Abstract
Factors such as genetic heterogeneity in the immune response contribute to respiratory syncytial virus (RSV) bronchiolitis severity. Such heterogeneity may manifest by an aberrant proliferation of phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) in response to lipopolysaccharide (LPS). The proliferation of PBMC was analysed in 52 infants: 21 ambulatory infants with mild RSV bronchiolitis (group I), 26 hospitalized infants with RSV bronchiolitis on ward (group II) and five intensive care unit (ICU) hospitalized infants (group III). Proliferation was analysed in response to negative control, PHA (LPS) and LPS/PHA. The TLR4 mutations were genotyped using reverse-transcriptase–polymerase chain reaction. The optical density (OD) post-LPS/PHA of group II (1·27 ± 0·63) was significantly higher than group II (0·65 ± 0·38, P = 0·005) or group I (0·63 ± 0·33, P = 0·003), suggesting hyporesponsiveness to the LPS attenuation effect. None of the ICU hospitalized infants demonstrated OD readings post-LPS/PHA under the 0·75 threshold as opposed to group I (67% under 0·75) and group II (69%) (P < 0·05). The responses to negative-control, LPS and PHA stimulation alone were similar across groups. The presence of TLR4 mutations (Asp299Gly and Thr399Ile) were associated with severe RSV bronchiolitis and were significantly over-represented in groups II and III. These findings suggest that impairments of PBMC function manifested by hyporesponsiveness to LPS as well as the presence of TLR4 mutations are associated with an increased risk for more severe RSV bronchiolitis in previously healthy infants. A certain threshold of LPS hyporesponsiveness may have a very high negative predictive value for ICU hospitalization, even better than the determination of known TLR4 mutations for this purpose.
Keywords: lipopolysaccharide (LPS), peripheral blood mononuclear cells (PBMC), respiratory syncytial virus (RSV), viral bronchiolitis
Introduction
Bronchiolitis is an acute infection of the lower respiratory tract that has been associated with respiratory syncytial virus (RSV) [1]. All infants become infected with RSV by the age of 3 years; however, only 1% are hospitalized, and 40–50% of the infants hospitalized will develop recurrent wheezing. Therefore, certain host factors may predispose to more severe illness and hospitalization following RSV infection. It has been suggested that the immune response induced by RSV may contribute to the pathogenesis and severity of the disease [1–4].
The immune response to RSV and specifically the response generated by innate immunity is connected to some properties of this virus, such as proteins F and G. These viral proteins are important in the induction of innate immunity. A major role of innate immunity is to sense pathogens and diverge the adaptive immunity into a Th1/Th2 response through secretion of cytokines and interaction of ligands. The human Toll-like family of proteins was found to be a critical link between immune stimulants produced by microorganisms and initiation of the type of immune response (Th1 versus Th2 response) [2,3,5,6].
Several studies have shown a predominant Th2-type response in infants with viral bronchiolitis [7,8]. Furthermore, the magnitude of disease severity was correlated with the Th2 response [7–9]. Thus, it seems that the more severe the disease, the greater the Th2-like response and the less the Th1-like response.
TLR4, which is one of the innate immunity receptors on the T lymphocytes, has been shown recently to be a major lipopolysaccharide (LPS) as well as RSV F protein receptors. Mutations in mice and humans TLR4 were found to be associated with hyporesponsiveness to LPS and to confer an increased risk of Gram-negative infections [10–12]. In addition to interacting with LPS, the TLR4/CD14 complex interacts with other exogenous and endogenous proteins, such as RSV [13–15]. Interestingly, TLR4-deficient mice had delayed clearance of RSV and a Th2 response, which correlated with disease progression [15].
Moreover, we have shown recently that TLR4 mutations in infants with RSV bronchiolitis were correlated with the magnitude of disease severity; the infants with the mutation causing decreased activity of TLR4 suffered from a more severe RSV infection [16]. As mutations in mouse and human TLR4 were found to be associated with hyporesponsiveness to LPS, we speculated that infants suffering from a more severe disease will express hyporesponsiveness to LPS. This LPS hyporesponsiveness has been shown to be correlated with a decreased Th1 and an increased Th2 response. Therefore, we hypothesized that the PBMC of the more severely affected infants will express hyporesponsiveness to LPS.
The aim of the present study was to investigate the PBMC proliferation response to LPS. LPS is known to inhibit PBMC proliferation. Therefore we measured the LPS inhibition of PBMC proliferation stimulated by a non-specific mitoge, phytohaemagglutinin (PHA).
This was conducted in three different patient populations: out-patients (mild bronchiolitis infection), infants hospitalized in a paediatric ward (moderately ill patients) and infants hospitalized in a paediatric intensive care unit (ICU) (severely ill patients). In addition we determined the frequency of TLR4 mutation in these different groups of RSV-infected infants.
Methods
Study design
This was a prospective one-side blinded study. The primary outcome was to determine the magnitude of PBMC proliferation stimulated by PHA, and the response to LPS inhibition.
These levels were compared between the three groups infected by RSV. Ambulatory infants with URI and/or mild bronchiolitis (group I), hospitalized infants with more severe bronchiolitis (group II) and infants suffering from very severe RSV bronchiolitis hospitalized in the paediatric ICU (group III).
All appropriate infants presenting with acute viral bronchiolitis during the winter of 2003–04 were recruited in the following groups: infants who were hospitalized in the Edith Wolfson Medical Center, Holon and children who presented to the Pediatrics Ambulatory Community Clinics of General Health Services of Bat-Yam and Holon for acute viral bronchiolitis which did not require hospitalization.
Signed informed consents were obtained from the parents of each child, after the human ethics committee (Helsinki) of the Wolfson Medical Center approved the study, as did the National Helsinki committee for genetic research in humans and the Israeli Ministry of Health. The inclusion criterion for the study populations was clinical presentation of viral bronchiolitis. Exclusion criteria for all study population were: cardiac disease, chronic respiratory disease and previous wheezing episode. Patients with RSV bronchiolitis were recruited and examined upon admission or arrival. Patients were re-examined daily until discharge (hospitalized patients) or for 5 days (ambulatory patients) by the investigators. All patients were enrolled within 24 h of admission to the hospital or upon arrival to the ambulatory clinics. Within 24 h of admission or arrival, a blood sample was retrieved by the investigators for further analysis.
RSV antigen detection
Antigen detection was performed by a commercial immunochromatographic assay (ImmunoCard STAT RSV; Meridian Diagnostics Europe, catalogue no. 750930). The sensitivity of the test is 80–90% [1]. The results were obtained 2–3 h after nasopharyngeal aspiration.
Proliferation assay
Whole blood was collected from the infants into heparin-containing syringes and diluted with physiological saline at a ratio of 1 : 1. The diluted blood was layered over a lymphocyte separation medium (LSM, Cappel Co., Aurora, OH, USA) in a centrifuge tube. The tube was centrifuged at 400 g for 30 min at room temperature, the buffy layer containing the PBMC was removed and an equal volume of buffered saline was added. The solution was then centrifuged for 10 min at 200 g. The cells were washed again with buffered saline solution and resuspended in medium and stored under liquid nitrogen until use.
Peripheral blood mononuclear cells (PBMC) were thawed and cultured in concentration of 106 cells per 1 ml RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal calf serum (FCS). The cells were then aliquoted and stimulated with PHA (5 µg/ml), LPS (100 ng/ml) or a combination of PHA (5 µg/ml) and LPS (100 ng/ml) and without mitogens (negative controls) for a final volume of 200 µl in a 96-well plate. All experiments were performed in triplicate cultures. The viable cells were measured with the cell proliferation kit (XTT) (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. Briefly, after incubation for 3 days, 50 µl XTT labelling mixture was added to each well. After 6 h incubation at 37°C, the optical density (OD) was measured at 450 nm.
Four different assays were analysed: (1) negative control, no stimulation; (2) the PHA group, after stimulation with PHA; (3) the LPS group, after stimulation with LPS; and (4) the LPS/PHA group, after combined stimulation with both LPS and PHA.
TLR4 genotyping
All subjects were genotyped for mutations in the TLR4 gene (Asp299Gly and Thr399Ile) using an ABI PRISM 7900HT Sequence Detection System as described previously [16].
Statistical analysis
Each variable was scanned visually for normalcy of distribution. Skewed variables were log-transformed prior to analysis. All continuous variables were examined using the two-tailed unpaired t-test as appropriate. Non-continuous variables were examined using the χ2 test or Fisher's exact test as appropriate. The mean ± s.d. express the central tendency of the data. P < 0·05 was considered significant.
Results
Fifty-three patients (mean age, 3·7 months ± 1·8) were recruited and their blood samples were analysed successfully. Twenty-one samples were of ambulatory patients (group I, mean age, 4·05 ± 1·9 months of hospitalized infants (group II, mean age, 3·7 months ± 1·5) and five of the ICU group (group III, mean age, 2·8 ± 2·4 months). There was no statistically significant difference in age distribution among the groups.
Results of the main primary outcome
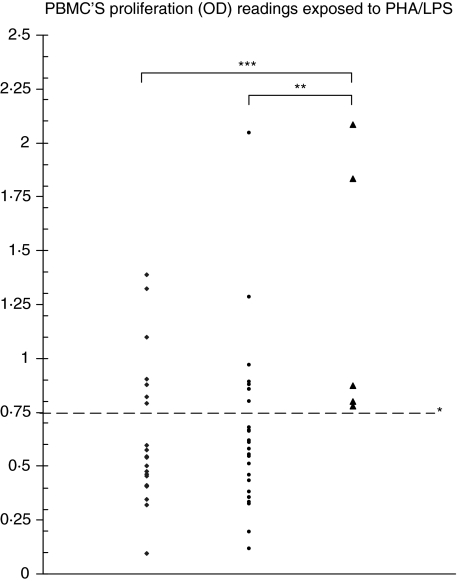
The magnitude of LPS inhibition of PBMC proliferation stimulated by PHA in the three groups of patients is given in Table 1 and Fig. 1. LPS inhibition of PBMC proliferation stimulated by PHA was attenuated significantly in the ICU hospitalized infants compared to both ambulatory and ward hospitalized infants: P = 0·003 and 0·005, respectively. There were no statistical differences among the groups in the response to negative controls, and to LPS and PHA stimulation alone.
Table 1.
Peripheral blood mononuclear cells (PBMC) proliferation [optical density (OD)] readings across groups and stimulations.
| Ambulatory (group I) (n = 21) | Hospitalized on ward (group II) (n = 26) | Hospitalized on ICU (group III) (n = 5) | P | |
|---|---|---|---|---|
| PHA/LPS (PHA + lPS) | 0·63 ± 0·33 | 0·65 ± 0·38 | 1·27 ± 0·63 | 0·003*, 0·005** |
| PHA (non-specific mytogen) | 1·00 ± 0·51 | 1·06 ± 0·42 | 1·13 ± 0·41 | n.s. |
| LPS | 0·35 ± 0·38 | 0·37 ± 0·24 | 0·45 ± 0·26 | n.s. |
| Negative control | 0·74 ± 0·50 | 0·83 ± 0·40 | 0·95 ± 0·54 | n.s. |
The magnitude of PBMC proliferation is given in all patient groups. LPS inhibits proliferation. However, this inhibition in PHA-stimulated PBMC (PHA/LPS) is attenuated significantly in infants suffering from very severe respiratory syncitial virus bronchiolitis hospitalized in intensive care unit (ICU) (group III). This is interpreted as LPS hyporesponsiveness in group III.
For the difference between ICU versus ambulatory infants;
for the difference between ICU versus ward infants. n.s.: Not significant.
Fig. 1.
Peripheral blood mononuclear cells proliferation [optical density (OD)] readings exposed to phytohaemagglutinin/lipopolysaccharide (PHA/LPS). All patients from group III compared with only 33% of group I and group II patients had an OD reading above 0·75*. This threshold had a 100% negative predictive value and 40% positive predictive value for intensive care unit (ICU) hospitalization. **P = 0·005 for the difference between ICU versus ward infants; ***P = 0·003 for the difference between ICU versus ambulatory infants. ♦, Group I, ambulatory; •, group II, on ward; ▴, group III, ICU.
TLR4 distribution in the three patient populations is given in Table 2. TLR4 mutations were significantly over-represented in groups of patients with a relatively severe illness compared to patients with a milder form of RSV infection.
Table 2.
TLR4-mutation distribution across groups.
| Ambulatory (group I) (n = 21) | Hospitalized on ward (group II) (n = 26) | Hospitalized on ICU (group III) (n = 5) | P | |
|---|---|---|---|---|
| TLR4-mutation | 0/21 | 4/26 | 2/5 | < 0·05*,**,*** |
TLR4 mutations were over-represented significantly in groups of patients with a relatively severe illness than in patients with a milder form of respiratory syncitial virus infection.
Difference between intensive care unit (ICU) versus ambulatory infants;
difference between hospitalized versus ambulatory infants;
difference between ICU versus hospitalized infants.
Distribution of OD values following LPS/PHA
The distribution of these values revealed that all patients from group 3 compared with only 33% of group I and group II patients had an OD reading above 0·75 (Fig. 1, P < 0·01 for the difference between group 3 and groups 1 and 2). This threshold had a 100% negative predictive value and 40% positive predictive value for ICU hospitalization.
There was no association between the presence of TLR4 mutation and the magnitude of LPS proliferation inhibition of the PHA-stimulated PBMC.
Discussion
The main finding in our study was decreased LPS sensitivity of stimulated mononuclear cells in patients with severe RSV bronchiolitis (group III: infants admitted to the ICU) than the rest of the patients (treated as ambulatory patients or admitted to the paediatric ward: groups I and II).
We have shown that LPS inhibition of PBMC proliferation stimulated by PHA is attenuated significantly in the ICU hospitalized infants than both ambulatory and ward hospitalized infants: P = 0·0067 and 0·03, respectively.
As LPS is known to inhibit PBMC proliferation [17], this attenuation is interpreted as hyporesponsiveness to LPS in the more severely affected ICU hospitalized infants.
Hyporesponsiveness to LPS has been shown to be correlated with decreased TH1 and increased TH2 response [7–9]. Because it has been demonstrated that the more severe the disease, the more Th2-like responses and less Th1-like responses are recruited [7–9] than in a more severe disease, there should be a lesser response to LPS (hyporesponsiveness) by the PBMC. Indeed, this is what we found: the PBMC of the most severely affected infants (ICU hospitalized) expressed hyporesponsiveness to LPS.
While hyporesponsiveness to LPS was detected in ICU hospitalized patients, we could not demonstrate such a response in the ward hospitalized infants. It could be speculated that hyporesponsiveness to LPS may account for the susceptibility of severely affected infants to RSV infection (such as ICU hospitalized patients), while other factors predispose moderately ill infants (such as ward hospitalized patients) to RSV bronchiolitis.
In the present study, the differential attenuated response to LPS was not evident following the addition of LPS to non-primed mononuclear cells but was unveiled after the stimulation of these cells with a non-specific mitogen, PHA.
There are several possible explanations for the difference in LPS response found in our study. First, an age-dependent response to LPS might have accounted for these results. However, the age of patients admitted to the ICU was not significantly different from the age of the rest of the patients. Secondly, patients admitted to the ICU are evidently more ill and may have temporary immune depression. However, the response of the mononuclear cells of infants admitted to the ICU to PHA was not different from the rest of the patients, which suggests specific hyporesponsiveness to LPS.
A third explanation for our finding is that the response to LPS is an indication of aberrant TLR4 activation. Indeed, it has been demonstrated previously that respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin [18]. We have shown previously that two specific mutations in TLR4 resulted in an increased risk for severe RSV bronchiolitis [16]. Indeed, in the present study we found that 40% of infants admitted to the ICU had this mutation: a significantly higher rate than in patients admitted to the ward and ambulatory patients (15% and 0%, respectively). These results confirm our latest findings that TLR4 mutations are associated with an increased risk of ward hospitalization of previous healthy infants with RSV bronchiolitis [16].
However, the known TLR4 mutations were present only in a minority of patients admitted to the ward or the ICU; therefore there are more factors that predispose for severe RSV infection. These as yet unknown, possibly intermittent factors may be manifested with LPS hyporesponsiveness (associated or not associated with different TLR4 polymorphism).
Thus, it may be speculated that PBMC of the most severely affected ICU infants are inherently more tolerant to LPS during RSV infection and therefore exert a diminished response to LPS ex-vivo, as shown in our study.
Moreover, in our assay a certain threshold of LPS hyporesponsiveness proved to be superior to genetic identification of the known TLR4 mutation in the identification of patients with the most severe RSV infections (P < 0·01). In our small sample of patients, this threshold had a 100% negative predictive value and 40% positive predictive value for ICU hospitalization.
This assay, if reproduced with higher numbers of patients, may be proved to be a useful tool for the identification of high-risk patients during RSV infection.
Therefore, we speculate that the hyporesponsiveness of mononuclear cells to LPS represents, at least in part, susceptibility to most severe RSV infection and that TLR4 mutations are associated with an increased risk for moderate to severe RSV infection (RSV infection that requires hospitalization) in previously healthy infants.
It is possible that other factors associated or not associated with different TLR4 polymorphisms are also important in discrimination between these three patient populations. Additional factors and more gene expressions will be proved to be involved in this issue. As RSV bronchiolitis is probably a multi-factor disease, more association studies are needed to clarify this issue.
Acknowledgments
The authors would like to thank Mona Boaz PhD, the bio-statisticians at the Edith Wolfson Medical Center, Holon, for the advice on the statistical analyses and the Israel Lung Association, Tel Aviv for financial support.
References
- 1.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- 3.Dabbagh K, Lewis DB. Toll-like receptors and T-helper-1/T-helper-2 responsiveness. Curr Opin Infect Dis. 2003;16:199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1979;89:405–21. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 5.Graham BS, Perkins MD, Wright F, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–62. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 6.Alwan WH, Record FM, Openshaw PJM. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus infection in mice. J Immunol. 1993;150:5211–8. [PubMed] [Google Scholar]
- 7.Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-γ expression in peripheral blood mononuclear cells of small children with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–8. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 8.Roman M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in small children is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 9.Bont L, Kavelaars A, Heijnen CV, Van Vught AJ, Kimpen JL. Monocyte interleukin-12 is inversely related to duration of respiratory failure in respiratory syncytial virus bronchiolitis. J Infect Dis. 2000;181:1772–5. doi: 10.1086/315433. [DOI] [PubMed] [Google Scholar]
- 10.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with Gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 12.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 13.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates PBMC chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167:2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 14.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tal G, Mandelberg A, Dalal I, et al. The common Toll-like receptor 4 mutations are associated with severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–63. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 17.Yaqub S, Solhaug V, Vang T, et al. A human whole blood model of LPS-mediated suppression of T cell activation. Med Sci Monit. 2003;9:BR120–6. [PubMed] [Google Scholar]
- 18.Monick MM, Yarovinsky TO, Powers LS, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to LPS. J Biol Chem. 2003;278:53035–44. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]