Abstract
Dendritic cell (DC) vaccines might induce both anti-tumour immunity and autoimmunity. In this report, we demonstrate elevated levels of anti-nuclear antibody (ANA) in the sera of patients with cancer who had received immunotherapy with a dendritic/tumour-fusion vaccine. Twenty-two patients were treated with DC vaccine of fusion cells composed of autologous DCs and tumour cells (DC/tumour-fusion vaccine), which was generated by treating each cell type with polyethylene glycol. Nine of the 22 patients were treated with both the DC/tumour-fusion vaccine and systemic administration of recombinant human interleukin (rhIL)-12. Serum levels of ANA were examined with an enzyme-linked immunosorbent assay kit. One patient with gastric carcinoma (patient 1, DC/tumour-fusion vaccine alone), one patient with breast cancer (patient 2, DC/tumour-fusion vaccine alone) and one patient with ovarian cancer (patient 3, DC/tumour-fusion vaccine + rhIL-12) showed significant elevations of serum ANA levels during treatment. In patient 1 malignant ascitic effusion resolved and serum levels of tumour markers decreased. Patients 2 and 3 remained in good physical condition during treatment for 24 and 9 months, respectively. Immunoblot analysis indicated antibody responses to autologous tumour cells after vaccination in patient 2. None of the treated patients showed clinical symptoms suggesting autoimmune disease. Patients with elevated serum levels of ANA had significantly longer treatment periods than those without it. Elevated serum levels of ANA after DC/tumour-fusion cell vaccine might be associated with anti-tumour immune response induced by the vaccination.
Keywords: anti-nuclear antibody, cancer vaccine, dendritic cell
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells that control primary and secondary immune responses to various exogenous antigens [1,2]. They also play important roles in establishing anti-tumour immunity and autoimmunity [3,4], both of which are immune responses to self-antigens through breakdown of immune tolerance. Because DCs might be used to induce antigen-specific anti-tumour immunity, various clinical trials of cancer immunotherapy using DC vaccines have been performed [5,6].
The identification of various tumour antigens, the immune responses to which might lead to tumour rejection, has stimulated the development of DC vaccines [7]. The effectiveness of DCs loaded with antigenic peptides of tumour antigens as cancer vaccines, transduced with genes encoding tumour antigens or pulsed with tumour cell lysates, has been investigated [6]. We and others [8–11] have reported that vaccination with fusion cells of dendritic cells and tumour cells (DC/tumour-fusion vaccine) can induce potent preventive and therapeutic anti-tumour immunity.
Although successful therapeutic responses to malignancies have not been achieved with all types of DC vaccines, no severe adverse effects have been reported to date [6,12]. However, DCs have been shown to play an important role in the development of autoimmune disease by activating autoreactive T cells in an antigen-specific fashion or inducing autoantibodies [13–19]. Preclinical studies in animal models suggest that DC vaccines can induce autoimmune disorders [20,21], which would be clinically important adverse effects if DC vaccine were to achieve significant therapeutic effects against malignancies.
Tumour antigens loaded onto DCs are usually derived from non-mutated proteins expressed on both normal and malignant cells [7,22]. Accordingly, if a potent immune response to such antigens is induced by DC vaccine, a destructive autoimmune reaction would also be induced against normal cells expressing the same antigen. In fact, successful immunotherapy with interleukin (IL)-2 against melanomas has resulted in the development of vitiligo [23], and adoptive transfer of gp-100-specific T cells to patients with melanoma on a non-myeloablative condition has led to vitiligo and uveitis [24] which were generated by immune responses to differentiated antigens expressed by melanoma cells and normal melanocytes.
DC/tumour fusion vaccines have several characteristics not seen in other types of DC vaccine. When the fusion cells are generated successfully, whole antigens of tumour cells, including known and unknown tumour antigens and many housekeeping proteins shared with normal cells, would be loaded onto DCs, which does not occur with pulsing of tumour cell lysate to DCs [25]. Consequently, if such a fusion-cell vaccine could induce potent anti-tumour activity, an autoimmune response might occur in treated hosts. We have demonstrated previously that vaccination of mice with fusion cells of DCs and well-differentiated hepatocellular carcinoma cells induces cytotoxic T lymphocytes responsive to syngeneic hepatocytes [26].
Anti-nuclear antibody (ANA) is a serum marker for autoimmune disease [27]. Although the precise significance of ANA in autoimmune diseases remains unclear, because ANA levels may be elevated in people without symptoms of antoimmune disease, elevated serum levels of ANA suggest a local or systemic autoimmune response. In the present study, we examined ANA levels in the sera of patients with cancer treated with DC/tumour fusion-vaccine to determine whether vaccination can induce both an autoimmune response and anti-tumour activity.
Materials and methods
Generation of DC/tumour cell fusion vaccine
We have reported previously the use of polyethylene glycol to generate fusion cells composed of autologous DCs and tumour cells derived from mice or humans [10,28,29]. Briefly, peripheral blood mononuclear cells (PBMCs) were obtained from patients’ blood using Ficoll density-gradient centrifugation (Pharmacia Diagnostics, Uppsala, Sweden). Adherent cells among PBMCs were incubated in a medium containing recombinant human (rh) granulocyte–macrophage colony-stimulating factor (10 ng/ml; BD Biosciences, Bedford, USA, MA, USA), rhIL-4 (10 ng/ml; BD Biosciences) and rh tumour necrosis factor-alpha (10 ng/ml; BD Biosciences) for 10 days. Non-adherent and loosely adherent cells were obtained as a DC-rich fraction [30]. Autologous tumour cells were collected after the enzymatic digestion of tumour tissue obtained at biopsy or surgery. Autologous DCs and irradiated tumour cells were mixed at a ratio of 2 : 1, centrifuged and treated with 50% polyethylene glycol, as described previously [28,31]. After overnight incubation, non-adherent and loosely adherent cells were obtained by gentle pipetting and used as a DC vaccine. The resultant polyethylene glycol-treated cells exhibited characteristics of both DCs and tumour cells [28,31].
Examination for ANA and other autoantibodies in patients’ sera
Sera from the above patients were collected and frozen every 2 weeks and examined for several autoantibodies, including ANA.
ANA in sera was examined using an enzyme-linked immunosorbent assay (ELISA) kit (Japan Lyophilization Laboratory, Tokyo, Japan). Crude nuclear components were obtained from HEp-2 cells and coated on wells in test plates. Antibodies in patients’ sera responding to the nuclear components were detected with peroxidase-conjugated goat anti-human immunoglobulin G. Sera from healthy control subjects showed an optical density (OD) less than 0·1. Levels in serum of an anti-DNA antibody was examined with radioimmunoassay (RIA), of an anti-centromere antibody with ELISA, of an anti-mitochondria M2 antibody with ELISA, of an anti-ribonuclear antibody with ELISA, and of an anti-actin antibody with the immunofluorescence techniques.
Immunoblot analysis
Aliquots of the tumour cell lysate (80 µg protein) were mixed with sample buffer and boiled for 5 min. Proteins were separated by sodium dodecylsulphate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). After blocking in solution containing 25 mM Tris-HCl (pH 8·0), 150 mM NaCl and 0·1% Tween 20 with 10% Difco skim milk (BD Biosciences Diagnostic Systems, Sparks, MD, USA), the membranes were incubated with the serum of each patient (×100 dilution). Then, each membrane was probed for 90 min with peroxidase-conjugated anti-human immunoglobulin (Bio-Rad Laboratories) and analysed with an electrochemiluminscence detection system (Amersham Biosciences, Buckinghamshire, UK).
Results
Patients receiving DC-vaccine showed no clinical features of autoimmune disease
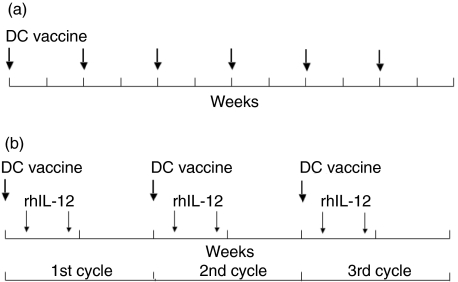
A pilot study for clinical research into cancer immunotherapy with a DC/tumour-fusion vaccine was established and implemented under the Jikei University's Ethics Committee approval no. 10–33 (2678), and performed in compliance with the Declaration of Helsinki. The DC/tumour fusion vaccine was inoculated subcutaneously every 2 weeks for a total of six times (Fig. 1a). The site of inoculation was either the neck or the groin. After performing one course, we evaluated the safety and efficacy of the treatment. Patients who could continue the treatment received a second course.
Fig. 1.
(a) Treatment with dendritic cell (DC)/tumour-fusion vaccine alone. In one course, DC/tumour-fusion vaccine was injected subcutaneously every 2 weeks, for a total of six times in 12 weeks. (b) Treatment with DC/tumour-fusion vaccine plus recombinant human interleukin (rhIL)-12. In the first week, DC/tumour-fusion vaccine was injected subcutaneously on day 1, and rhIL-12 on days 3 and 6 on the same place of the patients’ skin. The second week was a drug holiday. One cycle consisted of these 2 weeks. One course consisted of three cycles.
A pilot study for clinical research into cancer immunotherapy that concomitantly uses DC/tumour fusion-vaccine and rhIL-12 was established and implemented under the Jikei University's Ethics Committee approval no. 12–16 (2774), and performed in compliance with the Declaration of Helsinki. Subcutaneous inoculation of DC vaccine was performed on day 1, and subcutaneous injections of 30 ng/kg of rhIL-12 (Genetics Institute, Cambridge, MA, USA) were given on days 3 and 6. The site of inoculation was either the neck or the groin, and the DC vaccine and rhIL-12 were inoculated in the same place. The following week was made a drug holiday. This 2-week process was one cycle, and three cycles constituted one course (Fig. 1b). After one course had been given, the safety and efficacy of the treatment were assessed. Patients who could continue treatment received a second course [31].
Treatment, either the DC vaccine alone or the DC vaccine + rhIL-12, was stopped immediately if treatment-induced adverse effects more severe than grade 3 developed or if a patient showed significant progressive disease.
Twenty-two patients were treated with DC/tumour fusion vaccine, of whom nine received combination therapy with DC/tumour-fusion vaccine and systemic administration of rhIL-12. None of the 22 patients receiving DC/tumour-fusion vaccine therapy showed significant clinical symptoms indicating autoimmune diseases, neuropsychological symptoms without apparent causes, skin manifestations, joint or muscle symptoms, pleural or ascitic effusions not caused by malignancies or malnutrition, or renal dysfunction or proteinuria not associated with malignancies.
Detection of ANA in the serum of patients receiving DC/tumour-fusion vaccine
Serum levels of ANA were examined with an ELISA detection kit in all patients. Three patients showed elevation of serum levels of ANA more than threefold after treatment (Table 1). Patients 1 and 2 had gastric carcinoma and breast cancer, respectively, and were treated with DC/tumour-fusion vaccines alone. Patient 3 had ovarian cancer and received both a DC/tumour-fusion vaccine and rhIL-12. Two patients (a patient with breast cancer 3 and a patient with colon cancer 4) showed elevation of serum levels of ANA less than threefold (Table 1).
Table 1.
Patients treated with dendritic cell (DC)/tumour-fusion vaccine with or without a combination with recombinant human interleukin (rhIL)-12, and their serum levels of anti-nuclear antibody (ANA) before and after the treatments.
| Anti-nuclear antibody (OD) | ||||||
|---|---|---|---|---|---|---|
| Type of malignancy | Case, age, sex | Total DC (×106) | Total IL-12 (ng) | Treatment period (months) | Before treatment | After treatment |
| Gastric cancer | ||||||
| 1 (patient 1) | 74 years, M | 7·8 | 0 | 4 | 0·03 | 0·13 |
| 2 | 50 years, M | 7·0 | 0 | 6 | 0·1 | 0·07 |
| 3 | 61 years, F | 6·0 | 0 | 5 | 0·1 | 0·09 |
| Breast cancer | ||||||
| 1 | 32 years, F | 3·1 | 8 100 | 1·5 | 0·1 | 0·09 |
| 2 (patient 2) | 60 years, F | 21·8 | 0 | 24 | 0·04 | 0·12 |
| 3 | 54 years, F | 4·0 | 9 000 | 1·5 | 0·06 | 0·15 |
| Ovarian cancer | ||||||
| 1 (patient 3) | 65 years, F | 12·8 | 28 800 | 9 | 0·16 | 0·59 |
| 2 | 54 years, F | 20·7 | 52 104 | 9 | 0·26 | 0·27 |
| 3 | 58 years, F | 26·3 | 15 180 | 3 | 0·04 | 0·09 |
| 4 | 56 years, F | 9·6 | 11 340 | 2 | 0·06 | 0·04 |
| Colon cancer | ||||||
| 1 | 54 years, M | 3·7 | 8 916 | 8 | 0·09 | 0·09 |
| 2 | 31 years, F | 4·5 | 0 | 2 | 0·11 | 0·05 |
| 3 | 31 years, F | 3·1 | 19 965 | 2·5 | 0·14 | 0·1 |
| 4 | 62 years, M | 6 | 19 744 | 3 | 0·05 | 0·12 |
| Others | ||||||
| 1 | 35 years, F | 7·8 | 0 | 2 | 0·09 | 0·07 |
| 2 | 48 years, M | 5·5 | 0 | 1·5 | 0·07 | 0·09 |
| 3 | 54 years, M | 10·5 | 0 | 4 | 0·1 | 0·13 |
| 4 | 38 years, F | 6·8 | 0 | Unevaluable | 0·07 | 0·07 |
| 5 | 58 years, M | 8 | 0 | 3 | 0·07 | 0·06 |
| 6 | 59 years, M | 23 | 0 | 5 | 0·09 | 0·07 |
| 7 | 37 years, M | 18·2 | 0 | 5 | 0·05 | 0·04 |
| 8 | 45 years, M | 5·2 | 0 | 1·5 | 0·05 | 0·04 |
Others 1; pulmonary cancer, 2; oesophagial cancer, 3; hepatocellular carcinoma, 4; tongue cancer, 5; bladder cancer, 6; mesothelioma, 7; epithelioid sarcoma, 8; malignant histeocytosis.
Clinical features of patients with elevated serum levels of ANA
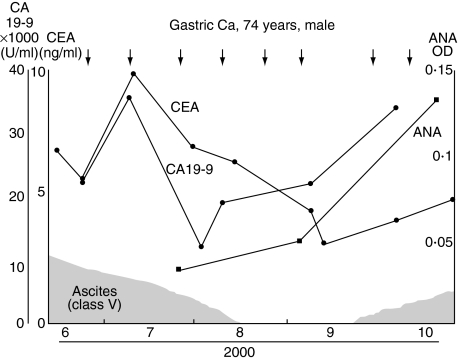
Clinical features and responses to treatment with DC/tumour-fusion vaccine of patient 1 have been reported previously [32]. In this patient, a tumour cell line established from autologous tumour tissue was used to generate the DC/tumour-fusion vaccine. Ascitic effusion caused by peritonitis carcinomatosa decreased markedly with treatment, and serum levels of tumour markers decreased significantly (Fig. 2). The PBMCs of the patient showed significant cytotoxic activity after the treatment with DC/tumour-fusion vaccine [32]. Serum ANA levels increased rapidly after treatment (Fig. 2). However, no clinical symptoms of autoimmune diseases were seen.
Fig. 2.
Changes in serum anti-nuclear antibody (ANA) levels in the clinical course of patient 1, a 74-year-old man with gastric cancer treated with dendritic cell (DC)/tumour-fusion vaccine.
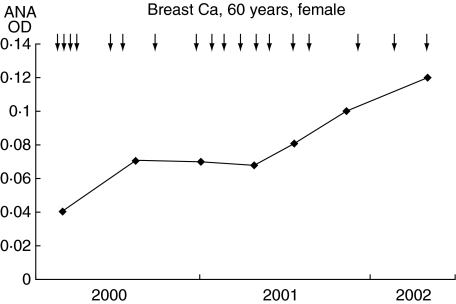
Patient 2 had breast cancer and was treated with a DC/tumour-fusion vaccine for 24 months, during which time her physical condition remained good without other treatments for breast cancer. Before treatment, a metastatic tumour in a cervical lymph node was resected and used as a source of tumour cells to generate a DC/tumour-fusion vaccine. Although serum ANA levels were elevated gradually and significantly (Fig. 3), this patient showed no clinical symptoms suggesting the development of autoimmune disease.
Fig. 3.
Changes in serum anti-nuclear antibody (ANA) levels in the clinical course of patient 2, a 60-year-old woman with breast cancer treated with dendritic cell (DC)/tumour-fusion vaccine.
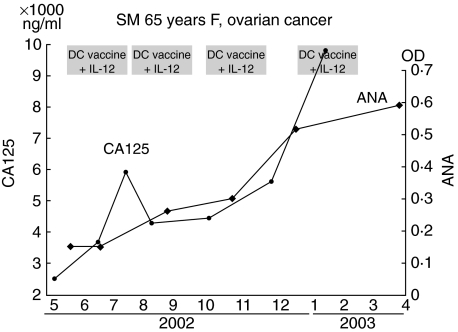
Patient 3 had ovarian cancer and received four courses of combination therapy with DC/tumour-fusion vaccine and rhIL-12. A tumour cell line derived from an autologous tumour had been established before treatment. Although the cancer metastasized to the peritoneum, lymph nodes and liver the patient had maintained a good performance status for 9 months, and serum levels of a tumour marker (CA125) decreased temporarily after treatment (Fig. 4). The patient showed no symptoms suggesting autoimmune disease during treatment.
Fig. 4.
Changes in serum anti-nuclear antibody (ANA) levels in the clinical course of patient 3, a 65-year-old woman with ovarian cancer treated with dendritic cell (DC)/tumour-fusion vaccine and recombinant human interleukin (rhIL)-12.
Cytotoxic activity against autologous tumour cells by PBMCs was induced after vaccine therapy in patient 1, but such activity could not be analysed in patients 2 or 3. No delayed hypersensitivity-like skin reactions were seen at the sites of vaccination in these three patients.
Two other patients with elevated serum levels of ANA (breast cancer 3 and colon cancer 4), but the increase in OD was less than threefold, showed no marked clinical responses to DC/tumour-fusion vaccine.
Examination of other autoantibodies in the three patients with elevated serum levels of ANA
Serum assays for other autoantibodies (anti-DNA antibody, anti-centromere antibody, anti-actin antibody, anti-mitochondria M2 antibody and anti-ribonuclear protein antibody) were negative in all three patients.
Comparison of treatment periods between groups of patients with or without an elevation of serum ANA
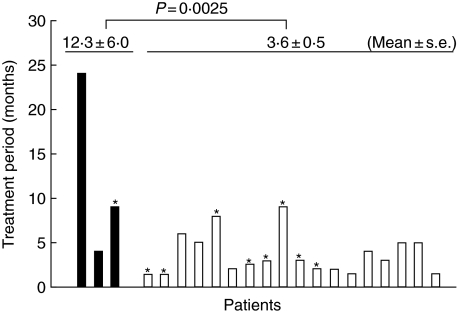
Figure 5 shows the treatment period of each patient who received DC/tumour-fusion cell vaccine. The treatment period of the patients showing elevated serum levels of ANA was significantly longer than that of patients without it (12·3 plus/minus 6·0 versus 3·6 plus/minus 0·5, mean plus/minus s.e., P = 0·025, Student's t-test).
Fig. 5.
Treatment periods of the patients who received dendritic cell (DC)/tumour-fusion cell vaccine with or without administration of recombinant human interleukin (rhIL)-12. Black bars show the patients with elevated serum anti-nuclear antibody (ANA) levels after the treatment, and white bars show the patients without it. *Cases administered with rhIL-12 in addition to the DC/tumour-fusion cell vaccine. Statistical analysis was performed by Student's t-test.
Detection of antibodies to tumour cells by immunoblot analysis
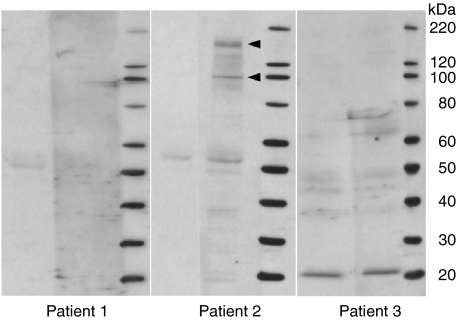
To determine whether antibodies to tumour cells were induced by the DC/tumour-fusion vaccine, the sera of the three patients with elevated ANA levels were examined with immunoblot analysis. Antibodies to tumour cells were present after treatment in patient 2 (Fig. 6).
Fig. 6.
Immunoblot analysis to detect antibodies against autologous tumour cells. Sera from the three patients showing elevated serum levels of anti-nuclear antibody (ANA) after dendritic cell (DC)/tumour-fusion vaccine therapy were examined. Arrows show the bands that appeared after the treatment. Left lane before therapy, and right lane after therapy.
Discussion
The close association between DCs and the development of autoimmune disease has been demonstrated by experiments in animal models of thyroiditis and hyperthyroidism in which disease-associated antibodies were induced by DC vaccination [16,18,19]. However, in other basic studies autoimmune disease was not induced by vaccination with DCs loaded with apoptotic cells or nuclear ribonucleoprotein, despite the induction of autoantibodies against nuclear double-strand DNA or ribonucleoprotein [33,34]. These results suggest that DCs can break down immune tolerance efficiently but that the resulting induction of autoimmunity does not always lead to the development of clinical autoimmune disease.
In the present study, we found that three of 22 patients treated with a DC/tumour-fusion vaccine had elevated serum levels of ANA after treatment, but none exhibited symptoms consistent with autoimmune disease. Because DC/tumour-fusion vaccines are composed of autologous DCs and whole tumour cells many antigens, including nuclear proteins expressed in tumour cells, are probably presented to T cells. Thus, autoimmune reactions to antigens in nuclear components of tumour cells might be induced and lead to elevated serum levels of ANA. However, in a study by Morse [35] immunotherapy with DCs pulsed with carcinoembryonic antigen peptide also increased serum ANA levels in cancer patients without producing autoimmune disease, suggesting that vaccination with DCs might induce ANA. They reported that three of 21 patients showed elevated serum levels of ANA after DC vaccination but did not describe how these elevations were related to clinical responses.
Because a DC/tumour-fusion vaccine would induce immune responses to known and unknown tumour antigens, an elevation of serum ANA levels after treatment might reflect the induction of an effective immune response to tumour cells. Indeed, the three patients with elevated serum levels of ANA showed clinical improvement or prolonged survival. Treatment with DC/tumour-fusion cell vaccine was stopped when the patient showed progressive disease. Therefore, a significantly longer treatment period of the patients with elevated serm levels of ANA suggests that these patients might acquire good anti-tumour immune response by the treatment. Good clinical responses to cancer immunotherapy are reportedly accompanied by both a cellular immune response and a humoral immune response to tumour cells [36]. In patient 2, immunoblot analysis demonstrated that antibodies to autologous tumour cells were generated after DC/tumour-fusion vaccine therapy, suggesting that humoral immunity to autologous tumour cells had been induced efficiently by the DC/tumour-fusion vaccine. We have previously reported that vaccination with DCs loaded with tumour cells by polyethylene glycol-treatment decreased the numbers of tumours in mice that have mutations of adenomatous polyposis coli gene and show spontaneous development of gastrointestinal tumours [29]. This anti-tumour activity is mediated by IgG induced by DC vaccination [29]. Thus, humoral immune responses to tumour cells might be involved in tumour suppression via immunological mechanisms.
Why only three of the 22 patients showed elevated serum levels of ANA after treatment is unclear. Bondanza et al. [37] have reported that renal failure caused by the autoimmune response induced by vaccination of mice with DCs that had phagocytosed apoptotic thymocytes developed only in susceptible mice, but not in normal BALB/c mice. Genetic factors that determine susceptibility to the induction of autoimmune responses might be associated with the ANA response induced by DC/tumour-fusion vaccines.
We have found that vaccination of mice with DCs loaded with well-differentiated hepatocellular carcinoma cells induces autoreactive T cells that can kill syngeneic hepatocytes without producing overt hepatitis [26]. However, when the DC-treated mice are given IL-12, significant hepatic inflammation develops [26]. The up-regulation of adhesion molecule and chemokine expression induced by treatment with IL-12 might be attributed to recruitment of autoreactive T cells into the liver [26]. Patient three received combination therapy with a DC/tumour-fusion vaccine and rhIL-12, but no symptoms of autoimmune disease were seen. This patient received ovarectomy that might avoid the autoimmune response against ovary, or expression of shared antigens on ovarian cancer cells used to generate the DC/tumour-fusion vaccine might not be high enough to induce autoimmune response.
The emergence and elevation of ANA in the sera of patients with cancer treated with DC/tumour-fusion vaccine suggest that DC vaccination could break down immune tolerance and induce both anti-tumour immunity and autoimmunity. However, the results of the present study suggest that autoimmune disease would not be induced in patients receiving DC/tumour-fusion vaccine therapy for malignant tumours despite elevation of serum ANA level. Rather, elevation of serum ANA level might possibly be associated with induction of effective anti-tumour immune response by DC/tumour-fusion cell vaccine.
Acknowledgments
This study was supported (in part) by ‘Bio-Venture Research Fund Project Aid’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman M. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Shurin MR. Dendritic cells presenting tumor antigen. Cancer Immunol Immunother. 1996;43:158–64. doi: 10.1007/s002620050317. [DOI] [PubMed] [Google Scholar]
- 4.Ludewig B, Odermatt B, Landmann S, et al. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 6.Lopez JA, Hart DN. Current issues in dendritic immunotherapy. Curr Op Mol Ther. 2002;4:54–63. [PubMed] [Google Scholar]
- 7.Wang RF. Tumor antigens discovery: perspectives for cancer therapy. Mol Med. 1997;3:716–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Gong J, Chen D, Kashiwaba M, et al. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–61. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 9.Akasaki Y, Kikuchi T, Homma S, et al. Antitumor effect of immunization with fusions of dendritic cells in a mouse brain tumor model. J Immunother. 2001;24:106–13. [PubMed] [Google Scholar]
- 10.Homma S, Toda G, Gong J, et al. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764–71. doi: 10.1007/s005350170019. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Koido S, Chen D, et al. Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice. Clin Immunol. 2001;101:192–200. doi: 10.1006/clim.2001.5112. [DOI] [PubMed] [Google Scholar]
- 12.den Brok MH, Nierkens S, Frigdor CG, et al. Dendritic cells: tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4:699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 13.Palucka AK, Banchereau J, Blanco P, et al. The interplay of dendritic cell subsets in systemic lupus erytheratosus. Immunol Cell Biol. 2002;80:484–8. doi: 10.1046/j.1440-1711.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 14.Simons PJ, Delemarre FGA, Jeucken PHM, et al. Pre-autoimmune thyroid abnormalities in the biobreeding diabetes-prone (BB-DP) rat: a possible relation with the intrathyroid accumulation of dendritic cells and the initiation of the thyroid autoimmune response. J Endocrinol. 1998;157:43–51. doi: 10.1677/joe.0.1570043. [DOI] [PubMed] [Google Scholar]
- 15.Dittel BN, Visintin I, Merchant RM, et al. Presentation of self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–9. [PubMed] [Google Scholar]
- 16.Knight SC, Farrant J, Chan J, et al. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1988;48:277–89. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- 17.Thomas R, MacDonald KP, Pettit AR, et al. Dendritic cells and the pathgenesis of rheumatoid arthoritis. J Leukoc Biol. 1999;66:286–92. doi: 10.1002/jlb.66.2.286. [DOI] [PubMed] [Google Scholar]
- 18.Farrant J, Bryant AE, Chan J, et al. Thyroglobulin-treated blood dendritic cells induce IgG anti-thyroglobulin antibody in vitro in Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986;41:433–42. doi: 10.1016/0090-1229(86)90014-0. [DOI] [PubMed] [Google Scholar]
- 19.Kita-Furuyama M, Nagayama Y, Pichurin P, et al. Dendritic cells infected with adenovirus expressing the thyrotropin receptor induce Graves’ hyperthyroidism in BALB/c mice. Clin Exp Immunol. 2003;131:234–40. doi: 10.1046/j.1365-2249.2003.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludewig B, Ochsenbein AF, Odermatt B, et al. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilboa E. The risk of autoimmunity associated with tumor immunotherapy. Nat Immunol. 2001;2:789–92. doi: 10.1038/ni0901-789. [DOI] [PubMed] [Google Scholar]
- 22.Engelhard VH, Bullock TN, Colella TA, et al. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use of cancer immnotherapy. Annu Rev Immunol. 2003;188:136–46. doi: 10.1034/j.1600-065x.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–4. [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homma S, Komita H, Sagawa Y, et al. Antitumor activity mediated by CD4+CTLs against MHC class II negative hepatocellular carcinoma by dendritic cell vaccine. Immunology. 2005;115:451–61. doi: 10.1111/j.1365-2567.2005.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamaki S, Homma S, Enomoto Y, et al. Autoimmune hepatic inflammation by vaccination of mice with dendritic cells loaded with well-differentiated hepatocellular carcinoma cells and administration of interleukin-12. Clin Immunol. 2005;117:280–93. doi: 10.1016/j.clim.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Reeves WH, Richards HB, Satoh M. Autoantibodies. In: Lahita RG, Chiorazzi N, Reeves WH, editors. Textbook of the autoimmune diseases. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 81–100. [Google Scholar]
- 28.Kikuchi T, Akasaki Y, Irie M, et al. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–44. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iinuma T, Homma S, Noda T, et al. Prevention of gastrointestinal tumors based on adenomatous polyposis coli gene mutation by dendritic cell vaccine. J Clin Invest. 2004;113:1307–17. doi: 10.1172/JCI17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homma S, Kikuchi TN, Ishiji N, et al. Cancer Immunotherapy by fusions of dendritic and tumor cells and rhIL-12. Eur J Clin Invest. 2005;35:279–86. doi: 10.1111/j.1365-2362.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 32.Homma S, Matai K, Irie M, Ohno T, Kufe D, Toda G. Immunotherapy using fusions of autologous dendritic cells and tumor cells showed effective clinical response in a patient with advanced gastric carcinoma. J Gastroenterol. 2003;38:989–94. doi: 10.1007/s00535-002-1183-3. [DOI] [PubMed] [Google Scholar]
- 33.Georgiev M, Agle LM, Chu JL, et al. Mature dendritic cells readily break tolerance in normal mice but do not lead to disease expression. Arthritis Rheum. 2005;52:225–38. doi: 10.1002/art.20759. [DOI] [PubMed] [Google Scholar]
- 34.Suen JL, Chuang YH, Chiang BL. In vivo tolerance breakdown with dendritic cells pulsed with U1A protein in non-autoimmune mice: the induction of high level of autoantibodies but not renal pathological changes. Immunology. 2002;106:326–35. doi: 10.1046/j.1365-2567.2002.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse MA, Deng Y, Coleman D, et al. A phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembrionic antigen. Clin Cancer Res. 1999;5:1331–8. [PubMed] [Google Scholar]
- 36.Relly RT, Emens LA, Jaffee EM. Humoral and cellular immune responses: independent forces or collaborators in the fight against cancer? Curr Opin Invest Drugs. 2001;2:133–5. [PubMed] [Google Scholar]
- 37.Bondanza A, Zimmermann VS, Dell’Antonio G, et al. Cutting edge: dissociation between antoimmune response and clinical disease after vaccination with dendritic cells. J Immunol. 2003;170:24–7. doi: 10.4049/jimmunol.170.1.24. [DOI] [PubMed] [Google Scholar]