Abstract
The balance between inflammatory mediators and their counter-regulatory molecules may be crucial for determining the outcome of immune pathology of periodontal diseases. Based on clinical and immunological findings, the immune response in stable gingivitis lesion is supposed to be in balance, whereas the response is skewed towards the predominance of proinflammatory reactivity in progressive periodontitis lesion. However, this hypothesis has not been verified. Therefore, the aim of this study was to compare the gene expression profile of inflammatory mediators including proinflammatory cytokines and other inflammatory molecules, and anti-inflammatory cytokines by using quantitative real-time polymerase chain reaction in gingivitis and periodontitis lesions showing distinct clinical entities. For inflammatory mediators, interleukin (IL)-1β, interferon (IFN)-γ and receptor activator of nuclear factor (NF)-κB ligand tended to be higher in periodontitis, whereas tumour necrosis factor (TNF)-α and IL-12 p40 showed no difference. Heat-shock protein 60 (HSP60) expression was up-regulated significantly in periodontitis. For anti-inflammatory cytokines, transforming growth factor (TGF)-β1 expression tended to be higher in periodontitis compared with gingivitis, whereas no difference was observed for IL-10 and IL-4. These findings support further our previous finding that autoimmune response to HSP60 may exert in periodontitis lesion, and suggest that perhaps subtle differences in the balance of cytokines may result in different disease expression.
Keywords: cytokines, gingiva, HSP60, RANKL, real-time PCR
Introduction
Chronic inflammatory periodontal disease manifests clinically as at least two distinct entities. Evidence based on microbiological, immunological and animal model studies has shown that some forms of periodontal disease in adults can remain stable throughout many years and not endanger the life of the dentition (gingivitis), whereas other forms, despite extensive treatment, continue to break down, leading ultimately to tooth loss (periodontitis) [1]. Although periodontal bacteria are the causative agents in periodontitis, subsequent progression and disease severity are thought to be determined by the host immune response [2].
Periodontopathic bacteria stimulate cells comprising periodontal tissue to express various inflammatory mediators such as interleukin (IL)-1, tumour necrosis factor (TNF)-α or receptor activator of nuclear factor κB ligand (RANKL) [3]. In addition to the bacterial products, the expression of endogenous heat-shock protein 60 (HSP60) is up-regulated in periodontitis lesions. Our previous studies demonstrated clearly that both cellular and humoral immune responses to HSP60 are elevated in periodontitis patients [4,5]. Also, interferon (IFN)-γ production by reactive T cells and TNF-α production by macrophages were enhanced by HSP60 stimulation [6]. Subsequently, these mediators may activate the production of matrix metalloproteinases and prostaglandin E2 and differentiation of osteoclasts, resulting in connective tissue destruction and alveolar bone resorption [7]. IL-1β and TNF-α production in macrophages will be up-regulated further by IFN-γ derived from activated type 1 helper T cells (Th1) and IFN-γ production can be modulated by IL-12 from antigen-presenting cells [8].
On the other hand, tissue destruction could be protected by suppressing the activity of proinflammatory cytokines by anti-inflammatory cytokines such as IL-4, IL-10 and transforming growth factor (TGF)-β1. IL-4 and IL-10 can be produced by Th2 effector T cells and TGF-β1 is produced by Th3 [9].
Thus, it is hypothesized that the balance between inflammatory mediators and their counter-regulatory molecules may be balanced in gingivitis lesions, whereas proinflammatory reactivity may be predominant in periodontitis lesions. However, few reports have examined the balance between pro- and anti-inflammatory activities in the lesions by measuring mRNA expression using a highly quantitative method.
In the present study, therefore, mRNA expression of the molecules which appear to be involved in the pathogenesis of periodontal diseases was analysed comprehensively by quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR).
Materials and methods
Patients and biopsies
Twenty-five patients with moderate to advanced chronic periodontitis, referred to the Periodontal Clinic of Niigata University Medical and Dental Hospital, took part in this study. Gingival biopsies were obtained at the time of periodontal surgery or extraction of severely involved teeth. As controls, 23 gingivitis tissues showing no supporting tissue destruction were also obtained from sites requiring extraction for reasons other than periodontitis, such as orthodontic treatment or pericoronitis. The modified diagnostic criteria of Kornman et al. [10] for periodontitis and gingivitis were used; periodontitis: subjects having at least one interproximal site with ≥ 50% bone loss and total mean bone loss of ≥ 16%; gingivitis: subjects having no probing pocket depth, no sites with bone loss > 10% and clinical signs of inflammation. Healthy control specimens were not included in this study because a number of studies demonstrated that histological inflammation was present not only prior to the accumulation of plaque resulting overt clinical signs of inflammation, but was often of quite significant extent [11,12]. The clinical status of the biopsy sites is shown in Table 1. The experimental protocol was approved by the Institutional Review Board of Niigata University and informed consent was obtained from all patients prior to inclusion in this study.
Table 1.
Clinical profile of gingival biopsy sites.
| Periodontitis | Gingivitis | ||
|---|---|---|---|
| (n = 25) | (n = 23) | P-value | |
| Age | 53·6 ± 9·5 | 28·4 ± 5·2 | < 0·0001 |
| Male/female | 14/11 | 12/11 | |
| Smokers/non-smokers | 5/20 | 4/19 | |
| Gingival index | 1·08 ± 0·76 | 0·30 ± 0·47 | 0·0001 |
| Probing depth (mm) | 7·56 ± 2·48 | 2·26 ± 0·54 | < 0·0001 |
| Loss of attachment (mm) | 8·56 ± 2·74 | 2·43 ± 0·51 | < 0·0001 |
| Tooth mobility | 1·24 ± 1·05 | 0·00 ± 0·00 | < 0·0001 |
| Bleeding on probing (%site) | 72·0 | 17·0 | |
| Bone loss (%) | 69·80 ± 24·64 | 1·09 ± 2·59 | < 0·0001 |
Data are expressed as mean ± s.d. The data were compared by unpaired t-test.
RNA isolation and real-time quantitative PCR
Total RNA was isolated from gingival tissues using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and treated with RNase-free DNase I (Invitrogen). The RNA was then reverse-transcribed to cDNA using random primers (Takara Shuzo Co. Ltd, Shiga, Japan) and M-MLV reverse transcriptase (Invitrogen). For real-time PCR, primers and probes were all purchased from Applied Biosystems (Foster City, CA, USA). Reactions were conducted in a 25 µl reaction mixture on the ABI Prism 7900HT sequence detection system (Applied Biosystems) using TaqMan gene expression assays (Applied Biosystems) and incubated for 10 min at 95°C, followed by 40 cycles of a two-step amplification procedure composed of annealing/extension at 60°C for 1 min and denaturation for 15 s at 95°C. The ABI Prism sds version 2·0 software (Applied Biosystems) was used to analyse the standards and to carry out the quantifications. The relative quantity of each mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA.
Statistical analysis
The difference of clinical parameters between the two groups was compared by unpaired t-test. The expression levels of cytokine, RANKL and HSP60 between gingivitis and periodontitis were compared by means of the Mann–Whitney U-test using StatView® for Windows software (version 5, SAS Institute Inc., Cary, NC, USA). Bonferroni's correction was applied to adjust the alpha level for the Mann–Whitney U-test, as multiple comparisons were made (with tests run for nine molecules, the alpha level was lowered to 0·005).
Results
Gene expression of proinflammatory cytokines
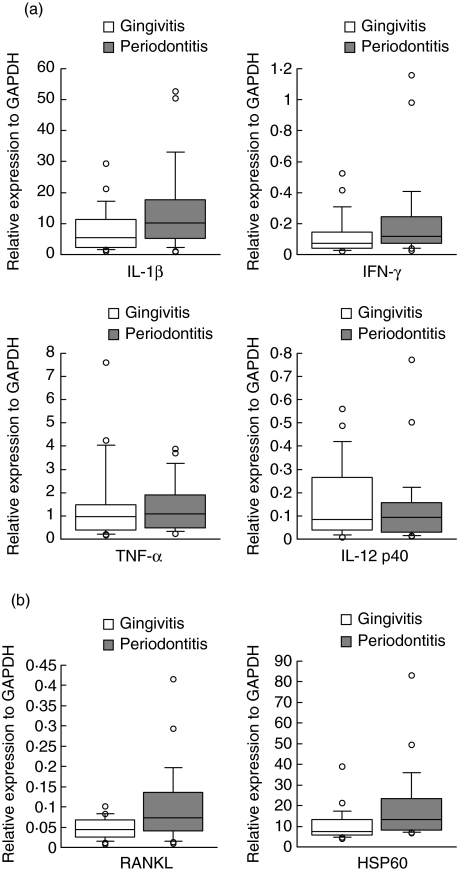
The mRNA expression levels of cytokines, RANKL and HSP60 are shown on the basis of disease status in Table 2. The mean ranks for IL-1β and IFN-γ tended to be higher in the periodontitis group compared with the gingivitis group (Mann–Whitney U-test, P = 0·046 and P = 0·028 for IL-1β and IFN-γ, respectively). The median values for the relative amounts of IL-1β and IFN-γ expression were 10·224 and 0·116 in the periodontitis group and 5·615 and 0·072 in the gingivitis group, respectively. No significant differences were seen in the mRNA levels of TNF-α (P = 0·516) and IL-12 p40 (P = 0·734), although median values of these cytokines were slightly higher in the periodontitis group compared with the gingivitis group (Fig. 1a).
Table 2.
Mean ranks of mRNA expressions in periodontitis group and gingivitis group.
| Periodontitis | Gingivitis | P-value | |
|---|---|---|---|
| IL-1β | 28·36 | 20·30 | 0·046 |
| IFN-γ | 28·76 | 19·87 | 0·028 |
| TNF-α | 25·76 | 23·13 | 0·516 |
| IL-12 p40 | 23·84 | 25·22 | 0·734 |
| RANKL | 29·12 | 19·48 | 0·017 |
| HSP60 | 30·36 | 18·13 | 0·003 |
| TGF-β1 | 29·56 | 19·00 | 0·009 |
| IL-10 | 27·64 | 21·09 | 0·105 |
| IL-4 | 24·54 | 24·46 | 0·984 |
The mean ranks were compared using the Mann–Whitney U-test.
Fig. 1.
Comparison of the relative gene expressions of proinflammatory cytokines (a) and receptor activator of nuclear factor κB ligand (RANKL) and heat-shock protein 60 (HSP60) (b) between periodontitis (n = 25) and gingivitis lesions (n = 23). The relative quantity of mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). The box plots show medians, 25th and 75th percentiles as boxes, 10th and 90th percentiles as whiskers. Outside values are shown as open circles.
Gene expression of RANKL and HSP60
The expression of RANKL, another important molecule involved in the periodontal tissue destruction, was not as high compared with other proinflammatory cytokines, but was substantial. The median value for the relative amounts of RANKL expression was 0·072 in the periodontitis group and 0·044 in the gingivitis group, respectively. The mean ranks for RANKL were higher in the periodontitis group compared with the gingivitis group, although they did not reach statistical significance (Mann–Whitney U-test, P = 0·017). Relative expression of HSP60 was even higher than that of IL-1β and the median values of HSP60 mRNA expressions in the periodontitis and gingivitis groups were 13·257 and 7·459, respectively. The expression of HSP60 in periodontitis was significantly higher compared with gingivitis (Mann–Whitney U-test, P = 0·003; Fig. 1b).
Gene expression of anti-inflammatory cytokines
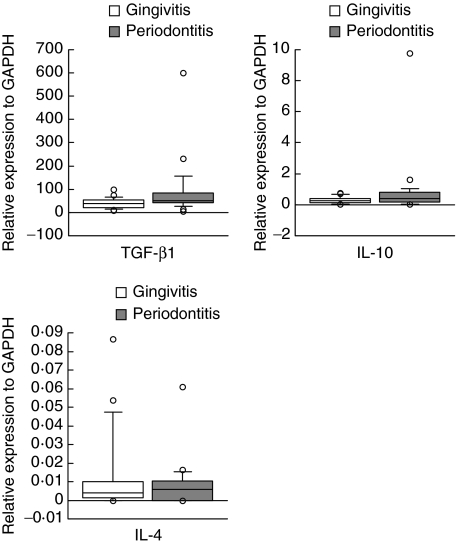
For immunosuppressive cytokines, the mean ranks of the expression levels of TGF-β1 and IL-10 was higher in periodontitis compared with gingivitis. The median values for the relative amounts of TGF-β1 and IL-10 expression were 51·848 and 0·422 in the periodontitis group and 38·556 and 0·282 in the gingivitis group, respectively. However, the difference in expression levels of these cytokines was not statistically significant between periodontitis and gingivitis despite the expression of TGF-β1 tending to be higher in periodontitis (Mann–Whitney U-test, P = 0·009). The mean ranks of IL-4 mRNA expression showed no difference between the periodontitis group and the gingivitis group. Although the median value of IL-4 expression in periodontitis was slightly higher compared with gingivitis, overall expression levels were much lower than the other cytokines (0·006 for periodontitis and 0·004 for gingivitis) (Fig. 2). The number of samples demonstrated that no transcript for IL-4 was higher than that of other cytokines in periodontitis (seven of 25) compared with gingivitis (four of 23).
Fig. 2.
Comparison of the relative gene expressions of anti-inflammatory cytokines between periodontitis (n = 25) and gingivitis lesions (n = 23). The relative quantity of mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). The box plots show medians, 25th and 75th percentiles as boxes, 10th and 90th percentiles as whiskers. Outside values are shown as open circles.
Discussion
The role of proinflammatory cytokines in the periodontal tissue destruction is well documented in the animal model [13]. However, cellular responses to the proinflammatory cytokines are regulated negatively by anti-inflammatory cytokines and the balance between these two categories of cytokine could be important in the outcome of inflammatory response. Our initial hypothesis was that the activity of proinflammatory cytokines may be suppressed effectively by anti-inflammatory cytokines in stable gingivitis lesions, whereas this may not be the case in progressive periodontitis. However, overall differences of the expressions for pro- and anti-inflammatory cytokines between gingivitis and periodontitis were unexpectedly small, except for RANKL and HSP60.
One of the factors which may affect interpretation of the results is the age of the study population. The systemic immune response is affected by age and the outcome of infection in old age may be more severe than in younger subjects, and this may be related to dysregulation of the immune system in the elderly. Whereas gingivitis is found preferentially in younger populations, the prevalence of periodontitis is high in elderly populations. In the present study, the mean ages of gingivitis and periodontitis patients were 28·4 and 53·6, respectively; this difference is likely to affect the interpretation of the results. However, studies examining alterations in proinflammatory cytokine production with age are contradictory [14–16]. Further, the differences were found in different cytokines with respect to each report. Therefore, although it is possible that gene expression of some cytokines might be influenced by the age difference, it may not be definitive. In support of this, experimental gingivitis study has shown only minor differences between young and old individuals in the inflammatory cell composition [17]. Taken together, the effect of age difference between the two groups in this study on interpretation of the results could be minimal.
Although the levels of IL-1β tended to be higher in periodontitis compared with gingivitis, the expression level in gingivitis was also high compared with other cytokines. On the other hand, the level of TNF-α expression was low compared with that of IL-1β and showed no difference between periodontitis and gingivitis. These findings obtained by using highly quantitative real-time RT-PCR are not consistent with previous reports. Roberts et al. [18] found that while the level of TNF-α mRNA was significantly higher in diseased tissues than in healthy tissues, there was no difference for IL-1β mRNA, although the median level in the diseased tissue was higher than the healthy tissues. Bickel et al. [19] have also shown that the expression level of IL-1β mRNA was high in the healthy sites as well as the progressive lesions of periodontitis patients. Although the expression pattern of proinflammatory cytokines is variable in different reports, they are expressed in either clinically healthy or diseased gingival tissues. These findings, together with our results, indicate clearly that IL-1β and other proinflammatory cytokines play roles not only in periodontitis lesions but also in gingivitis lesions, but the roles may be different. It is assumed that IL-1β and TNF-α may up-regulate normal tissue turnover, but not induce tissue destruction in gingivitis lesions. In fact, IL-1β is reported to stimulate collagen synthesis in cultured fibroblasts and matrix metalloproteinases at the same time [20]. However, exact roles of proinflammatory cytokines in healthy sites and gingivitis lesions remain to be elucidated.
The role of IFN-γ in periodontal tissue destruction has been controversial. This cytokine is produced by Th1 cells and induces IL-1β, TNF-α and prostaglandin E2 (PGE2) production by macrophage, indicating that it is primarily proinflammatory in nature. In contrast, it has been shown that IFN-γ inhibits osteoclastogenesis by interfering with the RANKL–RANK signalling pathway [21]. Consequently, IFN-γ is concluded to have a bilateral character, both destructive and protective. Eversole and Taubman [22] found that the IFN-γ message was expressed prominently by diseased gingival tissue cells. Further, significantly higher proportions of gingival mononuclear cells from periodontitis patients expressed IFN-γ mRNA than did those from healthy subjects. In the present study, however, the expression level of IFN-γ mRNA was much lower than that of IL-1β and TNF-α and even lower than that of IL-12 p40. This is consistent with the results by Roberts et al. [23] and Bickel et al. [19], and confirmed our previous result [24]. The role of Th1 cells on bone resorption has to be considered in conjunction with the expression of RANKL, as discussed below. Teng et al. [25] demonstrated clearly a positive co-expression relationship and interactions between IFN-γ and RANKL-mediated osteoclastogenesis in a mouse periodontitis model. Kotake et al. [26] found recently that IFN-γ+ human T cells induced osteoclast formation via the expression of RANKL. They speculated that individual effects of RANKL exceeded the inhibitory effects of IFN-γ. These findings suggest that the balance between IFN-γ and RANKL expressions may be critical in the activity of Th1 cells for osteoclastogenesis. Further, the number, but not the proportion of T cells in the infiltrate seems to be higher in periodontitis lesions compared with gingivitis lesions. The difference in the expression of T cell cytokines could be reflected by the size of inflammatory infiltrates.
RANKL expression also tended to be higher in periodontitis compared with gingivitis. In addition to osteoblast and stromal cells [27,28], both soluble and membrane-bound RANKLs could also be produced by activated CD4+ and CD8+ T cells [29]. We have shown recently that T cell clones established from gingival tissues expressed RANKL mRNA [30]. Therefore, the difference in the RANKL expression could be due to the difference in the activation stages or subsets of infiltrating T cells in the lesions.
HSP60, a potent proinflammatory protein, was expressed significantly higher in periodontitis compared with gingivitis. Previous reports, including ours, have demonstrated that human HSP60 has been shown to have a strong capability of stimulating proinflammatory reactivity in human cells of the innate immune system, such as macrophages [6,31]. In addition, it has been shown that the presence of a functionally active TLR4 molecule which is also expressed in periodontitis lesions [32] is essential for the inflammatory signalling of HSP60 [33]. Therefore, the role of HSP60 in the inflammatory process of periodontitis lesions may be more important than thought previously.
The expression of TGF-β1, which can be produced by a variety of cells including T cells and macrophages, was the most prominent in the present study. TGF-β1 has been shown to have both pro- and anti-inflammatory effects and plays an important regulating role in the inflammatory process [34]. However, how these contradictory effects are related to two distinct entities, namely, gingivitis and periodontitis, has yet to be determined. Considering that gingivitis and periodontitis lesions are stable and progressive, respectively, the primary role of TGF-β1, at least in gingivitis lesions, might be anti-inflammatory. On the other hand, concomitant elevation of various types of regulatory T cells and TGF-β1 was found in periodontitis lesions [35]. Therefore, in periodontitis lesions, on one hand inflammation progresses and on the other hand is suppressed. This may contribute to the occurrence of phases of active destruction and remission [36].
The expression of IL-10 tended to be higher in periodontitis compared with gingivitis. Conversely, expression of IL-4 was low and did not differ between periodontitis and gingivitis. IL-10 is a more potent suppressor of proinflammatory cytokine than IL-4 in vitro[37,38]. Whereas both IL-4 and IL-10 reduce the steady-state level of IL-1 mRNA, their mechanisms of suppression are reported to be different. While IL-10 inhibits IL-1 at the transcriptional level [39], IL-4 suppresses gene expression by decreasing mRNA stability [40]. Because we analysed proinflammatory cytokines at the transcriptional, but not at the translational, levels the effect of these anti-inflammatory cytokines is not obvious in periodontitis and gingivitis, even though the levels of proinflammatory cytokines and anti-inflammatory cytokines in the same sample tended to show reciprocal expression (data not shown). Collectively, although the difference in expression of each of the proinflammatory cytokines between two disease entities is small, it may be more substantial than that of anti-inflammatory cytokines, resulting in periodontitis lesions being prone to tissue destruction. Elevated expression of HSP60, and to a lesser extent RANKL, could be considered to promote tissue destruction further.
In conclusion, transcripts of anti-inflammatory as well as proinflammatory cytokines were elevated in gingivitis and periodontitis lesions. Perhaps subtle differences in the balance of cytokines may result in different disease expression. Alternatively, the importance of each cytokine or molecule in the pathogenesis may well be variable from patient to patient, and from time to time within the same patient.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Promotion of Niigata University Research Project.
References
- 1.Seymour GJ. Possible mechanisms involved in the immunoregulation of chronic inflammatory periodontal disease. J Dent Res. 1987;66:2–9. doi: 10.1177/00220345870660010401. [DOI] [PubMed] [Google Scholar]
- 2.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodont Res. 1993;28:478–86. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 3.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontology 2000. 1997;14:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–93. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki K, Ohsawa Y, Tabeta K, et al. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumor necrosis factor-α in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002;127:72–7. doi: 10.1046/j.1365-2249.2002.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz Z, Goultschin J, Dean DD, Boyan BD. Mechanisms of alveolar bone destruction in periodontitis. Periodontol 2000. 1997;14:158–72. doi: 10.1111/j.1600-0757.1997.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 8.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuirk P, Mills KHG. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23:450–5. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- 10.Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–7. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 11.Seymour GJ, Powell RN, Aitken JF. Experimental gingivitis in humans. A clinical and histologic investigation. J Periodontol. 1983;54:522–8. doi: 10.1902/jop.1983.54.9.522. [DOI] [PubMed] [Google Scholar]
- 12.Gemmell E, McHugh GB, Grieco DA, Seymour GJ. Costimulatory molecules in human periodontal disease tissues. J Periodont Res. 2001;36:92–100. doi: 10.1034/j.1600-0765.2001.360205.x. [DOI] [PubMed] [Google Scholar]
- 13.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–9. [PubMed] [Google Scholar]
- 14.Fagiolo U, Cossarizza A, Scala E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–8. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 15.Rich EA, Mincek MA, Armitage KB, et al. Accessory function and properties of monocytes from healthy elderly humans for T lymphocyte responses to mitogen and antigen. Gerontology. 1993;39:93–108. doi: 10.1159/000213519. [DOI] [PubMed] [Google Scholar]
- 16.O'Mahony L, Holland J, Jackson J, Feighery C, Hennessy TP, Mealy K. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol. 1998;113:213–9. doi: 10.1046/j.1365-2249.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransson C, Mooney J, Kinane DF, Berglundh T. Differences in the inflammatory response in young and old human subjects during the course of experimental gingivitis. J Clin Periodontol. 1999;26:453–60. doi: 10.1034/j.1600-051x.1999.260707.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts FA, Hockett RD, Jr, Bucy RP, Michalek SM. Quantitative assessment of inflammatory cytokine gene expression in chronic adult periodontitis. Oral Microbiol Immunol. 1997;12:336–44. doi: 10.1111/j.1399-302x.1997.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 19.Bickel M, Axtelius B, Solioz C, Attström R. Cytokine gene expression in chronic periodontitis. J Clin Periodontol. 2001;28:840–7. doi: 10.1034/j.1600-051x.2001.028009840.x. [DOI] [PubMed] [Google Scholar]
- 20.Lark MW, Walakovits LA, Shah TK, Vanmiddlesworth J, Cameron PM, Lin TY. Production and purification of prostromelysin and procollagenase from IL-1β-stimulated human gingival fibroblasts. Connect Tissue Res. 1990;25:49–65. doi: 10.3109/03008209009009812. [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi H, Ogasawara K, Hida S, et al. T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 22.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol. 2000 1994;5:112–41. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts FA, McCaffery KA, Michalek SM. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997;76:1833–9. doi: 10.1177/00220345970760120501. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki K, Nakajima T, Kubota Y, Gemmell E, Seymour GJ, Hara K. Cytokine messenger RNA expression in chronic inflammatory periodontal disease. Oral Microbiol Immunol. 1997;12:281–7. doi: 10.1111/j.1399-302x.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 25.Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitans-specific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun. 2005;73:3453–61. doi: 10.1128/IAI.73.6.3453-3461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotake S, Nanke Y, Mogi M, et al. IFN-γ-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35:3353–63. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 27.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 30.Ito H, Honda T, Domon H, et al. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiol Immunol. 2005;20:382–6. doi: 10.1111/j.1399-302X.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 31.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol Immunol. 2003;18:54–8. doi: 10.1034/j.1399-302x.2003.180109.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi K, Burkart V, Flohé S, Kolb H. Heat shock protein 60 is a putative endogeneous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 34.Wahl SM, Costa GL, Mizel DE, Allen JB, Skaleric U, Mangan DF. Role of transforming growth factor beta in the pathophysiology of chronic inflammation. J Periodontol. 1993;64:450–5. [PubMed] [Google Scholar]
- 35.Nakajima T, Ueki-Maruyama K, Oda T, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–43. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- 36.Socransky SS, Haffajee AD, Goodson JM, Lindhe J. New concepts of destructive periodontal disease. J Clin Periodontol. 1984;11:21–32. doi: 10.1111/j.1600-051x.1984.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 38.Sagawa K, Mochizuki M, Sugita S, Nagai K, Sudo T, Itoh K. Suppression by IL-10 and IL-4 of cytokine production induced by two-way autologous mixed lymphocyte reaction. Cytokine. 1996;8:501–6. doi: 10.1006/cyto.1996.0068. [DOI] [PubMed] [Google Scholar]
- 39.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor κB (NFκB) activation in human monocytes. J Biol Chem. 1995;270:9558–63. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 40.Donnelly RP, Fenton MJ, Kaufman JD, Gerrard TL. IL-1 expression in human monocytes is transcriptionally and posttranscriptionally regulated by IL-4. J Immunol. 1991;146:3431–6. [PubMed] [Google Scholar]