Abstract
We previously reported anti-PCNA autoantibodies in sera from patients with chronic HBV and HCV infection. To analyse the antigenic regions on proliferating cell nuclear antigen (PCNA) that confer autoantibody binding in patients with chronic hepatitis B (HBV) and C (HCV) infection, eight constructs including one wild type PCNA, one mutant type Y114A_PCNA and six C- or N-terminal PCNA truncations were generated. Sera from 185 patients with systemic lupus erythematosus (SLE), 178 with chronic HBV and 163 with chronic HCV infection, and 68 healthy individuals were examined for the presentation of anti-PCNA antibodies by enzyme linked immunosorbent assay (ELISA). By ELISA, anti-PCNA positive sera from patients with SLE, chronic HBV and HCV infection preferentially recognized the wild type PCNA more than the mutant type Y114A_PCNA (P< 0·05). The inhibition of binding by purified full-length rPCNA proteins with anti-PCNA positive sera was shown to exceed 70%. The inhibition of binding by purified truncated rPCNA proteins with sera from patients with chronic HBV and HCV infection and SLE was shown to confer dominant binding in TL2 and TL3. Moreover, the higher frequency of inhibition by using TL3 was found in patients with chronic HBV infection. These data indicate that anti-PCNA autoantibodies preferentially recognize C-terminal of PCNA in patients with chronic HBV infection and may also provide advanced understanding between viral infection and autoimmunity for further study.
Keywords: proliferating cell nuclear antigen (PCNA), autoantibody, hepatitis B virus (HBV), hepatitis C virus (HCV), antigenic regions
Introduction
The association of viral infection and autoimmune disease with autoantibodies has been strongly suggested [1–4]. Epstein-Barr virus, hepatitis C virus (HCV) and B virus (HBV), human immunodeficiency virus, and human parvovirus B19 appear to be associated with autoimmunity more commonly than other viruses. Several studies have suggested that chronic HBV or HCV may act as a trigger factor for the development of autoimmune rheumatic diseases [5–7]. Chronic HBV and HCV infection occur worldwide. They can cause acute or chronic hepatitis, cirrhosis of the liver and hepatocellular carcinoma [8]. Taiwan is a hyper-endemic area for chronic HBV and HCV infection. The hepatitis B surface antigen (HBsAg) carrier rate in its general population is about 10% to 20% [9], and anti-HCV seroprevalence is about 1·6% to 19·6% [10].
Autoantibodies to proliferating cell nuclear antigen (PCNA) were detected in the sera of 3–5% of patients with systemic lupus erythematosus (SLE) [11,12]. Anti-PCNA antibody has not been detected in other autoimmune diseases and was thought to be specific for SLE [13,14]. In spite of their low frequency, anti-PCNA antibodies are useful as a serologic marker for SLE. It can be detected by the characteristic speckled immunofluorescence pattern of variable immunolocalization during mitotic stages because the bulk of its expression occurs during late G1 and early S phase of the cell cycle just before DNA synthesis [15]. PCNA is very conserved in various eukaryotic organisms, and was required for both DNA replication and excision repair in eukaryotes [16,17]. Several reports indicated that SLE patients with anti-PCNA showed a high frequency of renal and central nerve system (CNS) involvements, nephropathy, thrombocytopenia, and seizure [18–20].
Epitopes of anti-PCNA autoantibodies have been studied in patients with SLE. Previous study using limited proteolysis indicated that both C- and N-terminal halves are crucial for recognition of autoantibodies [21]. Investigation using C-terminal truncated protein synthesized in vitro and mutant forms of PCNA expressed as fusion protein suggested complex heterogeneity of human autoantibodies and indicated the dependence of epitopes on conformation [22,23]. The sulphhydryl groups in PCNA proteins are involved in the antigenisity as the PCNA treated bythiol-modifying agent thimerosal was unable to be recognized by the autoantibodies [24]. Additionally, autoantibodies to PCNA belong to two classes in patients with SLE: one class comprising 80% of the positive sera whose recognition region depends nearly the full-length of PCNA for binding, and the other portion seems need to be further confined since their specificity was different in the amino terminus [25]. Previously, we have reported that anti-PCNA autoantibodies in sera from patients with chronic HBV and HCV infection, and had the frequencies of 12·3% and 18·7%, respectively [26]. Moreover, these anti-PCNA positive patients with chronic HBV or HCV infection had no evidence for diagnosis of SLE. However, the epitopes of anti-PCNA autoantibodies remain unclear in patients with chronic HBV and HCV infection. This study investigated the epitopes of PCNA recognized by autoantibodies from patients with chronic HBV and HCV infection. The difference of recognition between wild type PCNA and mutant Y114A_PCNA [27] that differs from its native counterpart by a single amino acid residue will also be compared.
Materials and Methods
Sera
Serum samples were obtained from affiliated hospital of Chung Shan Medical University and collected from 185 patients with SLE, 178 with chronic HBV and 163 with chronic HCV infection. The diagnosis of SLE was made in patients who met the American College of Rheumatology criteria for SLE [28]. The criteria of diagnosis of chronic HBV or HCV infection were based on the presence of hepatitis B surface antigen (HBsAg) or anti-HCV antibodies (anti-HCV) for over 6 months, respectively. Patients with chronic HBV or HCV infection had no evidence for diagnosis of SLE. Control sera were obtained from 68 normal individuals whose age and sex were matched. All the study participants provided written informed consent. An anti-PCNA monoclonal antibody (Ab-1/PC-10) that recognizes the amino acid 112–121 of PCNA was used as control (Oncogene Research products, Cambridge, MA, USA).
Preparation of truncated PCNA
Full-length of wild type rat PCNA expression vector (pET-30a(+)) was done as described previously [26] and used as the template for construction of truncations. The truncated forms of PCNA cDNA were generated by PCR. To synthesize the DNA templates for C-terminal deletions, which correspond to amino acid residues of 1–70, 1–200, and 1–235, TL was used as the common forward primer with three reverse primers, TR1, TR2 and TR3. Their oligonucleotide sequences were: TL (5′–GCCGGATCCATGTTTGAG GCACGC−3′), TR1 (5′–CCCGTCGACCACGCCCATGGC CAG−3′), TR2 (5′–CCCGTCGACATTCATCTCTATGGA CAC−3′), TR3 (5′–CCCGTCGACAAGGGGTACATCTGC−3′). In addition, to synthesize the DNA templates for N-terminal deletions, which correspond to amino acid residues of 100–261, 150–261, and 190–261, TR was used as the common reverse primer with three forward primers, TL1, TL2 and TL3. The sequences of primers were: TR (5′–CCCGTC GACCAACGCCTAAGATCC−3′), TL1 (5′–GCCGGATCC CTAGTA-TTTGAAGCA−3′), TL2 (5′–GCCGGATCCCT TAGCCATATTGGA−3′), TL3 (5′–GCCGGATCCGAAGAG GAAGCTGTG−3′). All forward and reverse primers have restriction enzyme sites of BamH I and Sal I that facilitate the cloning of six truncated PCNA fragments into pET-32a(+) expression vector. Similar schemes were used to construct a mutant PCNA expression vector [pET-30a (+)] within a mutant human cDNA, Y114A_PCNA [21] that contains a single amino acid substitution from tyrosine to alanine at position 114 and fails to form the toroidal structure. The BL21 (pLys) strain of E.coli was used as the bacterial host for transforming and expressing the full-length of wild type PCNA, mutant Y114A_PCNA and truncated PCNA proteins. The recombinant proteins were all purified by Ni2+ – NTA column system (Qiagen, Chatsworth, CA, USA) and used them as antigens for ELISA and immunoblotting study. All constructs were confirmed by sequencing.
ELISA
Ten mM of each recombinant PCNA (rPCNA) proteins with or without truncation were used and immobilized on the surface of each sample well of 96-well plates. ELISA was performed according to the method of Rubin et al. [29]. All sera were assayed at a dilution of 1/100 and added to each sample well. After incubation at 37 °C for one hour, liquid of each sample well was removed and was washed with phosphate buffered saline (PBS)-Tween and subsequently incubated with peroxidase conjugated goat antihuman immunoglobulin. The colour reaction was done with 1 mg/ml substrate ABTs[2,2′azino-di-(3-ethylbenzthiazolin-6-sulphonic acid)] in the presence of 0·005% H2O2 at room temperature for 15 min. For absorption experiments, 10 mM wild type rPCNA were coated and the sera were incubated with 50 mM of purified full-length or truncated PCNA for one hour at 37 °C before ELISA was performed.
Immunoblotting
Western blotting was performed as described in previous report [30]. Proteins were separated on a 12·5% SDS-PAGE and subsequently transferred onto nitrocellulose membrane. After blocking with 5% nonfat dry milk in (PBS), full-length or truncated rPCNA proteins were recognized by the antihistidine tag (Serotec, Oxford, UK) or anti-PCNA (Ab-1/PC-10, Calbiochem, Germany) mouse monoclonal antibody. Then goat anti-mouse IgG conjugated with alkaline phosphatase (Sigma, St. Louis, MO, USA) was used as secondary antibody. The substrate NBT/BCIP (nitro-blue retrazolium/5-bromo-4chloro-3 indolyl phosphate) was used to detect the antigen-antibody complexes.
Statistical analysis
Statistical analysis was determined by Students t-test. P < 0·05 was taken to indicate significant difference.
Results
Preparation of full-length and truncated rPCNA proteins
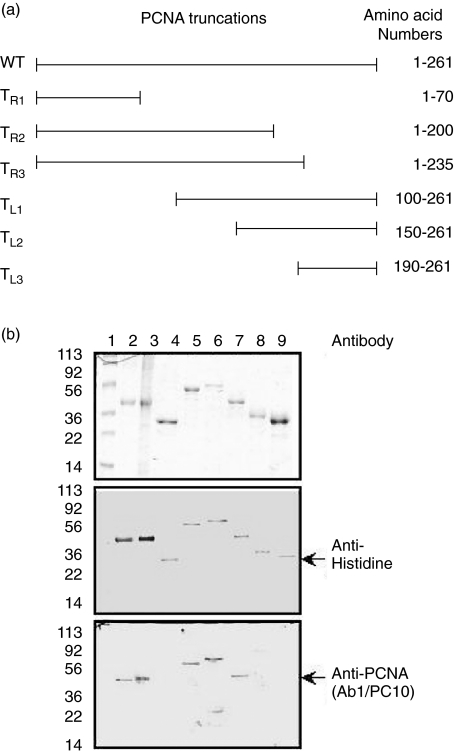
Figure 1a illustrates the schematic diagram of full-length and truncated PCNA with C- or N-terminal deletions. The wild type PCNA, mutant type Y114A_PCNA and six truncated rPCNA proteins were purified using the affinity chromatography on immobilized nickel column and demonstrated with SDS-PAGE (Fig. 1b upper panel). Anti-histidine and anti-PCNA monoclonal antibodies (Ab1/PC10) were used to identify the purified recombinant proteins (Fig. 1b, middle and bottom panels). TR1, TL2 and TL3, which contains no amino acids 112–121 of PCNA, were undetectable by Ab-1/PC-10 monoclonal antibody as expected (Fig. 1b, bottom panel).
Fig. 1.
Full-length and truncated rPCNA proteins. (a) Schematic representation of the full-length and deletions of PCNA. The C-terminal deletions of TR1, TR2 and TR3 were produced using primer TL, containing the BamH I restriction enzyme site, which paired individually with 3 different primers, TR1, TR2 and TR3, which contain the Sal I restriction enzyme site. The N-terminal deletions of TL1, TL2 and TL3 were produced using primer TR, containing the Sal I restriction enzyme site, which paired individually with 3 different primers, TR1, TR2 and TR3, which contain the BamH I restriction enzyme site. The six truncated PCNA recombinant proteins correspond to the amino acid sequences 1–70 (TR1), 1–200 (TR2), 1–235 (TR3), 100–261 (TL1), 150–261 (TL2) and 190–261 (TL3). (b) Purification and identification of full-length and mutant PCNA proteins. Upper panel shows the purified recombinant proteins separating in a 12·5% SDS-PAGE. Anti-histidine (middle panel) and PCNA (Ab1/PC10) (bottom panel) monoclonal antibodies were used to identify the purified recombinant proteins. Lane 1 shows the prestained protein marker, lane 2 is wild type PCNA, lane3 represent the Y114A_PCNA and lanes 4–9 correspond to the truncated deletions, TR1, TR2, TR3, TL1, TL2 and TL3, respectively.
Recognition of wild type and mutant type rPCNA proteins by anti-PCNA autoantibodies in patients with SLE, chronic HBV and HCV infection
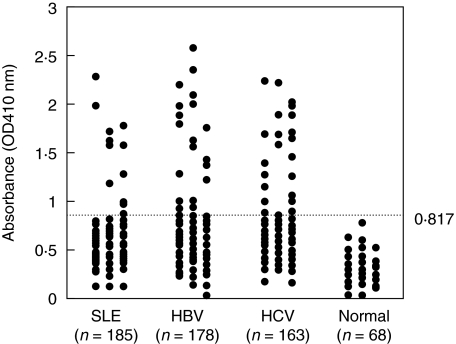
Previously, we have found that anti-PCNA antibodies were also present in patients with chronic HBV and HCV infection and these suggest that anti-PCNA antibody may not be specific for SLE [26]. To analyse the antigenic regions on PCNA that confer autoantibody binding in patients with chronic HBV and HCV infection, eight constructs including one wild type PCNA, one mutant type Y114A_PCNA and six C- or N-terminal PCNA truncations were generated. Figure 2 shows the results of anti-PCNA antibodies using wild type rPCNA as antigen by ELISA. The normal value of the absorbance was based on the results from 68 normal controls. The normal value of the absorbance was 0·370 ± 0·149 (mean ± 3 SD). Value above 0·817 was regarded as increased anti-PCNA. The frequency of increased anti-PCNA in patients with SLE, chronic HBV and HCV infection were 11 (5·9%) of 185, 22 (12·4%) of 178 and 27 (16·6%) of 163, respectively (Table 1). This result is consistent with our previous observations [26]. The distribution of immunoglobulin isotypes of anti-PCNA in patients with SLE, chronic HBV and HCV infection was shown in Table 2. All 11 SLE had elevated IgG anti-PCNA antibody only. Most sera from patients with chronic HBV or HCV infection had predominant IgG anti-PCNA. IgM and IgA anti-PCNA were present to varying extents in sera from patients with chronic HBV and HCV infection.
Fig. 2.
Anti-PCNA antibody was measured by enzyme-linked immunosrobent assay in sera from patients with SLE, chronic HBV and HCV infection. A cutoff value determined by testing sera from normal individuals is indicated by dot line (mean ± 3SD).
Table 1.
Recognition of wild type, mutant type, and six truncated rPCNA proteins in patients with SLE, chronic HBV and HCV infection.
| Full-length and truncated PCNA fragments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patients | WT | Mu | TR1 | TR2 | TR3 | TL1 | TL2 | TL3 |
| SLE (n = 11) | 11 (100) | 3 (27) | 4 (36) | 6 (55) | 6 (55) | 5 (46) | 3 (27) | 2 (18) |
| HBV (n = 22) | 22 (100) | 7 (32) | 3 (14) | 8 (36) | 11 (50) | 17 (77) | 16 (73) | 14 (64) |
| HCV (n = 27) | 27 (100) | 8 (30) | 7 (26) | 9 (33) | 10 (35) | 11 (41) | 8 (31) | 8 (30) |
Numbers in parentheses are percentages. WT, wild type PCNA; Mu,: mutant type Y114_PCNA.
Table 2.
Distribution of immunoglobulin isotypes of anti-PCNA in patients with SLE, chronic HBV and HCV infection.
| Immunoglobulin Isotype | SLE (n = 11) | HBV (n = 22) | HCV (n = 27) |
|---|---|---|---|
| Anti-PCNA | |||
| IgM | 0 (0) | 5 (23) | 4 (15) |
| IgG | 11 (100) | 19 (86) | 18 (67) |
| IgA | 0 (0) | 9 (41) | 6 (22) |
Numbers in parentheses are percentages.
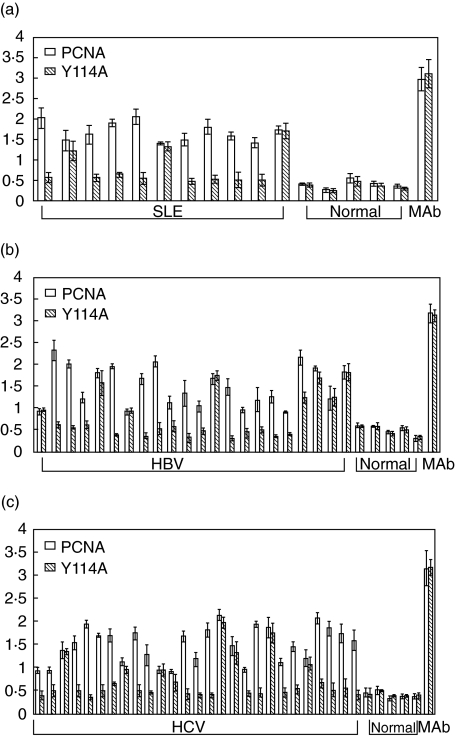
As we know that PCNA can form a trimeric ring structure and it is essential for the protein activity. Previous studies using proteolysis suggested that autoantibodies recognize either carboxyl or amino terminal or both of the PCNA protein [27]. Moreover, the mutant Y114A_PCNA contains a single substitution at position 114 where tyrosine is replaced with alanine, and this protein fails to form the trimeric structure and is dysfunctional. In our study, the results of anti-PCNA antibodies using mutant type rPCNA as antigen by ELISA were shown in Table 1. The frequency of anti-Y114_PCNA antibody in patients with SLE, chronic HBV and HCV infection were 3 (27%) of 11, seven (32%) of 22 and 8 (30%) of 27, respectively. In contrast, both wild type PCNA and mutant type Y114A_PCNA were equally recognized by the monoclonal antibody (Ab-1/PC-10). None of the normal individuals has increased anti-PCNA and anti-Y114A_PCNA antibodies. These results indicated that anti-PCNA positive sera from patients with SLE, chronic HBV and HCV infection more preferentially recognized the wild type PCNA than the mutant type Y114A _PCNA (P< 0·05) (Fig. 3).
Fig. 3.
Autoantibodies preferentially recognized wild type PCNA. Comparative ELISA using wild type or mutant type Y114A_PCNA as antigens. Serum sample with increased anti-PCNA autoantibodies by previous screen in patients with SLE, chronic HBV and HCV were used for comparative ELISA. Normal sera and the monoclonal antibody to PCNA (Ab1/PC10) were also used as negative and positive control, respectively. Each sample well was coated with 10 mM of wild type or mutant PCNA. ELISA results of (a) SLE, (b) chronic HBV and (c) HCV are shown.
Recognition of truncated rPCNA proteins by anti-PCNA autoantibodies in patients with SLE, chronic HBV and HCV infection
There were six truncated rPCNA proteins with either C- or N-terminal deletions. Table 1 lists the results of recognition of truncated rPCNA proteins by using sera from patients with SLE, chronic HBV and HCV infection. Among SLE patients, the frequency of anti-PCNA antibodies recognizing the truncated rPCNA proteins of TR1, TR2, TR3, TL1, TL2 and TL3 were 4 (36%), 6 (55%), 6 (55%), 5 (46%), 3 (33%) and 2 (18%) of 11, respectively. Among patients with chronic HBV infection, the frequency of anti-PCNA antibodies recognizingthe truncated rPCNA proteins of TR1, TR2, TR3, TL1, TL2 and TL3 were 3 (14%), 8 (36%), 11 (50%), 17 (77%), 16 (73%) and 14 (64%) of 22, respectively. Among patients with chronic HCV infection, the frequency of anti-PCNA antibodies recognizing the truncated rPCNA proteins of TR1, TR2, TR3, TL1, TL2 and TL3 were 7 (26%), 9 (33%), 10 (35%), 11 (41%), 8 (31%) and 8 (31%) of 27, respectively. These data indicated that TL2 and TL3 confer dominant binding reactivity in sera from patients with chronic HBV infection.
Absorption of anti-PCNA antibodies with full-length or truncated rPCNA proteins
The results of absorption experiments by ELISA are given in Table 3. The binding activity was inhibited by purified either full-length or truncated rPCNA proteins at the concentration of 50 mM. The inhibition of binding by purified full-length rPCNA proteins with sera from patients with chronic HBV and HCV infection and SLE was shown to exceed 70%. The inhibition of binding by purified truncated rPCNA with sera from patients with chronic HBV and HCV infection and SLE was shown to confer dominant binding reactivity in TL2 and TL3. Moreover, the higher frequency of inhibition by using TL3 was found in patients with chronic HBV infection (Table 3).
Table 3.
Absorption of anti-PCNA with 50 mM of purified full-length or truncated rPCNA proteins in patients with SLE, chronic HBV and HCV infection.
| Anti-PCNA after absorption (OD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patients | WT | WT | TR1 | TR2 | TR3 | TL1 | TL2 | TL3* |
| SLE | ||||||||
| 1 | 2·629 | 0·327 (88) | 1·709 (35) | 1·605 (39) | 1·333 (49) | 1·422 (46) | 1·401 (45) | 1·162 (39) |
| 2 | 1·485 | 0·401 (73) | 1·047 (29) | 0·988 (33) | 0·865 (41) | 0·891 (40) | 0·902 (39) | 1·002 (32) |
| 3 | 1·624 | 0·387 (76) | 1·233 (24) | 1·108 (32) | 0·872 (46) | 0·927 (43) | 1·123 (31) | 1·201 (26) |
| HBV | ||||||||
| 1 | 2·165 | 0·557 (74) | 2·025 (6) | 1·433 (34) | 0·956 (56) | 0·682 (68) | 0·801 (63) | 0·903 (58) |
| 2 | 1·920 | 0·583 (70) | 1·622 (15) | 1·409 (27) | 1·133 (41) | 0·833 (57) | 0·907 (53) | 0·945 (50) |
| 3 | 1·225 | 0·206 (83) | 1·088 (11) | 0·887 (28) | 0·656 (46) | 0·402 (67) | 0·502 (59) | 0·508 (59) |
| 4 | 1·860 | 0·496 (73) | 1·498 (19) | 1·222 (34) | 1·088 (41) | 0·912 (51) | 0·871 (53) | 1·027 (45) |
| 5 | 1·735 | 0·326 (81) | 1·104 (36) | 0·785 (55) | 0·563 (68) | 0·424 (75) | 0·496 (71) | 0·489 (72) |
| 6 | 3·011 | 0·318 (90) | 2·556 (15) | 1·862 (38) | 1·714 (43) | 1·002 (67) | 1·187 (60) | 1·282 (57) |
| HCV | ||||||||
| 1 | 1·548 | 0·387 (75) | 1·204 (22) | 0·928 (40) | 1·002 (35) | 0·889 (43) | 1·121 (28) | 1·014 (34) |
| 2 | 1·954 | 0·299 (85) | 1·638 (17) | 1·146 (41) | 1·098 (44) | 1·078 (45) | 1·153 (41) | 1·199 (39) |
| 3 | 1·714 | 0·412 (76) | 1·241 (28) | 1·272 (26) | 0·998 (42) | 0·883 (48) | 1·029 (40) | 1·229 (28) |
| 4 | 1·708 | 0·158 (91) | 1·328 (19) | 1·198 (30) | 1·020 (40) | 0·949 (44) | 0·998 (42) | 1·397 (18) |
| 5 | 2·105 | 0·598 (72) | 1·689 (20) | 1·301 (38) | 1·217 (42) | 1·424 (32) | 1·397 (34) | 1·388 (34) |
Numbers in parentheses are percentages of binding inhibition.
The average values of binding inhibition by TL3 in patients with SLE, chronic HBV and HCV infection were 32%, 58%, and 30%, respectively.
Discussion
The prevalence of anti-PCNA autoantibody in our patients with chronic HBV and HCV infection, and SLE was 12·4%, 16·6%, and 5·9%, respectively (Fig. 2). This result is similar to our previous finding [26]. Moreover, we also found that the frequency of anti-Y114_PCNA antibody in patients with SLE, chronic HBV and HCV infection were three of 11 (27%), seven of 22 (32%) and eight of 27 (30%), respectively (Table 1). To study the antigenic regions of PCNA that confer anti-PCNA autoantibody binding reactivity in patients with chronic HBV and HCV infection, six truncations were constructed for analysis (Fig. 1). We found TL2 and TL3 provide predominant higher binding reactivity to anti-PCNA autoantibodies in patients with chronic HBV infection (Table 1). Whereas sera from patients with SLE and chronic HCV infection revealed a random distribution of binding reactivity to the six truncated rPCNA. Additionally, the inhibition of binding in sera from patients with HBV infection was predominantly higher in competed with TL2 and TL3 (Table 3). These results strongly demonstrated that majority of anti-PCNA autoantibody recognize the C-terminal of rPCNA. It suggests the possibility of a different antigenic stimulation to produce anti-PCNA autoantibody in patients with HBV infection.
Previous reports indicated that some anti-PCNA positive sera did not react with the 34-kD PCNA polypeptide. However, it was conversely observed that serum of OK reacted exclusively with the 34-kD polypeptide [22]. Among the anti-PCNA positive sera, patients with SLE, chronic HBV and HCV infection more preferentially recognized the wild type PCNA than the mutant type Y114A _PCNA (Fig. 3 and Table 1). These results are similar to that anti-PCNA autoantibody in patients with SLE that indicated the majority of anti-PCNA autoantibodies recognize a conformational epitope [22,23]. The isotypes distribution of anti-PCNA antibody in patients with chronic HBV and HCV infection was examined and found to be predominantly IgG (86% and 67% in patients with chronic HBV and HCV infection, respectively, Table 2) that is consistent with our previous report [26]. IgM anti-PCNA antibody still represented a minor isotype (23% and 15% in patients with HBV and HCV infection, respectively). These results are similar to spontaneously arising autoantibodies in SLE, which was predominantly IgG with low level of IgM [31,32] and suggests that anti-PCNA antibody may occur in the autoimmune response.
In this study, we found that anti-PCNA autoantibodies from patients with chronic HBV infection major recognized the N-terminal truncated PCNA, TL2 and TL3, and the ratios were 73% and 64%, respectively (Table 1). Moreover, anti-PCNA autoantibodies preferentially recognize the C-terminal of PCNA in patients with chronic HBV infection (Table 3). In the Ogata et al. [21] study, both C- and N-terminal halves are crucial for recognition of human lupus autoantibodies. However, production of anti-PCNA autoantibody in patients with chronic HBV and HCV infection is still unclear. We still do not know when anti-PCNA antibody is produced and how long it will persist in patients with chronic HBV and HCV infection at this moment. Long-term longitudinal studies are required to determine the natural course of anti-PCNA autoantibody and whether its expression affects the natural history of chronic hepatitis B or hepatitis C. We will further define the role of C-terminal PCNA in anti-PCNA autoantibody production in patients with chronic HBV infection. This study is the first to report the antigenic regions on PCNA that confer autoantibody binding in patients with chronic HBV and HCV infection. These data may also provide advanced understanding between viral infection and autoimmunity for further study.
Acknowledgments
The authors would like to thank Dr U. Hubscher (University of Zurich-Irchel) for providing the mutant PCNA (Y114A) plasmid. The study was supported by grants VGHTH-86-017-2 and NSC 89–2314-B-040–021.
References
- 1.Hansen KE, Arnason J, Bridges AJ. Autoantibodies and common viral illnesses. Semin Arthritis Rheum. 1998;27:263–71. doi: 10.1016/s0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 2.Buskila D, Sikuler E, Shoenfeld Y. Hepatitis C virus, autoimmunity and rheumatic disease. Lupus. 1997;6:685–9. doi: 10.1177/096120339700600902. [DOI] [PubMed] [Google Scholar]
- 3.Schattner A, Rager-Zisman B. Virus-induced autoimmunity. Rev Inf Dis. 1990;12:204–21. doi: 10.1093/clinids/12.2.204. [DOI] [PubMed] [Google Scholar]
- 4.Panasiuk A. Autoantibodies in chronic liver diseases. Rocz Akad Med Bialymst. 2001;46:106–12. [PubMed] [Google Scholar]
- 5.Abu-Shakra M, El-Sana S, Margalith M, Sikuler E, Neumann L, Buskila D. Hepatitis B and C viruses serology in patients with SLE. Lupus. 1997;6:543–4. doi: 10.1177/096120339700600612. [DOI] [PubMed] [Google Scholar]
- 6.Inman R. Rheumatic manifestations of hepatitis B virus infection. Semin Arthritis Rheum. 1982;11:406–20. doi: 10.1016/0049-0172(82)90028-2. [DOI] [PubMed] [Google Scholar]
- 7.Prieto J, Yuste JR, Beloqui O, Civeira MP, Riezu JI, Augirre B, Sangro B. Anticardiolipin antibodies in chronic hepatitis C. implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199–204. doi: 10.1002/hep.510230201. [DOI] [PubMed] [Google Scholar]
- 8.Sung JL. Prevention of hepatitis B and C virus infection for prevention of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S370–6. doi: 10.1111/j.1440-1746.1997.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HY, Chang MH, Chen DS, et al. 1984: baseline seroepidemiology of hepatitis B virus infection in children in Taipei. A study just before the mass hepatitis B viral vaccination program in Taiwan. J Med Virol. 1986;18:301–7. doi: 10.1002/jmv.1890180402. [DOI] [PubMed] [Google Scholar]
- 10.Sun CA, Chen HC, Lu CF, et al. Transmission of hepatitis C virus in Taiwan: prevalence and risk factors based on a nationwide survey. J Med Virol. 1999;59:290–6. [PubMed] [Google Scholar]
- 11.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–34. [PubMed] [Google Scholar]
- 12.Takasaki Y, Fishwild D, Tan EM. Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med. 1984;159:981–92. doi: 10.1084/jem.159.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 14.Yeo JP, Toh BH. Cell cycle-associated autoantibodies: markers for autoimmunity and probes for molecular cell biology. Autoimmunity. 1994;18:291–300. doi: 10.3109/08916939409009531. [DOI] [PubMed] [Google Scholar]
- 15.Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA/cyclin) during the cell cycle. Exp Cell Res. 1986;166:209–19. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- 16.Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69:367–74. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- 17.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–43. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 18.Fritzler MJ, McCarty GA, Ryan JP, Kinsella TD. Clinical features of patients with antibodies directed against proliferating cell nuclear antigen. Arthritis Rheum. 1983;26:140–5. doi: 10.1002/art.1780260204. [DOI] [PubMed] [Google Scholar]
- 19.Ohata N. Clinical significance of antibodies to proliferating cell nuclear antigen (PCNA) Ryumachi. 1987;27:79–87. [PubMed] [Google Scholar]
- 20.Murashima A, Takasaki Y, Ohgaki M, Hashimoto H, Shirai T, Hirose S. Activated peripheral blood mononuclear cells detected by murine monoclonal antibodies to proliferating cell nuclear antigen in active lupus patients. J Clin Immuno. 1990;10:28–37. doi: 10.1007/BF00917495. [DOI] [PubMed] [Google Scholar]
- 21.Ogata K, Ogata Y, Takasaki Y, Tan EM. Epitopes on proliferating cell nuclear antigen recognized by human lupus autoantibody and murine monoclonal antibody. J Immunol. 1987;139:2943–6. [PubMed] [Google Scholar]
- 22.Huff JP, Roos G, Peebles CL, Houghten R, Sullivan KF, Tan EM. Insights into native epitopes of proliferating cell nuclear antigen using recombinant DNA protein products. J Immunol. 1990;172:419–29. doi: 10.1084/jem.172.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogure T, Takasaki Y, Takeuchi K, et al. Autoimmune responses to proliferating cell nuclear antigen multiprotein complexes involved in cell proliferation are strongly associated with their structure and biologic function in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:2946–56. doi: 10.1002/art.10606. [DOI] [PubMed] [Google Scholar]
- 24.Tsai WM, Roos G, Hugli TE, Tan EM. Influences of free thiol groups on autoantibody-defined epitopes of proliferating cell nuclear antigen. J Immunol. 1992;149:2227–33. [PubMed] [Google Scholar]
- 25.Brand SR, Bernstein RM, Mathews MB. Autoreactive epitope profiles of the proliferating cell nuclear antigen define two classes of autoantibodies. J Immunol. 1994;153:3070–8. [PubMed] [Google Scholar]
- 26.Tzang BS, Chen TY, Hsu TC, Liu YC, Tsay GJ. Presentation of autoantibody to proliferating cell nuclear antigen in patients with chronic hepatitis B and C virus infection. Ann Rheum Dis. 1999;58:630–4. doi: 10.1136/ard.58.10.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson ZO, Podust VN, Podust LM, Hubscher U. Tyrosine114 is essential for the trimeric structure and functional activities of human proliferating cell nuclear antigen. EMBO J. 1995;14:5745–51. doi: 10.1002/j.1460-2075.1995.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan EM, Cohen AS, Fries JF, et al. The revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 29.Rubin RL, Joslin FG, Tan EM. An improved ELISA for anti-native DNA by elimination of interference by antihistone antibodies. J Immunol Meth. 1983;63:359–66. doi: 10.1016/s0022-1759(83)80009-x. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E, Maniatis T. Detection and Analysis of Proteins Expressed from Cloned Genes, Molecular Cloning. Vol. 3. Cold Spring Habor: Cold Spring Habor Press; 1989. Molecular cloning; pp. 18.47–18.75. [Google Scholar]
- 31.Bootsma H, Spronk PE, Borg EJ, Hummel EJ, de Boer G, Limburg PC, Kallenberg CG. The predictive value of fluctuations in IgM and IgG class anti-dsDNA antibodies for relapses in systemic lupus erythematosus. A prospective long term observation. Ann Rheum Dis. 1997;56:661–6. doi: 10.1136/ard.56.11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colaco CB, Male DK. Anti-phoshpholipid antibodies in syphilis and athrombotic subset of SLE. distinect profiles of epitopes specificity. Clin Exp Immunol. 1985;59:449–56. [PMC free article] [PubMed] [Google Scholar]