Abstract
Onconeural antibodies are found in patients with cancer and are associated with paraneoplastic neurological syndromes (PNS). The objective of the present study was to assess the frequency of Yo antibodies in ovarian and breast cancer using a sensitive immunoprecipitation technique, and to look for any association of Yo antibodies with neurological symptoms and prognostic factors. A multiwell adapted fluid-phase immunoassay using radiolabelled recombinant cerebellar degeneration related protein (cdr2), produced by coupled in vitro transcription/translation was used for the detection of Yo antibodies. This technique combines high specificity and sensitivity with high sample analysing capacity for the antibody in question. Sera or EDTA-blood from 810 ovarian (n = 557) and breast cancer (n = 253) patients were analysed for Yo antibodies by immunoprecipitation, as well as immunofluorescence and immune blots. Two hundred healthy blood donors and sera from 17 patients with paraneoplastic cerebellar degeneration and Yo antibodies served as controls. Immunoprecipitation was more sensitive in detecting Yo antibodies than immunofluorescence and immune blots. The prevalence of Yo antibodies was 13/557 (2·3%) in ovarian cancer and 4/253 (1·6%) in breast cancer using immunoprecipitation. Yo antibodies were not correlated with specific histological subgroups. The Yo index of ovarian cancer patients in FIGO stage IV was higher compared to FIGO stage I-III. The prevalence of Yo antibodies was 3 times higher in patients with stage III breast cancer than in stage I and II. Only 2/17 (11·8%) patients with Yo antibodies detected during the screen of 810 cancer patients had PNS. The results show that the prevalence of Yo antibodies is low in ovarian and breast cancer. Yo antibodies may be associated with advanced cancer, but less often with PNS.
Keywords: paraneoplastic syndrome, Yo antibody, cdr2, breast cancer, ovarian cancer, immunoprecipitation
Introduction
Paraneoplastic neurological syndromes (PNS) arise as nonmetastatic manifestations in less than 1% of all cancers and are often associated with onconeural antibodies, which are highly specific markers of underlying malignancy. The targets of the antibodies in PNS are tumour antigens that are normally expressed by neurones alone. Whereas the immune response elicited by the onconeural antigens may be beneficial by keeping the tumour in check, it may also gain access to the nervous system and cause severe neuronal damage (for review, see [1]).
In female patients, paraneoplastic cerebellar degeneration (PCD) is in particular associated with tumours of the ovary or breast [2,3]. The large majority of PCD patients harbour high levels of Yo antibodies directed to the cytoplasmic antigen cdr2, and cdr2 specific cytotoxic lymphocytes are found in the blood and cerebrospinal fluid (CSF) of affected individuals [4,5].
PCD is a rare condition, despite the fact that the antigen cdr2 is widely expressed in gynaecological tumours [6]. Although onconeural antibodies are often associated with PNS, antibodies can also be found in cancer patients without neurological symptoms [7–9].
The common techniques used for detection of onconeural antibodies are immunhistochemistry and immune blots with neuronal extracts or recombinant proteins [10]. We have recently employed a very sensitive immunoprecipitation technique for the detection of Hu antibodies [8,11]. The aim of this study was to apply the same immunoprecipitation technique to examine the prevalence of Yo antibodies in a large cohort of patients with ovarian or breast cancer. In addition, we wished to correlate antibody positivity with neurological symptoms and with prognostic factors as CA-125, histology and stage of disease.
Patients and methods
Patients
EDTA blood or serum samples from altogether 557 ovarian cancer patients were investigated for the presence of Yo antibodies. EDTA blood from 458 of the patients were obtained from the Department of Gynaecological Oncology, Rikshospitalet-Radiumhospitalet Trust (RR), Oslo, Norway (ovary group I). Blood samples were obtained before and after surgery during the years 1994–98. In addition, sera from 99 patients with ovarian cancer were obtained at the Department of Obstetrics and Gynaecology, Haukeland University Hospital, Bergen in the years 2001–04 (ovary group II). Sera were obtained before tumour resection. Both groups consisted of consecutive patients in various stages of the disease, preoperative serum CA-125 was measured, and none had received chemotherapy when the sera were obtained.
Sera from 253 patients with breast cancer were obtained from the Department of Oncology, Haukeland University Hospital. The patients were treated during the years 1991–2001. Sera were obtained before surgery from 191 patients without metastases (stage II) (breast group I) and from 62 patients with metastatic breast cancer (stage III) (breast group II).
The medical records were available for all patients and were reviewed retrospectively in antibody-positive cases. Clinical data were available until 2005. The patients gave informed consent for inclusion in this study, which was approved by the ethical committee.
Sera from 17 patients (with PCD and ovarian or breast cancer) with Yo antibodies detected by immunofluorescence and dot blot for routine diagnostics were used as patients controls. Samples from 200 healthy blood-donors at the Haukeland University Hospital (100 sera and 100 EDTA blood) were used as normal controls. All samples were stored at −20 °C.
In vitro transcription-translation (ITT) and immunoprecipitation
The cdr2 gene was PCR amplified from a plasmid (kindly provided by Dr Josep Dalmau, University of Pennsylvania, Philadelphia, PA, USA) and the product was cloned into a pIVEX 2,3 vector (Roche Diagnostics GmbH, Mannheim, Germany) downstream of a T7 promoter. The vector containing full-length cdr2 was electroporated into Escherichia coli XL1-Blue MRF′ using a Bio-Rad gene pulser (Bio-Rad, Hercules, CA, USA) at 12·5 kV/cm and 25 µF. Bacteria containing the plasmid were grown in Luria Bertani medium and plasmid DNA was purified using the Qiagen plasmid midi kit (Qiagen, Hilden, Germany).
Recombinant [35S]-methionine labelled cdr2 protein was produced in a coupled transcription/translation system with a T7 RNA polymerase and nuclease-treated rabbit reticulocyte lysate, following the instructions of the manufacturer (Promega, Madison, WI, USA). The ITT product was quality-checked by SDS-PAGE followed by photostimulated luminescence imaging (Bio-image analyser Bas 2000, Fuji Photo Film, Tokyo, Japan). The radiolabelled protein had the expected molecular weight of approximately 62 kD [12].
MultiScreen 96-well filtration plates (MABV N0B50, Millipore, Bedford, MA, USA), which allow high throughput analysis, were used for immunoprecipitation experiments. Each well was washed and blocked as previously described [11]. Radiolabelled cdr2 protein (30 000 cpm/well) and patient sera (diluted 1 : 20) or EDTA-blood (diluted 1 : 10) in incubation buffer (20 mM Tris HCl, 150 mM NaCl, 0·001% Azide, 0·1% BSA, 0·15% Tween-20, pH 8·0) were incubated at 4 °C overnight in 96-well microtiter plates. The following day, 50 µl of a 50% (v/v) slurry of resuspended protein A-Sepharose (Amersham Biosciences AB, Uppsala, Sweden) in incubation buffer was added to each well of the treated filtration plates followed by the addition of the Yo-cdr2 complex. The immune complexes were immunoprecipitated with protein-A-sepharose in the 96-well plate, by incubation on a shaking platform for 45 min at 4 °C. Free antigen, residual [35S]-methionine and other reaction components were washed through the filter utilizing a vacum-operated 96-well plate washer. The MultiScreen filtration plates containing the radiolabelled immunoprecipitate were left to dry overnight. Finally 20 µl of scintillation fluid (MicroscintTM-0, Packard Biosciences B.V., Groningen, the Netherlands) was added to each well, and the plate was covered by TopSeal™-A (Packard). The emitted radioactivity, which correlates to the level of Yo antibodies in the patient sample, was measured in a β-counter (Topcount NXT microplate scintillation and luminescence counter, Packard) as counts per minute (cpm).
Pooled sera from 100 blood donors were used as a negative control. A polyclonal rabbit antibody (Eurogentec s.a., Seraing, Belgium) against two synthetic peptides corresponding to amino acid 123–138 (EELKSSGQGRRSPGKC) and amino acid c +429–443 (CDEQRTKYRSLSSHS) of the human cdr2 sequence was used as positive control. The peptides were coupled to KLH (keyhole limpet haemocyanin). Control tests using preimmune rabbit serum were negative. The cpm of the negative control is approximately 5% of the positive control and represent the background in this assay.
Each sample was run in triplicate and the mean value of these three was used. The results were expressed as:
Yo index greater than 117 was considered as positive, based on the mean ±3SD of 200 blood donors. Positive results were confirmed by a second immunoprecipitation experiment.
None of the Yo positive sera reacted when immunoprecipitation was run with a cdr2 negative ITT product (i.e an ITT reaction containing all reactants, but no plasmid). This exclude alternative explanations for immunoprecipitation of the Yo positive samples and confirms the specificity of the technique.
Immunofluorescence (IF)
Cryostat sections of snap-frozen rat cerebellar tissue were incubated with EDTA blood or serum, diluted 1 : 500 in phosphate-buffered saline (PBS) in a moist chamber at 4 °C overnight. The slides were subsequently washed in PBS and incubated with fluorescein isothiocyanate labelled rabbit anti-human immunoglobulin antibodies (F0200, DAKO A/S, Denmark) diluted 1 : 50 in PBS, for one hour at 20 °C. The slides were washed, mounted and examined by fluorescence microscopy (Leica DM IL, Leica Microsystems Ltd, Heerbrugg, Switzerland). Cytoplasmatic granular staining of Purkinje cells was suggestive of Yo antibody reactivity and titres of 500 were defined as positive, in line with general guidelines for the detection of onconeural antibodies [10].
Immune blot
Samples positive for Yo antibodies by immunoprecipitation or IF were tested by dot blot, employing a commercial kit (Anti-Onkoneurale Antigene Immuno Dot Blot Milenia, DPC Biermann GmbH, Germany) that contains the recombinant proteins HuD, Yo (cdr2), Ri (Nova-1) and amphiphysin. The samples were diluted 1 : 2000 (following the instructions of the manufacturer). Distinct bands corresponding to the cdr2 position in the immune blot were interpreted as positive.
The positive samples were also tested by Western blot using rat cerebellar homogenate or cdr2 protein produced by ITT. The proteins were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked, washed, cut into strips and incubated with a 1 : 100 dilution of serum or EDTA-blood samples. The strips were subsequently washed and incubated with HRP-conjugated rabbit antihuman IgG (DAKO Cytomation, Glostrup, Denmark), diluted 1 : 1000, followed by chromogenic detection by 4-chloro-1-naphtol. Distinct bands corresponding to a molecular weight of approximately 55 kD (cerebellar homogenate) and approximately 60 kD (recombinant protein) were considered positive.
Results
Controls
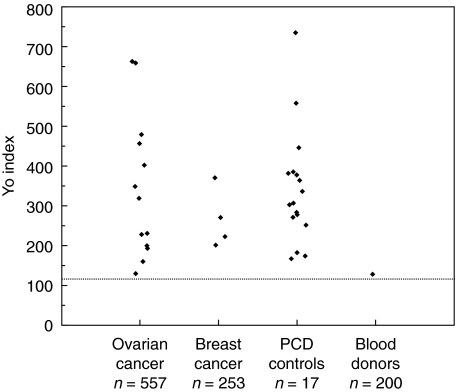
The mean Yo index of the 200 blood donors was −3 (range −100–129). A Yo index of 117 was set as the upper normal limit (mean Yo index of the blood donors + 3SD). The Yo index of the 100 EDTA blood samples (mean −9, range −71–86) were within the range of the 100 serum samples (mean 2, range −100–129). One of the 200 controls (0·5%) had a Yo index of 129, whilst the remaining 199 controls were below the cut-off limit. The control with increased Yo index was not positive by IF or immune blots. Yo antibodies were detected by immunoprecipitation in all the 17 PCD sera acting as patient controls, with a mean Yo index of 343 (range 169–740) (Fig. 1).
Fig. 1.
Positive Yo index for patients with ovarian cancer (13/557), breast cancer (4/253), control patients with paraneoplastic cerebellar degeneration (PCD) (17/17) and blood donors (1/200). Yo index of 117 was set as the upper normal limit (mean Yo index of the blood donors +3 SD), and is shown as a dotted line on the figure.
Ovarian cancer
Yo antibodies were detected by immunoprecipitation in 13 (2·3%) of 557 patients with ovarian cancer, with a mean Yo index of 344 (range 131–663) (Fig. 1). IF detected Yo antibodies in 5 of the samples (0·9%), all of which were also positive by immunoprecipitation. By immune blots, 5 were positive by dot blot, 8 by Western blot with cerebellar homogenate and 9 with Western blot with recombinant cdr2 protein (Table 1).
Table 1.
Yo antibodies detected by immunoprecipitation (Yo index), immunofluorescence (IF)/dot blot and Western blot with recombinant (ITT-cdr2) antigen (WBag) or cerebellar homogenate (WBch) at different dilutions. In ovary group I, EDTA-blood was obtained before or after tumour surgery, whereas in ovary group II sera were obtained before surgery. Breast group I and II represent stage II and stage III, respectively.
| Patient no. | Yo index 1 : 10/1 : 20 | IF/dot blot 1 : 500/1 : 2000 | WBag 1 : 100 | WBch 1 : 100 | Tumour |
|---|---|---|---|---|---|
| 1 | 659 | + | + | + | Ovary (I) |
| 2 | 479 | + | + | + | Ovary (I) |
| 3 | 232 | – | + | + | Ovary (I) |
| 4 | 342 | – | – | – | Ovary (I) |
| 5 | 229 | – | + | – | Ovary (I) |
| 6 | 663 | + | + | + | Ovary (I) |
| 7 | 457 | – | – | + | Ovary (I) |
| 8 | 131 | – | – | – | Ovary (I) |
| 9 | 161 | + | + | – | Ovary (II) |
| 10 | 201 | – | – | – | Ovary (II) |
| 11 | 402 | + | + | + | Ovary (II) |
| 12 | 195 | – | + | + | Ovary (II) |
| 13 | 319 | – | + | – | Ovary (II) |
| 14 | 272 | – | – | – | Breast (I) |
| 15 | 371 | – | – | – | Breast (I) |
| 16 | 202 | – | – | + | Breast (II) |
| 17 | 224 | – | – | – | Breast (II) |
Yo antibodies were approximately 3 times more frequent in ovary group II (5/99; 5%) than in ovary group I (8/458; 1·7%) determined by immunoprecipitation (P = 0·062, Fisher's exact test). The Yo index of the three patients with widespread ovarian cancer (FIGO stage IV) was higher (mean 479, range 402–659) compared to the 10 patients with FIGO stage I-III (mean 222, range 131–663) (P = 0·077, Man-Whitney test). Yo antibodies were found in ovarian cancers of different histology. There was no correlation between the CA-125 level and the Yo index among the Yo-positive ovarian cancer patients (P = 0·47, Spearmann correlation).
Two of the 13 patients with Yo antibodies had PCD (patients nos. 1 and 2). In both cases, the neurological symptoms preceded the tumour diagnosis (Table 2). All three methods detected Yo antibodies in these samples. Yo antibodies were also detected by all methods in two patients without neurological symptoms or signs (patients nos. 6 and 11). One of these patients died only six months after the cancer diagnosis, but the other had no neurological disease after 10 years of observation. All four patients had advanced disease and aggressive histoprognostic factors (Table 2). Two other patients developed chemotherapy-induced neuropathy (patients nos. 9 and 12) (Table 2).
Table 2.
Yo antibody positive patients with ovarian cancer. Clinical parameters and Yo index.
| Patient no. | Age (years) | Histology | FIGO stage | CA-125 | Neurological findings | Yo index |
|---|---|---|---|---|---|---|
| 1 | 49 | Serous cystadenocarcinoma | IV | 730 | PCD | 659 |
| 2 | 69 | Serous cystadenocarcinoma | IV | 348 | PCD | 479 |
| 3 | 64 | Granulosa cell | I | 50 | None | 232 |
| 4 | 67 | Mucinous cystadenocarcinoma | I | 5 | None | 342 |
| 5 | 68 | Serous cystadenocarcinoma | I | 427 | None | 229 |
| 6 | 55 | Undifferentiated carcinoma | III | 28 | None | 663 |
| 7 | 83 | Mixed adenocarcinoma | III | 2100 | None | 457 |
| 8 | 84 | Mixed adenocarcinoma | III | 69 | None | 131 |
| 9 | 78 | Endometroid adenocarcinoma | III | 461 | Chemotherapy-induced neuropathy | 161 |
| 10 | 53 | Mucinous/Borderline | III | 6 | None | 201 |
| 11 | 58 | Serous cystadenocarcinoma | IV | 156 | None | 402 |
| 12 | 54 | Clear cell carcinoma | I | 10 | Chemotherapy-induced neuropathy | 195 |
| 13 | 62 | Endometroid adenocarcinoma | III | 250 | None | 319 |
Patients 1–8, Ovary group I; patients 9–13, Ovary group II; Patients 5 and 10 were treated by surgery alone, the others had adjuvant chemotherapy. Yo antibodies were detected in pre-operative serum or EDTA blood samples in all patients, except for patients 4, 7 and 8, in whom the samples were drawn after surgery. Borderline = adenocarcinoma with atypia. FIGO = international federation of gynaecology and obstetrics.
Breast cancer
Yo antibodies were detected by immunoprecipitation in 4 of 253 (1·6%) patients with breast cancer, with a mean Yo index of 267 (range 202–371) (Fig. 1). Yo antibodies were not detected in any of these samples by IF or immune blots (Table 1). The antibodies were approximately 3 times more frequent in patients with stage III disease (2/62; 3·2%) (breast group II) than in patients with stage II disease (2/191; 1·0%) (breast group I) (P = 0·21, Fisher's exact test) (Table 1). There was almost no difference in Yo index between these patients. Yo antibodies were found in patients with lobular or ductal carcinoma. None of the Yo positive breast cancer patients had neurological symptoms (Table 3).
Table 3.
Yo antibody positive patients with breast cancer. Clinical parameters and Yo index.
| Patient no. | Age (years) | Histology | Cancer stage | Neurological findings | Yo index |
|---|---|---|---|---|---|
| 14 | 50 | Ductal carcinoma | II | None | 272 |
| 15 | 74 | Ductal carcinoma | II | None | 371 |
| 16 | 67 | Unknown | III | None | 202 |
| 17 | 52 | Lobular carcinoma | III | None | 224 |
Discussion
In the present study, we have analysed EDTA-blood or serum samples from 557 patients with ovarian cancer, 253 patients with breast cancer and 200 blood donors for the presence of Yo antibodies by an ITT based immunoprecipitation technique. This is, to our knowledge, the largest material that has been systematically analysed for Yo antibodies.
In the ITT reaction, cdr2 is transcribed using the T7 polymerase which has highly specific sequence requirements, and translated using a rabbit reticulocyte lysate depleted of endogenous mRNA. This makes the protein synthesis very specific and quite similar to in vivo mammalian protein synthesis.
The immunoprecipitation technique was more sensitive in detecting Yo antibodies than IF and immune blots. The increased sensitivity may be due to the use of radioactive labelling as well as lower dilutions of serum or EDTA-blood, which was in the same range as used for the detection of other antibodies such as GAD65 and 21 hydroxylase [13,14]. Furthermore, lower dilution of serum or EDTA-blood samples used in Western blot probably increased the sensitivity of this assay compared to dot blot.
The ability to detect antibodies could also differ slightly between the methods employed: First, proteins in rat cerebellar tissue are detected by human antibodies in IF. The cdr2 sequence in rat is approximately 88% identical to human, which may lead to lack or changes in some of the epitopes. However, within the leucine zipper, which is the only known epitope region so far [15], the rat and human protein sequence are identical. Second, in the immune blots, antibodies detect epitopes in denatured cdr2, while the protein is native in IF and immunoprecipitation. The recombinant proteins used in dot blot are made by expression in E. coli while the recombinant protein made by ITT is expressed in a mammalian cell lysate. Furthermore, the amount of cdr2 in the dot blot and cerebellar homogenate is not available so the cdr2 content on the immune blots probably differ. A third factor is the polyclonal nature of the Yo antibody, which may account for a range of affinities and perhaps also individual variations in avidity of the antibodies. Finally, fluid-phase assays probably have some advantage over solid-phase assays as some common autoantibodies are not reliable detected by solid-phase assays [16,17]. As the results from the different techniques employed were more comparable in detecting Hu antibodies [8,11], we suspect that the cdr2-protein has conformational epitopes as well as linear epitopes.
One of the blood donors (female) harboured Yo antibodies detected by immunoprecipitation. Unfortunately, there is no clinical information on this individual. Yo antibodies were found in 2·3% of patients with ovarian cancer and 1·6% of patients with breast cancer. Yo antibodies have previously been detected in 4 (2·2%) of 181 patients with ovarian cancer [18], which is approximately the same prevalence as we have found in this material by immunoprecipitation. However, in the report of Drlicek et al. [18], the sera were assayed by IF on human cerebellum and Western blot with human Purkinje cell extract, and recombinant cdr2 antigen was not used. When using IF on rat cerebellum and dot blot with recombinant cdr2 protein as antigen, we could only detect Yo antibodies in 0·9% of the ovarian cancer patients.
Recently, we have found that approximately 25% of patients with small cell lung cancer (SCLC) harbour Hu antibodies, detected by immunoprecipitation [8]. In comparison, we found Yo antibodies in only a small percentage of patients with ovarian and breast cancer. The more common prevalence of onconeural antibodies in SCLC is unknown, but may be associated with the neuroendocrine phenotype of this tumour. SCLC is associated with several different PNS and numerous onconeural antibodies [19], and the HuD antigen is expressed by all SCLC tumours [20,21]. On the other hand, gynaecological tumours are associated with fewer PNS and antibodies, of which PCD associated with Yo antibodies is the most common. Cdr2 is expressed by most, but not all, tumours of the ovary and breast [6].
We found that Yo antibodies were less prevalent in the group of patients where blood samples were obtained before or after surgery (ovary group I) compared to the group where all samples were obtained before tumour resection (ovary group II). Prior surgery may have resulted in a decline in antibody levels, resulting in fewer Yo antibody positive patients in this group. This is in line with previous results where Yo antibody levels have been found to decrease after surgery [22]. The assays were performed on EDTA-blood from ovary group I and serum from ovary group II. However, we did not find that this represented a bias as the Yo index was within the same range in the two control groups of serum and EDTA-blood.
Drlicek et al. [18] reported that most of their ovarian cancer patients had serous tumours and advanced disease at the time of diagnosis (FIGO stage III-IV). We found Yo antibodies in various cancer types and FIGO stages, but the highest Yo index was found in the patients with the most advanced disease (FIGO IV). We also found that patients with stage III breast cancer harboured Yo antibodies more frequently than patients with cancer stage I-II. Although the number of Yo positive samples was small, the results suggest that Yo antibodies can be associated with more widespread cancer.
None of the Yo positive patients with breast cancer had PNS. Of the 13 patients with ovarian cancer and Yo antibodies, only two had PCD, both of whom had advanced disease. This observation is in line with a former report [23]. We have recently found that other onconeural antibodies such as anti-Hu or anti-VGCC are not associated with the prognosis of SCLC [8]. Our results support earlier reports [9,18,24], and suggest that Yo antibodies are a more reliable marker of cancer than of PNS.
Acknowledgments
We thank Mette Haugen, Kibret Mazengia and Cecilie Totland for technical help. This study was supported by grants from the Norwegian Cancer Society, Haukeland University Hospital and the University of Bergen, Norway.
References
- 1.Roberts WK, Darnell RB. Neuroimmunology of the paraneoplastic neurological degenerations. Curr Opin Immunol. 2004;16:616–22. doi: 10.1016/j.coi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. A clinical analysis of 55 anti-Yo antibody positive patients. Neurology. 1992;42:1931–7. doi: 10.1212/wnl.42.10.1931. [DOI] [PubMed] [Google Scholar]
- 3.Shams’ili S, Grefkens J, de Leeuw B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies. analysis of 50 patients. Brain. 2003;126:1409–18. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 4.Albert ML, Darnell JC, Bender A, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–4. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 5.Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann Neurol. 2000;47:9–17. [PubMed] [Google Scholar]
- 6.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occurring human tumor immunity, is widely expressed in gynecological tumours. Cancer Res. 2000;60:2136–9. [PubMed] [Google Scholar]
- 7.Graus F, Dalmau J, Rene R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 8.Monstad SE, Drivsholm L, Storstein A, et al. Hu and Voltage-Gated Calcium Channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol. 2004;22:795–800. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56:715–9. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 10.Moll JW, Antoine JC, Brashear HR, et al. Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies: report from the Workshop to the Fourth Meeting of the International Society of Neuro-Immunology on paraneoplastic neurological disease, held October 22–23, 1994, in Rotterdam, The Netherlands. Neurology. 1995;45:1937–41. doi: 10.1212/wnl.45.10.1937. [DOI] [PubMed] [Google Scholar]
- 11.Storstein A, Monstad SE, Nakkestad HL, et al. Paraneoplastic antibodies against HuD detected by a sensitive radiobinding assay. J Neurol. 2004;251:197–203. doi: 10.1007/s00415-004-0303-9. [DOI] [PubMed] [Google Scholar]
- 12.Fathallah-Shaykh H, Wolf S, Wong E, et al. Cloning of a leucine-zipper protein recognized by the sera of patients with antibody-associated paraneoplastic cerebellar degeneration. Proc Natl Acad Sci. 1991;88:3451–4. doi: 10.1073/pnas.88.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falorni A, Örtqvist E, Persson B, Lernmark Å. Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Meth. 1995;186:89–99. doi: 10.1016/0022-1759(95)00139-2. [DOI] [PubMed] [Google Scholar]
- 14.Falorni A, Nikoshkov A, Laureti S, et al. High diagnostic accuracy for idiopathic Addison's disease with a sensitive radiobinding assay for autoantibodies against recombinant human 21-hydoxylase. J Clin Endocrinol Metab. 1995;80:2752–5. doi: 10.1210/jcem.80.9.7673419. [DOI] [PubMed] [Google Scholar]
- 15.Sakai K, Ogasawara T, Hirose G, et al. Analysis of autoantibody binding to 52-kd paraneoplastic cerebellar degeneration-associated antigen expressed in recombinant proteins. Ann Neurol. 1993;33:373–80. doi: 10.1002/ana.410330407. [DOI] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay. results of the Fourth International Workshop on the standardization of Insulin Autoantibodies Measurements. J Clin Endocrinol Metab. 1992;74:1040–4. doi: 10.1210/jcem.74.5.1569152. [DOI] [PubMed] [Google Scholar]
- 17.Schmidli RS, Colman PG, Bonifacio E, et al. High level of concordance between assays for glutamic acid decarboxylase antibodies. The First International Glutamic Acid Decarboxylase Antibody Workshop. Diabetes. 1994;43:1005–9. doi: 10.2337/diab.43.8.1005. [DOI] [PubMed] [Google Scholar]
- 18.Drlicek M, Bianchi G, Bogliun G, et al. Antibodies of the anti-Yo and anti-Ri type in the absence of paraneoplastic neurological syndromes: a long-term survey of ovarian cancer patients. J Neurol. 1997;244:85–9. doi: 10.1007/s004150050054. [DOI] [PubMed] [Google Scholar]
- 19.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronpathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Dalmau J, Graus F, Cheung NK, et al. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75:99–109. doi: 10.1002/1097-0142(19950101)75:1<99::aid-cncr2820750117>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Greenlee JE, Dalmau J, Lyons T, et al. Association of anti-Yo (type I) antibody with paraneoplastic cerebellar degeneration in the setting of transitional cell carcinoma of the bladder. Detection of Yo antigen in tumor tissue and fall in antibody titres following tumour removal. Ann Neurol. 1999;45:805–9. doi: 10.1002/1531-8249(199906)45:6<805::aid-ana18>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Rojas I, Graus F, Keime-Guibert F, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55:713–5. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- 24.Brashear HR, Greenlee JE, Jaeckle KA, Rose JW. Anticerebellar antibodies in neurologically normal patients with ovarian neoplasms. Neurology. 1989;39:1605–9. doi: 10.1212/wnl.39.12.1605. [DOI] [PubMed] [Google Scholar]