25], nor among the IAA-positive young children in the DIPP study [24].
The present study has a limited statistical power, since there were only 15 progressors available for the analysis. Therefore we combined the present series with the sibling cohort from the DiMe study published earlier [16]. When analysed together the progressors tested initially significantly more often positive for JM antibodies (47%versus 17%; P < 0·004) and the higher frequency of IgE class antibodies among the nonprogressors during the follow-up turned highly significant (64%versus 19% in the progressors; P < 0·001). Accordingly to two studies provide conspicuously consistent results.
The present data show that genetically susceptible young children who progress to clinical TID are characterized by a broad epitope response and strong JM reactivity to the IA-2 antigen, reflecting an aggressive immune response, which rapidly leads to progression to clinical disease. The emergence of an IgE antibody response to IA-2 is associated with relative protection against overt TID, even in the case of positivity for IA-2 JM antibodies. Accordingly, IA-2 antibodies of the IgE class may reflect a regulatory or protective autoimmune response in young children carrying HLA-conferred susceptibility to TID.
Acknowledgments
We are indebted to Tuula Simell, Maija Törmä, Birgitta Nurmi, Kaija-Leena Rasimus, Sari Airismeri, Elina Mäntymäki, Minna Romo, Hanna Kukkula, Minna Linnavalli, Katja Orpana, Riikka Sihvo, Hilkka Pohjola, Aino Stenius, Kaisu Riikonen, Eija Koivistoinen, Susanna Lunkka, Merja Koskinen, Ulla Markkanen, Seija Väyrynen, Paula Asunta, Mia Nyblom, and Kaisa Salminen for their commitment to the study. We are also grateful to Leni Joutsjoki, Jukka Rinkinen, Elina Ronkainen, Riitta Päkkilä, Susanna Heikkilä, Päivi Salmijärvi, Tuovi Mehtälä, Sirpa Anttila and Sirpa Pohjola for their skilful technical assistance.
This research was supported by Type 1 Diabetes Targeted Program cofunded by the Research Council for Health, Academy of Finland, the Juvenile Diabetes Foundation International and the Sigrid Jusélius Foundation, the Medical Research Fund, Tampere University Hospital, the National Graduate School of Clinical Investigation, the Foundation for Diabetes Research in Finland, and Finska Läkaresällskapet. The DIPP study has been supported by the Juvenile Diabetes Research Foundation International (grants 197032 and 4-1998-274), the Medical Research Funds, Oulu, Tampere and Turku University Hospitals, the Research Council for Health, Academy of Finland, the Diabetes Research Foundation in Finland, the Päivikki and Sakari Sohlberg Foundation, Helsinki, Finland, the Novo Nordisk Foundation and an EU Biomed 2 grant (BMH4-CT98-3314).
References
- 1.Knip M. Disease-associated autoimmunity and prevention of insulin-dependent diabetes mellitus. Ann Med. 1997;29:447–51. doi: 10.3109/07853899708999375. [DOI] [PubMed] [Google Scholar]
- 2.Lampasona V, Bearzatto M, Genovese S, Bosi E, Ferrari M, Bonifacio E. Autoantibodies in insulin-dependent diabetes recognize distinct cytoplasmic domains of the protein tyrosine phosphatase-like IA-2 autoantigen. J Immunol. 1996;157:2707–11. [PubMed] [Google Scholar]
- 3.Notkins AL, Zhang B, Matsumoto Y, Lan MS. Comparison of IA-2 with IA-2β and with six other members of the protein tyrosine phosphatase family: recognition of antigenic determinants by IDDM sera. J Autoimmun. 1997;10:245–50. doi: 10.1006/jaut.1997.0132. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacio E, Lampasona V, Bingley PJ. IA-2 (islet cell antigen 512) is the primary target of humoral autoimmunity against type 1-associated tyrosine phosphatase autoantigens. J Immunol. 1998;161:2648–54. [PubMed] [Google Scholar]
- 5.Hatfield EC, Hawkes CJ, Payton MA, Christie MR. Cross reactivity between IA-2 and phogrin/IA-2β in binding of autoantibodies in IDDM. Diabetologia. 1997;40:1327–33. doi: 10.1007/s001250050828. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki E, Yu L, Rewers MJ, Hutton JC, Eisenbarth GS. Definition of multiple ICA512/phogrin autoantibody epitopes and detection of intramolecular epitope spreading in relatives of patients with type 1 diabetes. Diabetes. 1998;47:733–42. doi: 10.2337/diabetes.47.5.733. [DOI] [PubMed] [Google Scholar]
- 7.Naserke HE, Ziegler AG, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA−2 and their association with progression to type 1 diabetes. J Immunol. 1998;161:6963–9. [PubMed] [Google Scholar]
- 8.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 9.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 10.Kolb H. Benign versus destructive insulitis. Diab Metab Rev. 1996;3:139–46. doi: 10.1002/(sici)1099-0895(199709)13:3<139::aid-dmr190>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Pilström B, Björk L, Böhme J. Demonstration of Th1 cytokine profile in the late phase of NOD insulitis. Cytokine. 1995;8:806–14. doi: 10.1006/cyto.1995.0097. [DOI] [PubMed] [Google Scholar]
- 12.Kallmann BA, Huther M, Tubes M, Feldkamp J, Bertrams J, Gries FA, Lampeter EF, Kolb H. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves’ disease. Diabetes. 1997;46:237–43. doi: 10.2337/diab.46.2.237. [DOI] [PubMed] [Google Scholar]
- 13.Kallmann BA, Lampeter EF, Hanifi-Moghaddam P, Hawa M, Leslie RDS, Kolb H. Cytokine secretion patterns in twins discordant for type 1 diabetes. Diabetologia. 1999;42:1080–5. doi: 10.1007/s001250051274. [DOI] [PubMed] [Google Scholar]
- 14.Kupila A, Muona P, Simell T, et al. Feasibility of genetic and immunological prediction of Type1 diabetes in a population-based birth cohort. Diabetologia. 2001;44:290–7. doi: 10.1007/s001250051616. [DOI] [PubMed] [Google Scholar]
- 15.Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies – a sensitive marker of type 1 diabetes with clinical onset in childhood and adolescence. Diabetologia. 1998;41:424–9. doi: 10.1007/s001250050925. [DOI] [PubMed] [Google Scholar]
- 16.Hoppu S, Härkönen T, Ronkainen MS, Åkerblom HK, Knip M the Childhood Diabetes in Finland Study Group. IA-2 antibody epitopes and isotypes during the prediabetic process in siblings of children with type 1 diabetes. J Autoimmun. 2004;23:361–70. doi: 10.1016/j.jaut.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;304:1279–8l. doi: 10.4049/jimmunol.1701303. [DOI] [PubMed] [Google Scholar]
- 18.Lernmark A, Molenaar JL, van Beers WA, Yamaguchi Y, Nagataki S, Ludvigsson J, Maclaren NK. The fourth international serum exchange workshop to standard cytoplasmic islet cell antibodies. The immunology and diabetes workshops and participating laboratories. Diabetologia. 1991;34:534–5. doi: 10.1007/BF00403293. [DOI] [PubMed] [Google Scholar]
- 19.Ronkainen M, Hämäläinen AM, Koskela P, Åkerblom HK, Knip M. the Finnish TRIGR Study Group. Pregnancy induces non-immunoglobulin insulin-binding activity in both maternal and cord blood serum. Clin Exp Immunol. 2001;124:190–6. doi: 10.1046/j.1365-2249.2001.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M. Autoantibodies associated with type 1 diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–7. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 21.Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifacio E, Scirpoli M, Kredel K, Füchtenbusch M, Ziegler AG. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J Immunol. 1999;163:525–32. [PubMed] [Google Scholar]
- 23.Seissler J, Eikamp K, Schott M, Scherbaum WA the DENIS Study Group. IA-2 autoantibodies restricted to the IgG4 subclass are associated with protection from type 1 diabetes. Horm Metab Res. 2002;34:186–91. doi: 10.1055/s-2002-26708. [DOI] [PubMed] [Google Scholar]
- 24.Hoppu S, Ronkainen MS, Kimpimäki T, Simell S, Korhonen S, Ilonen J, Simell O, Knip M. Insulin autoantibody isotypes during the prediabetic process in young children with increased genetic risk of type 1 diabetes. Pediatr Res. 2004;55:236–42. doi: 10.1203/01.PDR.0000100905.41131.3F. [DOI] [PubMed] [Google Scholar]
- 25.Hoppu S, Ronkainen MS, Kulmala P, Åkerblom HK, Knip M the Childhood Diabetes in Finland Study Group. GAD65 antibody isotypes and epitope recognition during the prediabetic process in siblings of children with type I diabetes. Clin Exp Immunol. 2004;136:120–8. doi: 10.1111/j.1365-2249.2004.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]