Abstract
Atopic dermatitis (AD) is a common, fluctuating skin disease that is often associated with atopic conditions such as asthma and IgE-mediated food allergy and whose skin lesions are characterized by a Th-2 cell-mediated response to environmental antigens. The increasing prevalence and severity of atopic diseases including AD over the last three decades has been attributed to decreased exposure to microorganisms during early life, which may result in an altered Th-1/Th-2-balance and/or reduced T cell regulation of the immune response. Patients with AD exhibit defects in innate and acquired immune responses resulting in a heightened susceptibility to bacterial, fungal and viral infections, most notably colonization by S. aureus. Toxins produced by S. aureus exacerbate disease activity by both the induction of toxin-specific IgE and the activation of various cell types including Th-2 cells, eosinophils and keratinocytes. Allergens expressed by the yeast Malazessia furfur, a component of normal skin flora, have also been implicated in disease pathogenesis in a subset of AD patients.
Microorganisms play an influential role in AD pathogenesis, interacting with disease susceptibility genes to cause initiation and/or exacerbation of disease activity.
Introduction
Atopic dermatitis (AD) is a common, chronic fluctuating skin disease with prevalence in children of between 7 and 17%. The disease presents commonly within the first 2 years of life, and in approximately two-thirds of cases will persist into adulthood. A high proportion of infants with AD have a positive family history of AD, asthma or allergic rhinitis in one or both parents, and will go on to develop further atopic complications later in childhood. AD is a multifactorial disease in which both hereditary and environmental factors play a role. Four chromosomal regions of linkage to the disease, which differ from atopy-associated loci, have been identified in genome scans of children with AD [1,2]. Interestingly, these linkage sites closely correspond to four loci reported to contain susceptibility genes for psoriasis suggesting that they may contain genes that control skin inflammation and immunity in both diseases [2]. In addition, several candidate genes coding for proteins with immunological function, such as the high affinity IgE receptor present on mast cells, Th-2 cytokines, RANTES and IRF2 [3–8], and receptors involved in the response to microorganisms, CARD4/Nod1, CARD15/Nod2, TLR2 and TLR4 [9–11] have been implicated in AD. Environmental factors that interact with susceptibility genes in AD, inducing the production of IgE antibodies and activation of Th-2 cells, include foods, house dust mite (HDM) allergens, secondary microbial infections and stress. This review focuses on the role of microorganisms in AD, and discusses the mechanisms involved in the prevention, initiation and exacerbation of disease activity by microbial components.
The hygiene hypothesis
Over the last 30 years there has been a continuously increasing prevalence of AD and other atopic allergic disorders, especially in western industrial countries where it can be as high as 20–37% of the population [12]. Environmental factors such as increased air pollution, indoor exposure to HDM antigens in less ventilated modern homes, and dietary changes have been implicated, but there is little consistent evidence that these factors account for the observations. A more relevant factor appears to be a reduction in the exposure to infections during early childhood, which may result from modern lifestyle factors such as the use of antibiotics, a reduction in family size (allergic sensitization is higher in the first-born, but is less frequent in children from large families), and an increase in hygiene and living standards [13]. The relationship between decreased exposure to microbial antigens associated with a western lifestyle and the increasing severity and prevalence of atopic diseases has become known as the ‘hygiene hypothesis’ [14]. Innate immune cells, such as macrophages and dendritic cells, express pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) associated with microorganisms; activation of these receptors induces a Th-1 type response. It was therefore suggested that a lack of microbial antigen-induced immune deviation from the Th-2 cytokine profile that predominates at birth to a Th-1 type profile could explain the development of enhanced Th-2 cell responses to allergens (Fig. 1) [15]. However, this explanation did not did not take into account the fact that chronic parasitic worm (helminth) infections which induce strong Th-2 responses and high IgE levels are not associated with allergy, or that the prevalence of Th-1-associated autoimmune diseases have also increased at the same time as allergic diseases [16].
Fig. 1.
Two possible mechanisms for the increased prevalence of allergy as a result of decreased exposure to microbial antigens.
Subsequently, a second mechanism was proposed for the switch to an atopic phenotype; a defect in the stimulation of dendritic cells by nonpathogenic microorganisms in gut-associated lymphoid tissue leading to reduced production of IL-10-producing regulatory T cells (Fig. 1) [16,17]. Differences in the bacterial colonization of the gut have been reported in children with AD; Enterococci and Bifidobacteria were reduced, and Clostridia and Staphylococcus aureus numbers increased [18,19]. These changes have been attributed, at least in part, to a frequent use of antibiotics, which has consistently been shown to markedly increase the risk of developing AD, even into the antenatal period [20]. This has led to the use of diet supplementation with Lactobacillus (marketed as probiotics) in both the prevention and treatment of AD [20]. A randomised, controlled trial in which oral Lactobacillus CG supplementation was given prenatally to mothers with an allergic family history, and postnatally for 6 months to their offspring, resulted in an almost 50% reduction in AD frequency [21]. Furthermore, a significant reduction in SCORAD (disease severity scoring of atopic dermatitis) after one month of Lactobacillus GG supplementation was observed in a randomised controlled trial of 31 infants with AD and cow's milk intolerance [22]. It was subsequently demonstrated that L. reuteri and L. casei prime monocyte-derived dendritic cells, via DC-SIGN (DC-specific intercellular adhesion molecule 3-grabbing nonintegrin), to drive the development of IL-10-producing regulatory T cells [23]. These findings suggested a possible mechanism for the beneficial effects of Lactobacillus administration in AD, and provided support for the proposal that reduced T cell regulatory activity may be responsible for the increased prevalence of allergy.
Although a recent review of 64 studies published between 1966 and August 2004 failed to find any convincing evidence that exposure to a specific infection such as tuberculosis reduces the risk of AD, there was, in contrast, some evidence that common viral and bacterial childhood infections were positively associated with an increased risk of AD expression [20]. However, a protective effect was associated with endotoxin exposure in a farming environment, day care attendance or having a dog during infancy, situations in which chronic (nonpathological) microbial stimulation may take place. Thus it is possible that the effects of infections in early childhood on the development of AD may depend partly upon the nature and degree of exposure, rather than infection per se. Clearly further research into the early priming of a child's immune system by microorganisms is necessary in order to establish the factors required to induce the development of AD.
Microbial colonization of AD skin
Patients with AD are highly susceptible to certain cutaneous bacterial, fungal and viral infections [24]. AD patients have an increased incidence of warts caused by the human papillomavirus, and of cutaneous fungal infections such as that caused by Trichophyton rubrum. They are particularly susceptible to severe infections caused by herpes simplex type 1 virus (eczema herpeticum or Kaposi's varicelliform), vaccinia virus (eczema vaccinatum) coxsackieA virus and molluscum contagiosum virus. These viral infections can represent serious complications in AD, and if not treated promptly have the potential to be life threatening. However, bacterial colonization with Staphylococcus aureus is the most common skin infection in AD (> 90% of patients compared to 5% of normal individuals) and occurs on both lesional and, to a lesser extent, nonlesional AD skin [25,26]. Furthermore, there appears to be a causative relationship between the numbers of bacteria present on the skin and the severity of disease in AD patients, whilst treatment-induced removal of the bacteria is associated with improvement in skin lesions in most cases [27,28]. Various factors are involved in the altered skin colonization by S. aureus in AD including an altered epidermal barrier, increased bacterial adhesion, defective bacterial clearance, and decreased innate immune responses.
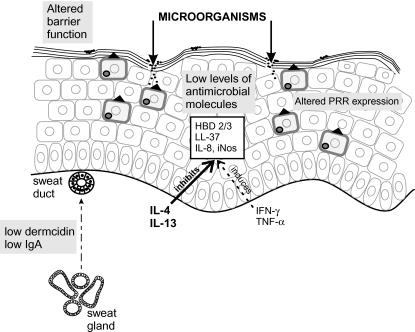
S. aureus are tightly attached to the uppermost corneocytes, and can penetrate the epidermis via the intercellular spaces probably as a result of lipid deficiencies in AD skin. In AD, the average pH of the skin is slightly more alkaline, and sphingosine levels are decreased in both lesional and nonlesional stratum corneum [29,30]. In addition, the dryness and cracking of AD skin, as a result of transepidermal water loss caused by altered lipid content, may facilitate bacterial colonization. Furthermore, Th-2 cytokines such as IL-4 in atopic skin increase expression of fibronectin and fibrinogen, receptors that mediate the adhesion of S. aureus to stratum corneum [31]. In a proportion of AD patients who respond poorly to anti-inflammatory treatment, persistent S. aureus colonization is associated with higher total IgE levels suggesting that IgE may contribute to an increased susceptibility to infection [28]. Indeed, IgE has been shown to inhibit neutrophil adhesion, phagocytosis and respiratory burst, which may affect clearance of microorganisms from the skin [32]. A major factor in increased S. aureus colonization of AD skin is a defective cutaneous innate immune response, involving decreased production of antimicrobial peptides and the expression of functional variants of the TLR and Nod/CARD receptors for microbial components (Fig. 2).
Fig. 2.
Defective innate immunity in AD skin.Altered intracellular and extracellular PRR expression, and decreased antimicrobial molecule production (partly due to inhibition by Th-2 cytokines) results in an impaired innate immune response to microorganisms, which gain entry to the epidermis as a result of an altered barrier function. HBD-2/HBD-3, human β-defensin-2/3; LL-37, a member of the cathelicidin family; iNos, induced nitric oxide synthetase.
Defective innate immunity in AD: antimicrobial peptides
The innate immune system of the epidermis is the first line of defence against invasion by microorganisms, which gain entry after the skin is damaged. Anti-microbial peptides form part of this defence system, three of which are triggered by injury or inflammation of the skin: the β defensins HBD-2 and HBD-3, and a cathelicidin, hCAP18/LL-37 [33]. HBD-2 shows microbicidal activity against predominately Gram-negative organisms such as E. coli and P. aeruginosa, and yeasts, but is relatively ineffective against Gram-positive bacteria such as S. aureus [34]. In contrast, HBD-3 and hCAP18/LL-37 are more potent, broad-spectrum antibiotics that kill both Gram-positive and Gram-negative organisms and the yeast C. albicans [35,36]. In addition, HBD-2 can enhance the innate immune response of the epidermis, and provide a link with the acquired immune response by inducing up-regulation of costimulatory molecules and the maturation of immature dendritic cells in a TLR4-dependent manner [37]. Beta-defensins can also act as chemoattractants for immature dendritic cells and memory T lymphocytes via the chemokine receptor CCR6 [38].
Immunostaining, measurement of specific mRNA by real-time reverse-transcriptase-PCR or GeneChip microarray analysis for HBD-2, HBD-3 and hCAP18/LL-37 in acute and chronic lesions from patients with AD showed a significant decrease in expression as compared to that of psoriasis patients [39,40]. IL-4 and/or IL-13, has been shown to suppress the TNF-α or IFN-γ-induced up-regulation of HBD-2 and HBD-3 mRNA in keratinocytes and normal skin explants suggesting that the reduced levels of antimicrobial peptides may be explained by the predominance of Th-2 type cytokines in AD skin lesions [40]. Clinical isolates of S. aureus from AD patients can be killed by a combination of HBD-2 and hCAP18/LL-37 at the concentrations found in psoriatic lesions, but the levels present in AD skin are too low to be effective.
The innate skin defence system of patients with AD is further compromised by a deficiency of dermcidin-derived antimicrobial peptides in sweat, which correlates with infectious complications [41]. Dermcidin, a peptide with no homology to other antimicrobial peptides, is specifically and constitutively expressed in sweat glands in the dermis of skin, secreted into sweat and transported to the epidermal surface [42]. In common with HBD-3 and hCAP18/LL-37, dermcidin has a broad spectrum of activity against a variety of pathogenic microorganisms. In healthy individuals, a significant reduction in viable bacterial cells on the skin surface occurs after sweating, but this is not the case in patients with AD [41]. Furthermore, the rate of sweat production, and the secretion of IgA in sweat are reduced in AD patients contributing to the impaired innate immune response [43,44].
The expression of the innate immune response genes, IL-8 (CXCL8) and induced nitric oxide synthetase (iNos) was also decreased in AD compared to psoriatic skin [40]. IL-8 is a chemokine that attracts polymorphonuclear leucocytes into the skin where they phagocytose and kill bacteria, whilst iNos can kill viruses, bacteria and fungi through production of nitric oxide. In common with the antimicrobial peptides, production of IL-8 and iNos is also inhibited by Th-2 cytokines.
Defective innate immunity in AD: TLR and Nod/CARD proteins
Toll-like receptor 2 (TLR2) and TLR4 are members of a family of PRRs that recognize various conserved microbial components or PAMPs. TLR2 recognizes components of Gram-positive bacteria and yeasts, such as lipotechoic acid, peptidoglycan (although recent evidence suggests that this may be contaminating lipoteichoic acid [45]), lipoproteins or zymosan, whilst the PAMPs detected by TLR4 include the Gram-negative bacterial component, LPS [46]. Recognition of PAMPs by TLRs initiates a signalling cascade that results in the production of proinflammatory cytokines, chemokines, antimicrobial peptides and inducible enzymes in the skin, via activation of transcription factors, activator protein (AP)-1 and nuclear factor (NF)-κB [47]. Two single nucleotide polymorphisms (SNPs) have been described for each of the receptors, which result in changes in amino acid sequences. One of the TLR2 polymorphisms (Arg753Gln), which is located within the intracellular part of the receptor and has been particularly associated with S. aureus infections, was found to be present in a higher frequency in AD patients compared to controls [11]. The subgroup of AD patients carrying this polymorphism had increased disease severity characterized by markedly elevated IgE antibodies to S. aureus superantigens and HDM allergens. In addition, a further subgroup of AD patients expressed a higher frequency of the two TLR4 polymorphisms, Asp299Gly and Thr399Ile, than controls [11]. These cosegregating polymorphisms, located in the extracellular domain of the receptor, have previously been reported in patients with septic shock, particularly that induced by Gram-negative bacteria, and are linked to LPS hyporesponsiveness [48].
Intracellular PRRs are represented by the Nod (nucleotide-binding oligomerization domain) family, which includes Nod2/caspase recruitment domain containing protein (CARD) 15 and the closely related Nod1/CARD4 protein. Both proteins detect peptidoglycan, the major component of the bacterial cell wall, although the specificities of the two receptors are distinct and nonoverlapping [49]. Polymorphisms in the Nod2/CARD15 gene that result in changes in peptidoglycan recognition have been reported to be associated with susceptibility to Crohn's disease, a Th-1-mediated inflammatory disease of the bowel [50,51]. Three of these Crohn’s-associated Nod2/CARD15 polymorphisms have been investigated in children with asthma and allergy by PCR-based restriction enzyme assays [10]. Children with the polymorphic C2722 allele had a more than 3-fold risk to develop allergic rhinitis and an almost 2-fold risk for AD. More recently, an association of Nod1/CARD4 polymorphisms with AD have been reported in a study that examined the effects of 11 SNPs, covering the complete gene, on atopy phenotypes [9]. One Nod1/CARD4 haplotype and three polymorphisms (rs2907748, rs2907749, rs2075822) were significantly associated with AD in a population-based cohort, case-control population, and/or family–based association analysis. These polymorphisms were also associated with asthma and total serum IgE levels, but not with allergic rhinoconjunctivitis or specific sensitization.
Peptidoglycan from S. aureus has been shown to induce the production of various keratinocyte-derived mediators including GM-CSF, a cytokine that is overproduced in AD skin lesions [52]. It remains to be established whether this occurs via PRR stimulation; however, keratinocytes are known to express several members of the TLR family [47], and intracellular Nod/CARD receptors may also be present in these cells.
Thus the reduced production of antimicrobial peptides and other innate immune factors, together with an impaired recognition of microbial antigens as a result of functional polymorphisms in the genes coding for PRRs, are major factors contributing to the susceptibility to infection and resulting exacerbation of disease activity in patients with AD.
Cellular activation by staphylococcal superantigens
The main consequence of increased colonization of AD skin by S. aureus is exacerbation of the inflammatory immune response, which is largely mediated by the release of staphylococcal enterotoxins (SE), such as SEA, SEB and toxic shock syndrome toxin (TSST)-1, also referred to as ‘superantigens’ [53] (Table 1). SEB applied to intact normal skin or the nonlesional skin of patients with AD can induce erythema and dermatitis, and, in some AD patients, a flare of their disease in the elbow flexure of the same arm to which the toxin was applied [54]. Furthermore, 14 of 68 patients recovering from toxic shock syndrome caused by TSST-1, but no patients recovering from Gram-negative sepsis, developed chronic eczematous dermatitis [55]. These findings suggest that superantigens can initiate, exacerbate and maintain inflammation associated with AD.
Table 1.
Role of staphylococcal superantigens in AD.
| Superantigen-induced effects | Functional consequences |
|---|---|
| Application of SEB to uninvolved skin | Induces local erythema and dermatitis |
| Sometimes causes disease flare | |
| Superantigen-specific IgE production | Release of histamine by mast cells and basophils |
| Facilitates toxin presentation by Langerhans cells to T cells | |
| Correlates with disease severity | |
| SEC1-specific IgG2 production | Decreased in AD; may affect phagocytosis of S. aureus by |
| polymorphonuclear leucocytes | |
| Selective Vβ family expansion in lesional skin and blood | Activation of Th-2 cells in a Vβ-specific manner |
| CLA expression on activated Th-2 cells, circulating and in lesional skin | Induces homing of T cells to skin |
| Apoptosis of superantigen-reactive T cells | SEB-reactive Th-2 cells in AD are apoptosis-resistant, thus helping to maintain information |
| Inhibition of CLA+CD4+CD25+ regulatory T cell activity | Increases inflammation |
| Dermal eosinophils | Inhibits apoptosis, increases expression of surface activation antigens and enhances oxidative burst |
| Langerhans cells and macrophages | Stimulates production of IL-1, TNF-α and IL-12 |
| HLA-DR+ keratinocytes | Transient Ca2+ mobilization |
| TNF-α production | |
| Presentation of superantigen to Th-2 cells |
The major effects of staphylococcal superantigens in AD are likely to be mediated via the polyclonal activation of superantigen-specific TCR Vβ families of T cells. T cells expressing Vβ chains specific for the superantigen accumulate selectively in superantigen-treated skin, but not in skin treated with sodium lauryl sulphate, supporting this hypothesis [56]. In the peripheral blood of AD patients whose skin is colonized by superantigen-secreting S. aureus, a relevant skewing of superantigen-reactive Vβ families was observed in CD4+ and CD8+ T cells coexpressing cutaneous lymphocyte antigen (CLA), a skin homing receptor [57]. Superantigens up-regulate CLA expression by T cells via stimulation of IL-12 production, thus promoting their homing to the skin [58].
T cell activation by superantigens may be further augmented by an inhibitory effect (at least by SEB) on the immunosuppressive activity of circulating CLA+CD4+ CD25+ regulatory T cells, which, surprisingly, are increased in patients with AD [59]. Furthermore, SEB-reactive (Vβ3+, Vβ12+ or Vβ17+) CD4+ T cells producing Th-2 cytokines in AD patients are more resistant to SEB-induced apoptosis than corresponding SEB-reactive Th-1 cells from healthy individuals [60].
In addition to T cells, superantigens can also mediate effects on other cell types such as eosinophils, Langerhans cells, macrophages and keratinocytes. During flares of AD, eosinophils are recruited to the skin by chemoattractants such as RANTES (regulated on activation, normal T expressed and secreted) and eotaxin, where they are activated and undergo degranulation and cytolytic degeneration, the products of which promote inflammation and tissue damage [61,62]. It has been demonstrated that SEB in AD skin lesions is localized predominately to eosinophils in the dermis, as well as to a lesser extent on Langerhans cells and IgE-bearing cells [63]. Superantigens modulate the effector function of eosinophils, and probably the course of AD, by inhibiting eosinophil apoptosis, increasing expression of activation antigens on the eosinophil surface, and enhancing oxidative burst of eosinophils in vitro [64]. They also bind to HLA-DR on Langerhans cells and macrophages and stimulate them to produce IL-1, TNF-α and/or IL-12. These cytokines either up-regulate the expression of adhesion molecules on endothelial cells, or increase CLA expression on T cells, respectively, thus facilitating the recruitment of CLA+ memory T cells to the skin. In addition, keratinocytes that have been induced to express MHC Class II molecules by stimulation with IFN-γ can interact with superantigens, resulting in transient intracellular Ca2+ mobilization and the release of proinflammatory TNF-α [65,66]. HLA-DR+ keratinocytes can also present superantigens to T cells; because KCs do not synthesize IL-12, this results in the activation of Th-2 rather than Th-1 cells [67]. Two other S. aureus products, staphylococcal protein A and α-toxin also induce the production of TNF-α by keratinocytes, with additional cytotoxic effects exerted by the latter [68].
Superantigen-specific IgE and IgG2 antibodies
Superantigen-secreting S. aureus have been isolated from over 50% of AD patients, and many of these patients produce IgE antibodies specific for the toxins found on their skin [69,70]. In contrast, although SEA, SEB or TSST – secreting S. aureus have also been isolated from the lesional skin of psoriasis patients, their sera did not contain IgE antibodies to the toxins [69]. Basophils and mast cells from AD patients with antitoxin IgE antibodies release histamine on exposure to toxins, but only those toxins against which they have raised specific IgE antibody [69]. Furthermore, there is a correlation between the presence of IgE antibodies specific for staphylococcal superantigens and both the severity of AD, and total serum IgE levels [71]. Thus toxins produced by S. aureus also exacerbate AD by activating mast cells, basophils and other Fcɛ-receptor bearing cells carrying the relevant antitoxin IgE antibody.
Conversely, a subgroup of patients with AD showed a deficiency in the production of IgG2, but not of IgG1 or IgG4 antibodies against toxin SEC1, which was associated with a severe disease phenotype [71]. This appeared to be specific for SEC1 as levels of IgG2 antibodies against SEB, or another bacterial antigen, pneumococcal capsular polysaccharide, were normal in these patients. IgG2 antibodies are poor complement activators, but can effectively mediate polymorphonuclear leucocyte phagocytosis of S. aureus via the FcγRIIa receptor on the cell surface [72]. Although the functional role of anti-SEC1 IgG2 antibodies in AD pathogenesis remains unclear, it is possible that such a selective decrease in some patients with AD may contribute to the persistence of SEC1-producing S. aureus on lesional skin and the resulting exacerbation of disease activity.
Malassezia-induced IgE- and Th-2 cell-mediated responses
Malassezia (formerly known as Pityrosporum orbiculare/ovale) is part of the normal human skin flora and is most abundant at sites of high sebum production such as the scalp, chest and back where it colonizes the stratum corneum. Most healthy individuals have developed IgG antibodies to Malassezia, but in 30–80% of AD patients, IgE and/or T cell reactivity to the organism is present [73]. Patients with AD affecting mainly the head and neck region appear to be more likely to produce Malassezia-specific IgE antibodies, coinciding with the higher levels of yeast colonization in these areas, than patients with AD located elsewhere on the body [74]. Malassezia-specific IgE antibodies are rarely produced by atopic patients whose skin is unaffected [75,76]. This, together with the high prevalence of type I hypersensitivity to Malassezia as compared to other fungi in AD suggests that Malassezia-specific antibodies are pathogenically significant [77,78]. Histamine release tests have confirmed the biological activity of circulating Malassezia-specific IgE antibodies in approximately 70% of AD patients, supporting a role for these antibodies in the disease process [78].
The defective skin barrier in AD may allow the whole Malassezia yeast cells and their allergens, nine of which have been isolated and cloned so far, to enter the skin and be taken up by Langerhans cells. In vitro studies have shown that the process of internalization of Malassezia causes maturation of dendritic cells and the production of proinflammatory and immunoregulatory cytokines, but not IL-12, thereby favouring the induction of a Th-2 type response [79]. Indeed, higher blood and skin Th-2 type responses to Malassezia have been demonstrated in vitro in AD patients compared to that of normal controls [80]. Furthermore, it has been demonstrated that a positive atopy patch test to Malassezia in vivo correlates with a Th-2-like peripheral blood mononuclear cell response in AD patients in vitro [81].
Malassezia also exerts other proinflammatory effects such as activation of the alternative complement pathway, and the stimulation of keratinocytes to produce a variety of inflammatory cytokines such as IL-6, IL-8 and TNF-α, which may contribute to its role in AD pathogenesis [82,83]. However, antifungal treatment studies have so far proved inconclusive as to the clinical relevance of Malassezia-induced allergy in AD. This may be explained by a lack of selection of relevant patient subgroups and the use of inappropriate measures of clinical outcome [84].
Conclusion
AD is a chronic inflammatory skin disease whose initiation and clinical activity is modified by exposure to, and interaction with, microorganisms. In genetically predisposed individuals a lack of chronic exposure to microbial antigens in early life increases the risk of disease. Once manifested, defects in skin immune surveillance mechanisms such as decreased production of antimicrobial peptides and the expression of functionally altered PRRs result in exacerbation of disease activity by skin colonizing microorganisms, particularly S. aureus and Malassezia furfur. Manipulation of the innate immune response to microorganisms could provide a novel approach to the treatment of AD, as suggested by the beneficial effects of administration of probiotic bacteria in preliminary studies. TLR ligands are also being tested for their ability to shift an allergen-specific Th-2 immune response to that of protective Th-1 immunity, although the long-term effects of such a strategy are, at present, unknown [85].
References
- 1.Lee YA, Wahn U, Kehrt R, et al. A major susceptibility locus for atopic dermatitis maps to chromosome 3q21. Nat Genet. 2000;26:470–3. doi: 10.1038/82625. [DOI] [PubMed] [Google Scholar]
- 2.Cookson WO, Ubhi B, Lawrence R, et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet. 2001;27:372–3. doi: 10.1038/86867. [DOI] [PubMed] [Google Scholar]
- 3.Cox HE, Moffatt MF, Faux JA, Walley AJ, Coleman R, Trembath RC, Cookson WO, Harper JI. Association of atopic dermatitis to the beta subunit of the high affinity immunoglobulin E receptor. Br J Dermatol. 1998;138:182–7. doi: 10.1046/j.1365-2133.1998.02108.x. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima T, Noguchi E, Arinami T, Yamakawa-Kobayashi K, Nakagawa H, Otsuka F, Hamaguchi H. Linkage and association of an interleukin 4 gene polymorphism with atopic dermatitis in Japanese families. J Med. 1998;35:502–4. doi: 10.1136/jmg.35.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He JQ, Chan-Yeung M, Becker AB, Dimwit-Ward H, Ferguson AC, Manfreda J, Watson WT, Sandford AJ. Genetic variants of the IL13 and IL4 genes and atopic diseases in at-risk children. Genes Immun. 2003;4:385–9. doi: 10.1038/sj.gene.6363985. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Nickel R, Beyer K, et al. An IL13 coding region variant is associated with a high total serum IgE level and atopic dermatitis in the German multicenter atopy study (MAS-90) J Allergy Clin Immunol. 2000;106:167–70. doi: 10.1067/mai.2000.107935. [DOI] [PubMed] [Google Scholar]
- 7.Nickel RG, Casolaro V, Wahn U, et al. Atopic dermatitis is associated with a functional mutation in the promoter of the C-C chemokine RANTES. J Immunol. 2000;164:1612–6. doi: 10.4049/jimmunol.164.3.1612. [DOI] [PubMed] [Google Scholar]
- 8.Nishio Y, Noguchi E, Ito S, Ichikawa E, Umebayashi Y, Otsuka F, Arinami T. Mutation and association analysis of the interferon regulatory factor 2 gene (IRF2) with atopic dermatitis. J Hum Genet. 2001;46:664–7. doi: 10.1007/s100380170018. [DOI] [PubMed] [Google Scholar]
- 9.Weidinger S, Klopp N, Rummler L, et al. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol. 2005;116:177–84. doi: 10.1016/j.jaci.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Kabesch M, Peters W, Carr D, Leupold W, Weiland SK, von Mutius E. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol. 2003;111:813–7. doi: 10.1067/mai.2003.1336. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, et al. The Toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- 12.Ring J, Kramer U, Schafer T, Behrendt H. Why are allergies increasing? Curr Opin Immunol. 2001;13:701–8. doi: 10.1016/s0952-7915(01)00282-5. [DOI] [PubMed] [Google Scholar]
- 13.Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged? Br Med. 1994;308:1132–5. doi: 10.1136/bmj.308.6937.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan DP. Hay fever, hygiene and household size. Br Med. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matricardi PM, Bonini S. High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the Hygiene hypothesis? Clin Exp Allergy. 2000;30:1506–10. doi: 10.1046/j.1365-2222.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 16.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 17.Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunol. 2004;112:352–63. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergic development and the intestinal micro-flora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, Kumemura M. Differences in faecal micro-flora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–91. doi: 10.1067/mai.2003.105. [DOI] [PubMed] [Google Scholar]
- 20.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–16. doi: 10.1111/j.1365-2133.2004.06436.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 22.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179–85. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 23.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Lubbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol. 2003;4:641–54. doi: 10.2165/00128071-200304090-00006. [DOI] [PubMed] [Google Scholar]
- 25.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 26.Ring J, Abeck D, Neuber K. Atopic eczema: role of microorganisms on the skin surface. Allergy. 1992;47:265–9. doi: 10.1111/j.1398-9995.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Am Acad Dermatol. 1992;27:29–34. doi: 10.1016/0190-9622(92)70151-5. [DOI] [PubMed] [Google Scholar]
- 28.Guzik TJ, Bzowska M, Kasprowicz A, Czerniawska-Mysik G, Wójcik K, Szmyd D, Adamek-Guzik T, Pryjma J. Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: relationship to clinical and immunological parameters. Clin Exp Allergy. 2005;35:448–55. doi: 10.1111/j.1365-2222.2005.02210.x. [DOI] [PubMed] [Google Scholar]
- 29.Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus aureus. Am J Clin Dermatol. 2004;5:217–23. doi: 10.2165/00128071-200405040-00002. [DOI] [PubMed] [Google Scholar]
- 30.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119:433–9. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 31.Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th-2 mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658–63. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 32.al-Mohanna F, Parhar R, Kawaasi A, Ernst P, Sheth K, Harfi H, al-Sedairy S. Inhibition of neutrophil functions by human immunoglobulin E. J Allergy Clin Immunol. 1993;92:575–66. doi: 10.1016/0091-6749(93)90020-g. [DOI] [PubMed] [Google Scholar]
- 33.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–31. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 34.Schroder J-M, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–51. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 35.Harder J, Bartels J, Christophers E, Schroder J-M. Isolation and characterisation of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 36.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leuk Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 37.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:10255–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 39.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 40.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 41.Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K, Garbe C, Schittek B. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174:8003–10. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- 42.Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 43.Stern UM, Hornstein OP, Salzer B. Do training-dependent differences in perspiration exist between healthy and atopic subjects? J Dermatol. 2000;27:491–9. doi: 10.1111/j.1346-8138.2000.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 44.Imayama S, Shimozono Y, Hoashi M, Yasumoto S, Ohta S, Yoneyama K, Hori Y. Reduced secretion of IgA to skin surface of patients with atopic dermatitis. J Allergy Clin Immunol. 1994;94:195–200. doi: 10.1016/0091-6749(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 45.Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Reports. 2004;5:1000–6. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda K, Akira S. Toll-like receptors in innate immunity Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 47.Pivarcsi A, Nagy I, Kemeny L. Innate immunity in the skin: how keratinocytes fight against pathogens. Curr Immunol Rev. 2005;1:29–42. [Google Scholar]
- 48.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Int Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 49.Girardin SE, Hugot J-P, Sansonetti PJ. Lessons from Nod2 studies: towards a link between Crohn's disease and bacterial sensing. Trends Immunol. 2003;24:652–8. doi: 10.1016/j.it.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 51.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara M, Harada D, Manabe H, Hasegawa K. Staphylococcus aureus peptidoglycan stimulates granulocyte macrophage colony-stimulating factor production from human epidermal keratinocytes via mitogen-activated protein kinases. FEBS Lett. 2004;566:195–200. doi: 10.1016/j.febslet.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 53.Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol. 2003;133:299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- 55.Michie CA, Davie T. Atopic dermatitis and staphylococcal superantigens. Lancet. 1996;347:324. doi: 10.1016/s0140-6736(96)90498-5. [DOI] [PubMed] [Google Scholar]
- 56.Skov L, Olsen JV, Giorno R, Schlievert PM, Baadsgaard O, Leung DY. Application of Staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T cells by a superantigen-mediated mechanism. J Allergy Clin Immunol. 2000;105:820–6. doi: 10.1067/mai.2000.105524. [DOI] [PubMed] [Google Scholar]
- 57.Davison S, Allen M, Vaughan R, Barker J. Staphylococcal toxin-induced T cell proliferation in atopic eczema correlates with increased use of superantigen-reactive Vbeta-chains in cutaneous lymphocyte-associated antigen (CLA)-positive lymphocytes. Clin Exp Immunol. 2000;121:181–6. doi: 10.1046/j.1365-2249.2000.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–53. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–63. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 60.Lin Y-T, Wang CT, Hsu CT, Wang LF, Shau WY, Yang YH, Chang BL. Differential susceptibility to Staphylococcal superantigen (SsAg)-induced apoptosis of CD4+ T cells from atopic dermatitis patients and healthy subjects: The inhibitory effect of IL-4 on SsAg-induced apoptosis. J Immunol. 2003;171:1102–8. doi: 10.4049/jimmunol.171.2.1102. [DOI] [PubMed] [Google Scholar]
- 61.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. N Engl J Med. 1985;313:282–5. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 62.Maeda K, Yamamoto K, Tanaka Y, Anan S, Yoshida H. The relationship between eosinophils, OKT6-positive cells and house dust mite (HDM) antigens in naturally occurring lesions of atopic dermatitis. J Dermatol Sci. 1992;3:151–6. doi: 10.1016/0923-1811(92)90029-b. [DOI] [PubMed] [Google Scholar]
- 63.Morishita Y, Tada J, Sato A, Toi Y, Kanzaki H, Akiyama H, Arata J. Possible influences of Staphylococcus aureus on atopic dermatitis – the colonizing features and the effects of staphylococcal enterotoxins. Clin Exp Allergy. 1999;29:1110–7. doi: 10.1046/j.1365-2222.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- 64.Wedi B, Wieczorek D, Stunkel T, Breuer K, Kapp A. Staphylococcal exotoxins exert proinflammatory effects through inhibition of eosinophil apoptosis, increased surface antigen expression (CD11b, CD45, CD54, and CD69), and enhanced cytokine-activated oxidative burst, thereby triggering allergic inflammatory reactions. J Allergy Clin Immunol. 2002;109:477–84. doi: 10.1067/mai.2002.121702. [DOI] [PubMed] [Google Scholar]
- 65.Wakita H, Tokura Y, Furukawa F, Takigawa M. Staphylococcal enterotoxin B upregulates expression of ICAM-1 molecules on IFN-gamma-treated keratinocytes and keratinocyte cell lines. J Invest Dermatol. 1995;105:536–42. doi: 10.1111/1523-1747.ep12323426. [DOI] [PubMed] [Google Scholar]
- 66.Ezepchuk YV, Leung DY, Middleton MH, Bina P, Rieser R, Norris DA. Staphylococcal toxins and protein A induce cytotoxicity and release of tumor necrosis factor from human keratinocytes. J Invest Dermatol. 1996;107:603–9. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- 67.Goodman RE, Nestle F, Naidu YM, Green JM, Thompson CB, Nickoloff BJ, Turka LA. Keratinocyte-derived T cell costimulation induces preferential production of IL-2 and IL-4 but not IFN-gamma. J Immunol. 1994;152:5189–98. [PubMed] [Google Scholar]
- 68.Travers JB, Norris DA, Leung DYM. The keratinocyte as a target for staphylococcal bacterial toxins. J Invest Dermatol Symp Proc. 2001;6:225–30. doi: 10.1046/j.0022-202x.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 69.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. J Clin Invest. 1993;92:1374–80. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nissen D, Pedersen LJ, Skov PS, Vejlsgaard GL, Poulsen LK, Jarlov JO, Karlsmark T, Nolte H. IgE-binding components of staphylococcal enterotoxins in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 1997;79:403–8. doi: 10.1016/s1081-1206(10)63033-5. [DOI] [PubMed] [Google Scholar]
- 71.Mrabet-Dahbi S, Breuer K, Klotz M, Herz U, Heeg K, Werfel T, Renz H. Deficiency in immunoglobulin G2 antibodies against staphylococcal enterotoxin C1 defines a subgroup of patients with atopic dermatitis. Clin Exp Allergy. 2005;35:274–81. doi: 10.1111/j.1365-2222.2005.02192.x. [DOI] [PubMed] [Google Scholar]
- 72.Bredius RG, de Vries CE, Troelstra A, van Alphen L, Weening RS, van de Winkel JG, Out TA. Phagocytosis of Staphylococcus aureus and Haemophilus influenzae type B opsonized with polyclonal human IgG1 and IgG2 antibodies. Functional hFc gamma RIIa polymorphism to IgG2. J Immunol. 1993;151:1463–72. [PubMed] [Google Scholar]
- 73.Scheynius A, Johansson C, Buentke E, Zargari A, Linder MT. Atopic eczema/dermatitis syndrome and Malassezia. Int Arch Allergy Immunol. 2002;127:161–9. doi: 10.1159/000053860. [DOI] [PubMed] [Google Scholar]
- 74.Devos SA, van der Valk PG. The relevance of skin prick tests for Pityrosporum ovale in patients with head and neck dermatitis. Allergy. 2000;55:1056–8. doi: 10.1034/j.1398-9995.2000.00782.x. [DOI] [PubMed] [Google Scholar]
- 75.Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis. a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol. 1999;104:1273–9. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 76.Broberg A, Faergemann J, Johansson S, Johansson SG, Strannegard IL, Svejgaard E. Pityrosporum ovale and atopic dermatitis in children and young adults. Acta Derm Venereol. 1992;72:187–92. [PubMed] [Google Scholar]
- 77.Kim TY, Jang IG, Park YM, Kim IIO, Kim CW. Head and neck dermatitis: the role of Malassezia furfur, topical steroid use and environmental factors in its causation. Clin Exp Dermatol. 1999;24:226–31. doi: 10.1046/j.1365-2230.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 78.Nissen D, Petersen LJ, Esch R, Svejgaard E, Skov PS, Poulsen LK, Nolte II. IgE-sensitization to cellular and culture filtrates of fungal extracts in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 1998;81:247–55. doi: 10.1016/S1081-1206(10)62821-9. [DOI] [PubMed] [Google Scholar]
- 79.Buentke E, Scheynius A. Dendritic cells and fungi. APMIS. 2003;111:789–96. doi: 10.1034/j.1600-0463.2003.11107810.x. [DOI] [PubMed] [Google Scholar]
- 80.Tengvall Linder M, Johansson C, Zargari A, Bengtsson A, van der Ploeg I, Jones I, Harfast B, Scheynius A. Detection of Pityrosporum orbiculare reactive T cells from skin and blood in atopic dermatitis and characterisation of their cytokine profiles. Clin Exp Allergy. 1996;26:1286–97. doi: 10.1046/j.1365-2222.1996.d01-281.x. [DOI] [PubMed] [Google Scholar]
- 81.Johansson C, Eshagi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cell response. J Invest Dermatol. 2002;118:1044–51. doi: 10.1046/j.1523-1747.2002.01758.x. [DOI] [PubMed] [Google Scholar]
- 82.Belew PW, Rosenberg EW, Jennings BR. Activation of the alternative pathway of complement by Malassezia ovalis (Pityrosporum ovale) Mycopathologica. 1980;70:187–90. doi: 10.1007/BF00443030. [DOI] [PubMed] [Google Scholar]
- 83.Watnabe S, Kano R, Sato II, Nakamura Y, Ilasegawa A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest Dermatol. 2001;116:769–73. doi: 10.1046/j.1523-1747.2001.01321.x. [DOI] [PubMed] [Google Scholar]
- 84.Aspres N, Anderson C. Malassezia yeasts in the pathogenesis of atopic dermatitis. Austr J Dermatol. 2004;45:199–207. doi: 10.1111/j.1440-0960.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 85.Leung T-f, Tang NLS, Wong GWK, Fok T-f. CD14 and Toll-like receptors: Potential contribution of genetic factors and mechanisms to inflammation and allergy. Curr Drug Targets – Inflamm Allergy. 2005;4:169–75. doi: 10.2174/1568010053586336. [DOI] [PubMed] [Google Scholar]