Abstract
Tissue transglutaminase (tTG) autoantibodies decline after gluten-free diet in patients with coeliac disease. We tested the hypothesis that gluten-free diet-induced change in tTG autoantibody levels affects subsets of peripheral blood lymphocytes. Peripheral blood was obtained from 20 children with biopsy-proven active coeliac disease. Gluten-free diet was initiated and the children examined again after three and six months. tTG autoantibodies were measured in radioligand binding assays and lymphocyte subsets by flow cytometry. IgA-tTG levels at diagnosis, 2204 U/ml (median, range 113–24990), were reduced over six months to 76 U/ml (median, range 1–1261) (P< 0·001). At six months, 12/20 (60%) children had reduced their IgA-tTG levels to < 100 U/ml and these children showed a decrease in B cells (mean change −3·8%, P = 0·014), CD4+ T cells (mean −4·32%, P = 0·011) and CD4+ T cells expressing CD25high (mean change −0·62%, P = 0·036). In contrast, the CD4+CD25highCCR4+ T cell population increased during the same period (mean change 11·5%, P = 0·0036). The decline in IgA-tTG levels correlated to the decrease in B cells (r= 0·56, P = 0·01), CD4+ T cells (r= 0·66, P = 0·004) as well as CD4+CD25high T cells (r= 0·59, P = 0·01). A negative correlation was found between the decline in IgA-tTG and CD4+CD25high T cells expressing CD45RO (r = –0·49, P = 0·03) and CCR4 (r = –0·54, P = 0·01). This is the first observational study on the effect of gluten-free diet on concurrent changes of tTG autoantibodies and specific peripheral blood lymphocyte subsets. Our data suggest that flow cytometry may be a useful complement to tTG autoantibodies when studying the effects of gluten-free diet in children with coeliac disease.
Keywords: autoantibodies, autoimmunity, B cells, T cells, mucosa
Introduction
Coeliac disease (CD) is characterized by an inflammatory infiltrate of lymphocytes in the small intestinal mucosal epithelium of genetically predisposed individuals carrying primarily the HLA-DQ2 (DQA1*0501-B1*0201) or DQ8 (DQA1*0301-B1*0302) haplotypes [1]. While the disease is thought to be triggered by a T cell response against gliadin and related prolamines [2], CD is also strongly associated with autoantibodies against tissue transglutaminase (tTG) [3]. As a marker for CD, circulating IgA class autoantibodies against tTG show high predictive value, whereas the diagnostic sensitivity of IgG-tTG varies between different immunoassays [4].
tTG belongs to a widely distributed group of calcium-dependent enzymes that catalyse post-translational transamidation of proteins and peptides [5]. The enzyme may selectively modify gliadin peptides that are recognized by gut-derived T cells thought to play a central part in CD [6,7]. Autoantibodies against tTG bind to several conformational epitopes [8–11]. The mechanism by which autoimmunity against tTG is important to the pathogenesis of CD is a matter of debate [12–14]. It remains unclear how tTG modification of gliadin leads to an immune response against tTG and the formation of IgA and IgG autoantibodies. In other autoimmune disorders it has been demonstrated that human autoantibodies are able to modulate immunodominant epitopes interacting with self-reactive T cells [15,16].
The T cell response against gluten in the lamina propria in CD is predominantly associated with high release of the Th1 cytokine IFN-γ [17], but it is also associated with increased expression of the Th2 cytokine IL-10 [18]. Although the up-regulation of IL-10 in the lamina propria appears a compensatory mechanism to induce an immunosuppressive effect on Th1 cells, the response is insufficient in re-establishing tolerance against gliadin during the active phase of CD [19]. Increased levels of IFN-γ have also been measured in peripheral blood from patients with active CD [20]. More recently, an IFN-γ induced CD4+ T cell response to a transglutaminase deamidated immunodominant α-gliadin peptide was detected in peripheral blood of treated CD patients shortly after oral gluten challenge, indicating that specific memory T cells persist also in the circulation [21].
A subset of peripheral CD4+ T cells expressing IL-2 receptor (CD4+CD25+) has been demonstrated to possess suppressor functions in both human and rodent models and is now regarded as a population of naturally occurring regulatory T cells [22]. It has been demonstrated that the majority of these T regulatory cells in human peripheral blood reside within the CD4+ T cells expressing high level of CD25 (CD4+CD25high) [23]. CD4+CD25high T cells also appear important in retaining intestinal immune homeostasis in the lamina propria [24]. Decreased frequency [25] and defective function [26,27] of peripheral blood CD4+CD25high T cells have been further described in other organ-specific autoimmune diseases such as multiple sclerosis and type 1 diabetes.
Cytokines and their receptors are regulators of the immune system with chemotactic function guiding T cells into the affected tissue [28]. T regulatory cells have been characterized by the surface expression of chemokine receptor like CCR4 and CCR8 [29], which have been shown to be important for the interaction between T regulatory cells and antigen-presenting cells secreting the CCR4-ligand macrophage-derived chemokine (MDC). It is also known that the chemokine receptor CCR9 is expressed at high levels of CD4+ and CD8+ T cells in the small intestine [30]. The CCR9 has further been detected in a small subset of memory peripheral blood CD4+ T cells with characteristics of intestinal lymphocytes, indicating that circulating lymphocytes might mirror the inflammation in the gut [31].
To our knowledge, there are no reports describing a quantitative correlation between changes in tTG autoantibody levels and regulatory T cells in peripheral blood in CD patients before and after gluten-free diet. The aim of this observational study was to test the hypothesis that gluten-free diet-induced decline in tTG autoantibody levels is associated with changes in subsets of peripheral blood lymphocytes.
Materials and methods
Patients
Between November 2003 and October 2004, peripheral blood samples were obtained from 40 children consecutively admitted to the Department of Paediatrics, University Hospital MAS in Malmö, Sweden for investigation with small intestinal biopsy. The first blood sample was collected on the day of the intestinal biopsy after all parents gave their informed consent. Children with an intestinal biopsy showing typical villous atrophy according to the Marsh criteria [1] were placed on a gluten-free diet and followed prospectively. A total of 14 children were found to have a normal biopsy and were included as disease controls for baseline comparison. The remaining 26 children had an abnormal biopsy and a gluten-free diet was thus introduced. In 20 of these children (13 females, 7 males, median age 3·9 years, range 1·3–13·9 years) (Table 1), both a second and a third blood sample were available after a median 3·1 months (range 2·9–3·8 months) and 6·1 months (median, range 5·9–7·0 months) of gluten-free diet, respectively. tTG autoantibodies and endomysial autoantibodies (EMA) were measured to assess the dietary compliance. A follow-up examination after six months revealed that all 20 children had relief of gastrointestinal symptoms and the diagnosis was established according to the ESPGHAN (European Society of Paediatric Gastroenterology Hepatology and Nutrition) criteria [32]. The Ethics Committee of the Medical Faculty, Lund University, approved this study.
Table 1.
Patient gender, age, HLA haplotypes (DQA1-DQB1) and autoantibody levels at diagnosis of 20 children with coeliac disease.
| Patient | Sex | Age (years) | DQA1-B1 haplotype 1 | DQA1-B1 haplotype 2 | Biopsy | EMA (titre) | IgA-tTG (U/ml) | IgG-tTG (U/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 3·9 | *05-*02 | *03-*0302 | TVA | 1600 | 5 331 | 806 |
| 2 | M | 2·8 | *ND-*0302 | *ND-*0603 | SVA | 1600 | 8 041 | 10 665 |
| 3 | M | 1·8 | *05-*02 | *X-*0602 | TVA | 1600 | 11 310 | 5 150 |
| 4 | F | 12·0 | *05-*02 | *03-*0302 | TVA | 1600 | 1 451 | 892 |
| 5 | F | 10·1 | *0201-*02 | *05-*02 | TVA | 1600 | 24 991 | 11 785 |
| 6 | F | 2·6 | *05-*02 | *X-*0502/0504 | SVA | 1600 | 1 571 | 4 705 |
| 7 | F | 12·0 | *05-*02 | *X-*02/0609 | SVA | 1600 | 215 | 318 |
| 8 | F | 2·6 | *05-*02 | *X-*0602 | SVA | 1600 | 1 560 | 25 007 |
| 9 | M | 13·9 | *05-*02 | *X-*0602 | PVA | 100 | 113 | 27 |
| 10 | M | 8·0 | *05-*02 | *X-*04 | SVA | 1600 | 2 375 | 1 387 |
| 11 | M | 8·6 | *05-*02 | *03-*0302 | PVA | 400 | 982 | 1 322 |
| 12 | M | 1·6 | *05-*02 | *X-*02/0609 | SVA | 1600 | 3 181 | 7 911 |
| 13 | F | 3·9 | *05-*02 | *X-*0602 | SVA | 1600 | 2 032 | 2 572 |
| 14 | F | 11·5 | *0201-*02 | *05-*02 | PVA | 1600 | 1 446 | 315 |
| 15 | F | 2·3 | *05-*02 | *03-*0302 | SVA | 1600 | 6 844 | 8 433 |
| 16 | F | 10·8 | *05-*02 | *X-*0602 | TVA | 400 | 12 763 | 3 145 |
| 17 | F | 1·3 | *05-*02 | *X-*02/0609 | TVA | 1600 | 4 620 | 5 295 |
| 18 | F | 1·8 | *05-*02 | *X-*0602 | TVA | 1600 | 12 351 | 6 540 |
| 19 | F | 9·9 | *05-*02 | *X-*0602 | PVA | 400 | 247 | 409 |
| 20 | F | 2·5 | *05-*02 | *X-*0602 | PVA | 400 | 113 | 64 |
EMA, endomysial autoantibodies; F, female; M, male; PVA, partial villous atrophy; SVA, subtotal villous atrophy; tTG, tissue transglutaminase; TVA, total villous atrophy. X denotes HLA other than the mentioned alleles and ND is not determined.
Transglutaminase autoantibody radioligand binding assay
Human tTG C was synthesized in the presence of 20 µCi 35S-methionine (Perkin Elmer Life Sciences, Inc., Boston, MA, USA) by in vitro transcription and translation as previously described [33,34]. The labelled proteins were stored at −80°C, used within 2 weeks and the radioligand binding assay (RBA) was run as described previously [34] with several modifications. The IgA-tTG antigen/antibody complexes were isolated with 10% goat antihuman IgA Agarose (Sigma, St. Louis, MO, USA) and the IgG-tTG antigen/antibody complexes was separated with 30% protein A Sepharose (PAS) conjugate 4B (Zymed Laboratories, Inc., San Francisco, CA, USA). The relative amount of tTG autoantibodies was expressed as U/ml calculated from standard curves constructed to contain 4, 8, 16, 31, 62, 125, 250, 500 and 1000 U/ml of respective IgA-tTG and IgG-tTG. High binding samples were tested in further dilutions. Cut-off level for a positive value was set at >16 U/ml for IgA-tTG and at > 4 U/ml for IgG-tTG, which represented the 95th percentile of 398 blood donors. Distinction between high and low tTG autoantibody levels was arbitrary set at the level used to define the cohort at the start of the study and represented 100 U/ml for IgA-tTG and 25 U/ml for IgG-tTG.
Endomysial autoantibody immunofluorescence staining
EMA were analysed at Clinical Microbiology and Immunology, Lund University Hospital, Sweden, by an indirect immunofluorescence method [35]. Patient serum was diluted 1 : 10 in PBS/Tween (Euroimmun, Lubeck, Germany) and applied to reaction fields of reagent tray containing tissue slides of primate intestine, liver and oesophagus (Euroimmun). EMA were detected with fluorescein isothiocyanate conjugated goat anti-human IgA antibodies. Results were expressed as the highest dilution factor giving a positive fluorescence pattern in microscope. All sera manifesting fluorescence titre of 1 : 10 were considered to be positive and titres of 1 : 100 were considered high.
Lymphocyte subsets analysis by flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated using Lymphoprep (Ficoll-Isopaque) (Axis-Shield PoC AS, Oslo, Norway) overlayed by heparin blood diluted 1 : 2 in PBS (phosphate buffer saline) and centrifuged at 475 × g for 30 min. The mononuclear layer was gently collected and washed twice in wash buffer (PBS with 0·5% BSA and 2 mM EDTA). The cells were resuspended at 106 cells/ml in wash buffer. PBMC were stained directly with various fluorochrome-conjugated antibodies directed against the following markers: CD3 (FITC-conjugated, clone SK7), CD4 (PerCP-conjugated, clone SK3), CD8 (PE- or PerCP-conjugated, clone SK1), CD16 (PE-conjugated, clone SK7, 1), CD19 (PerCP-conjugated, clone 4G7), CD25 (FITC-conjugated, clone M-A251), CD62L (APC-conjugated, clone DREG-56), CD45RA (PE-conjugated, clone HI100), CD45RO (APC-conjugated, clone UCHL1), CD56 (PE-conjugated, clone MY31), CCR4 (PE-conjugated, clone 1GI), Integrin B7 (PE-conjugated, clone FIB504), irrelevant isotype controls IgG2a (FITC-conjugated, clone X39) and IgG1 (PE-conjugated, clone X40)(all from Becton-Dickinson/PharMingen, CA, USA) and CCR9 (APC-conjugated, clone 248621, R & D System, Minneapolis, MN, USA). PBMC were incubated at 2–8°C for 30 min, washed and centrifuged at 400 × g in 10 min, and diluted with wash buffer to appropriate volumes. Prior to CCR9 and Integrin B7 staining, cells were incubated for 30 min with human IgG in order to block Fc receptors. The samples were acquired in a four-colour FACScalibur® (Becton Dickinson, CA, USA) and analysed using CellQuest® software (Becton-Dickinson). Isotype-matched control antibodies were used to set the dot plot quadrant and calculate the percent of lymphocyte populations.
HLA-DQA1 and DQB1 genotyping
High resolution HLA typing was carried out by PCR followed by hybridization with allele specific probes as described [36]. Three probes were used to define the presence of DQA1*0201, *0301–0303 and *0501–0505 alleles and five probes the DQB1*02, *0301–0304, and *0602–0604 alleles [37].
Statistical methods
All graphs were drawn by GraphPad PRISM 4·0 (GraphPad Software, San Diego, CA, USA) and analyses performed in Splus6·1 (Insightful Corp., Seattle, USA). Changes in percent of lymphocytes over time were expressed as mean (95% CI) and tested for significance using the one-sample t-test. Changes in antibody levels were highly skewed and the Wilcoxon signed rank test was used instead. Association between changes in tTG autoantibody log10 levels and percent of lymphocytes were performed using the Spearman rank correlation (r). P-values less than 0·05 were considered significant.
Results
Changes in autoantibody levels during gluten-free diet
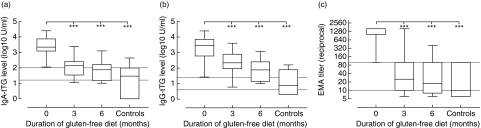
All 20 children followed prospectively from diagnosis either carried HLA-DQ2 or DQ8, had high levels of all three autoantibodies or had a biopsy showing villous atrophy (Table 1). At diagnosis, both levels of IgA-tTG (median 2204 U/ml, range 113–24990) (Fig. 1a) and IgG-tTG (median 2858 U/ml, range 27–25010 U/ml) (Fig. 1b) were higher compared to disease controls (IgA-tTG median 29 U/ml, range 1–440 U/ml) (IgG-tTG median 7 U/ml, range 2–162 U/ml) (P< 0·001, respectively). After three months of gluten-free diet, levels of both IgA-tTG and IgG-tTG were reduced in 19/20 children. One child, however, showed an increase in IgG-tTG levels. After six months, IgA-tTG levels further declined in 16/20 children and IgG-tTG in 19/20 children. The same child that had increased IgG-tTG levels at three months also increased after six months in IgA-tTG levels. Overall, levels of IgA-tTG at six months were reduced to 76 U/ml (median, range 1–1261 U/ml) (Fig. 1a) and IgG-tTG to 78 U/ml (median, range 1–1206) (Fig. 1b) (P< 0·001, respectively). By six months, 12/20 children had IgA-tTG levels below 100 U/ml and 7/20 had IgG-tTG levels below 25 U/ml. Over the same period, EMA titres showed a decline in all children from 1 : 1600 (median, range 1 : 100–1 : 1600) to 1 : 10 (median, range < 1 : 10–1 : 400) (P< 0·001) and after six months on gluten-free diet 14/20 reduced their EMA titres to < 1 : 100 (Fig. 1c).
Fig. 1.
Levels of tTG autoantibody and EMA in CD children (n = 20) before and after gluten-free diet compared with disease controls (n = 14). The horizontal lines represent the cut-off level of normal and the limit of high autoantibody level. ***Significant change from baseline P < 0·001.
Changes in lymphocytes subsets during gluten-free diet
The proportion of total B or T cells showed no significant change after either three or six months of gluten-free diet. At six months however, a gradual decrease of CD4+CD25high T cells (mean change −0·43% of the CD4+ T cells, P = 0·015) was observed with an increased proportion of CD4+CD25high expressing CCR4 (mean change 6·22% of the CD4+CD25high, P = 0·041) (Table 2).
Table 2.
Mean percent change of peripheral B cells (CD3-CD19+) and subsets of T cells (CD3+) at diagnosis (baseline) and after gluten-free diet in children with coeliac disease (n = 20).
| Subset | Baseline Mean (%) (95% CI) | Three months Δ mean (%) (95% CI) | P-value | Six months Δ mean (%) (95% CI) | P-value |
|---|---|---|---|---|---|
| CD3-CD19+ | 11·0 (7·4–14·6) | − 1·1 (−3·6–1·4) | NS | − 2·5 (−5·2–0·1) | NS |
| CD3+ | 71·5 (66·7–76·2) | 1·3 (−7·1–17·3) | NS | 1·6 (−5·7–9·0) | NS |
| CD3+ CD4+ | 57·7 (53·4–62·0) | − 1·1 (−4·0–1·7) | NS | − 1·6 (−4·8–1·6) | NS |
| CD3+ CD4+ CD25+ | 8·4 (6·4–10·4) | − 0·7 (−3·5–2·1) | NS | − 0·9 (−3·0–1·3) | NS |
| CD3+ CD4+ CD25high | 0·9 (0·5–1·4) | − 0·2 (−0·6–0·1) | NS | − 0·4 (−0·8–0·1) | 0·015 |
| CD3+ CD4+ CCR4 + | 20·9 (15·4–26·4) | − 3·8 (−9·3–1·7) | NS | − 2·3 (−8·0–3·4) | NS |
| CD3+ CD4+ CD25+ CCR4 + | 84·8 (79·3–90·3) | − 5·1 (−11·7–1·5) | NS | − 1·2 (−5·5–3·0) | NS |
| CD3+ CD4+ CD25high CCR4 + | 74·4 (64·3–84·6) | 1·2 (−9·8–12·2) | NS | 6·2 (0·3–12·1) | 0·041 |
Δ denotes difference from baseline means, NS is not significant.
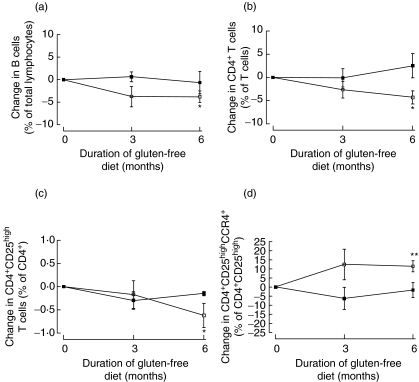
Changes in lymphocyte subsets over time were also examined within the two groups having either low or high autoantibody levels after treatment, respectively. Children with low IgA-tTG levels had a temporary increase in CD8+CD45RA+ T cells after three months (mean change 1·15% of the CD8+, P = 0·0048). After six months they also had a significant decrease in B cells (mean change −3·79% of the total lymphocytes, P = 0·014) (Fig. 2a), CD4+ T cells (mean −4·32% of the T cells, P = 0·011) (Fig. 2b) and CD4+ T cells expressing CD25high (mean change −0·62% of the CD4+, P = 0·034) (Fig. 2c). In contrast, the proportion of CD4+CD25high T cells expressing CCR4 increased (mean change 11·46% of the CD4+CD25high, P = 0·0036) during the same period (Fig. 2d).
Fig. 2.
The mean percent change ± SEM of lymphocyte subset in children with low (□) and high (▪) IgA-tTG levels after gluten-free diet. *P < 0·05; **P < 0·005.
In children with low IgG-tTG levels after six months, there was a decrease in B cells (mean change −5·0% of the total lymphocytes, P = 0·025) and an expansion of the CD4+CD25highCCR4+ subpopulation (mean change 12·43% of the CD4+CD25high, P = 0·039). Similarly, in children who seroconverted to low EMA titres there was a decrease in B cells (mean change −2·89% of the total lymphocytes, P = 0·041) and an increase in CD4+CD25highCCR4+ T cells (mean change 10·27% of the CD4+CD25high, P = 0·0072). Of the remaining patients, there was only a decrease in the T cell subsets CD4+CD25high (mean change −0·15% of the CD4+, P = 0·014) and CD8+CCR9+ (mean change −0·058% of the CD8+, P = 0·0091) in children who retained high IgA-tTG levels after gluten-free diet.
Correlation between changes in lymphocyte subsets and autoantibody levels during gluten-free diet
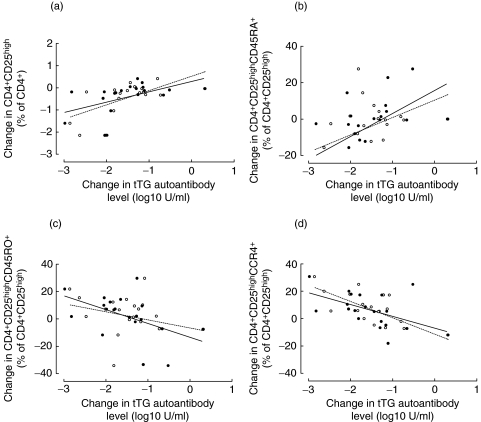
The degree of changes in log levels of tTG autoantibodies were compared with changes in lymphocyte subsets after six months of gluten-free diet. Changes in B cells correlated with both changes in IgA-tTG (r= 0·56, P = 0·01) and IgG-tTG (r= 0·49, P = 0·03), whereas there was no correlation between T cells and IgA-tTG (r= 0·21 P = 0·35) or IgG-tTG (r= 0·05, P = 0·82). However, CD4+ T cells were positively correlated with change in IgA-tTG (r= 0·66, P = 0·004) and IgG-tTG (r= 0·48, P = 0·04), whereas there was no association between CD8+ T cells and IgA-tTG (r = –0·32, P = 0·15) or IgG-tTG (r = –0·43, P = 0·06). A greater increase in the CD4+CD25high T cell subset was associated with a lesser decline in IgA-tTG (r= 0·59, P = 0·01) and IgG-tTG (r= 0·52, P = 0·02) (Fig. 3a). The change in CD4+CD25highCD45RA+ T cells was only correlated with change in IgA-tTG (r= 0·46, P = 0·04) (Fig. 3b). Moreover, the change of subset CD4+CD25highCD45RO+ T cells correlated negatively with change in IgA-tTG (r = –0·49, P = 0·03) in contrast to IgG-tTG (r = –0·29, P = 0·20) (Fig. 3c). An inverse relationship was observed between change in CD4+CD25highCCR4+ T cells and change in both IgA-tTG (r = –0·54, P = 0·01) and IgG-tTG (r = –0·67, P = 0·003) (Fig. 3d).
Fig. 3.
Correlations between changes in log10 levels of IgA-tTG (•, ——) and IgG-tTG (○, ···········) and changes in subsets of peripheral blood lymphocytes in children with coeliac disease after six months of gluten-free diet (n = 20).
Discussion
It is well known that the alleviation of symptoms and clinical signs related to CD is associated with a reduction in tTG autoantibody levels [38]. However, it is not clear to what extent the reduction in tTG autoantibodies might be reflected by changes in the frequency of peripheral blood B and T lymphocytes. The recent rapid development of highly specific cell surface marker reagents for different subsets of B and T cells has made it possible to test the hypothesis that gluten-free diet is affecting the proportion of certain lymphocyte subsets in CD patients.
RBA for quantitative measurement of tTG autoantibodies can detect diet-induced changes in autoantibody levels with high accuracy [39]. Using the RBA for IgA-tTG and IgG-tTG, we could objectively assess and document disease activity over time to delineate how the patients responded to their diet. Even though we found a dramatic decline in tTG autoantibody levels after treatment with gluten-free diet, some children still showed high levels after six months. The absence of an effect on autoantibody levels might be explained by the ability of the patients to follow the recommended diet guidelines during the first months after diagnosis.
The distinct but variable decline in tTG autoantibody levels in this study allowed us to test whether the reduction in autoantibodies was associated with changes in the proportion of lymphocyte subsets. We are well aware of the fact that we are observing changes in proportion of certain subsets in parallel with the reduction of autoantibodies. It is therefore not possible to determine whether the observed changes in lymphocyte proportions precede the reduction of autoantibody levels or are a consequence of reduced autoantibody levels.
Nevertheless, our results suggest that a significant decline in tTG autoantibody levels is associated with change in both B cells and CD4+ T cells. We speculate that the reduction in the proportion of B cells in children demonstrating low tTG autoantibody levels at six months may be due to absence of the antigenic stimulation by gliadin leading to a contraction of circulating B lymphocytes. Similar to the reduction of B cells following gluten-free diet and a concomitant reduction in tTG autoantibodies, a decrease in the CD4+ T cell population would suggest that the immune system retracts after healing of the intestinal mucosa.
We also observed a decrease in CD4+CD25high T cells that correlated with the decline in tTG autoantibody levels. CD4+CD25high T cells are regarded as a circulating regulatory T cells with suppressor function on autoreactive T cells [23,40]. The decrease in CD4+ T cells and circulating regulatory T cells could reflect the accumulation of those cells in the intestinal mucosa during healing. However, this is in contrast to a recent report where patients with inactive compared to active inflammatory bowel disease showed an expansion of peripheral blood CD4+CD25high T cells [41]. This is of interest since gliadin as a sole source of antigen activates CD after re-introduction perhaps because the gluten-free diet is associated with a decrease rather than an increase in this supposedly regulatory T cell subset.
Although we found a decrease in CD4+CD25high T cells, the proportion of cells within this subpopulation expressing CCR4 was increased, suggesting a re-circulation of primed regulatory T cells. CCR4 is highly expressed on differentiated regulatory T cells and is an important chemokine receptor for the recruitment of T cells in the site of inflammation and their interaction with dendritic cells [29,42]. The specific increase in the proportion of CD4+CD25highCCR4+ subpopulation might indicate a possible mechanism of peripheral tolerance induced after removal of the antigenic stimulation during remission, which could be mediated by the specific regulation of highly specialized regulatory T cells.
The chemokine receptor CCR9 and the B7 integrin are expressed selectively on T cells in the intestinal mucosa and in a subset of circulating T cells [43]. Therefore, CCR9 and B7 integrin have been proposed as a marker for T cells homing to the intestinal lamina propria. These cells may play an important role in the pathogenesis of inflammatory bowel disorders and the presence of these cells in the periphery might reflect an active inflammatory process in the gut [44]. Overall, we were not able to find any differences in the expression of CCR9 or B7 integrin on either CD4+ or CD8+ T cells before or after gluten-free diet (data not shown). However, a moderate reduction in CD8+CCR9+ T cells was observed only in children remaining high in tTG autoantibody levels after six months. This may indicate that gluten-free diet nonresponders may retain cytotoxic T cells in the gut, but the significance of this finding will need further investigation.
Interestingly, it has been shown that in peripheral blood gliadin-specific T cells secreting IFN-γ and expressing B7 integrin are only detectable for a few days shortly after oral gluten challenge in CD patients that were previously on gluten-free diet [21,45]. These findings could explain our difficulties in detecting changes in T cells expressing markers associated with homing to the intestinal lamina propria after three and six months of gluten-free diet and are in line with our observation of a specific increase of primed regulatory T cells after antigen removal. In future studies, we will monitor children with more frequent blood samples to test the hypothesis that changes in peripheral B and T cell subsets will precede the reduction in tTG autoantibody levels in response to gluten-free diet.
In summary, this prospective observational study adds credible evidence that the decline in tTG autoantibody levels induced by gluten-free diet is associated with a shift in specific peripheral blood lymphocyte subsets in children with newly diagnosed CD. We further found that the children who significantly reduced their tTG autoantibody levels after six months of treatment, also showed the greatest changes in peripheral B and T cell subset. From a clinical point of view, this study suggests that flow cytometry might be a useful complement to tTG autoantibodies to monitor the disease activity of childhood CD. Indeed, peripheral blood lymphocyte subsets need to be further explored in both clinical and functional studies to evaluate the role of B cells and regulatory T cells, not only during the development of CD but also in response to gluten-free diet.
Acknowledgments
The study was funded by the Faculty of Medicine, Lund University, the Skåne Council Foundation for Research and Development, University Hospital MAS in Malmö, the Majblomman Fund, the Swedish Research Council (Grants ♯2002–6535, ♯2004–1102), the Swedish Society of Medical Research (SSMF), and Direktör Albert Påhlssons foundation.
References
- 1.Marsh M. Gluten, major histocompability complex and small intestine. Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 2.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E, Detlef S. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 4.Rostom A, Dube C, Cranney A, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Aeschlimann D, Paulsson M. Transglutaminases: protein cross-linking enzymes in tissues and body fluids. Thromb Haemost. 1994;71:402–15. [PubMed] [Google Scholar]
- 6.Molberg O, McAdam SN, Körner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 7.Vader LW, de Ru A, van der Wal Y, et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med. 2002;195:643–9. doi: 10.1084/jem.20012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Niro R, Ferrara F, Not T, Bradbury AR, Chirdo F, Marzari R, Sblattero D. Characterizing monoclonal antibody epitopes by filtered gene fragment phage display. Biochem J. 2005;388:889–94. doi: 10.1042/BJ20041983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sblattero D, Florian F, Azzoni E, et al. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur J Biochem. 2002;269:5175–81. doi: 10.1046/j.1432-1033.2002.03215.x. [DOI] [PubMed] [Google Scholar]
- 10.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–6. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 11.Seissler J, Wohlrab U, Wuensche C, Scherbaum WA, Boehm BO. Autoantibodies from patients with coeliac disease recognize distinct functional domains of the autoantigen tissue transglutaminase. Clin Exp Immunol. 2001;125:216–21. doi: 10.1046/j.1365-2249.2001.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halttunen T, Maki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology. 1999;116:566–72. doi: 10.1016/s0016-5085(99)70178-2. [DOI] [PubMed] [Google Scholar]
- 13.Esposito C, Paparo F, Caputo I, et al. Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut. 2002;51:177–81. doi: 10.1136/gut.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieterich W, Trapp D, Esslinger B, Leidenberger M, Piper J, Hahn E, Schuppan D. Autoantibodies of patients with coeliac disease are insufficient to block tissue transglutaminase activity. Gut. 2003;52:1562–6. doi: 10.1136/gut.52.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49:1621–6. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 16.Quaratino S, Ruf J, Osman M, Guo J, McLachlan S, Rapoport B, Londei M. Human autoantibodies modulate the T cell epitope repertoire but fail to unmask a pathogenic cryptic epitope. J Immunol. 2005;174:557–63. doi: 10.4049/jimmunol.174.1.557. [DOI] [PubMed] [Google Scholar]
- 17.Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarstrom S, Hammarstrom M. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology. 2002;123:667–78. doi: 10.1053/gast.2002.35355. [DOI] [PubMed] [Google Scholar]
- 19.Salvati V, Mazzarella G, Gianfrani C, et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut. 2005;54:46–53. doi: 10.1136/gut.2003.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A. Cytokine profile in coeliac disease. Scand J Immunol. 1999;49:441–6. doi: 10.1046/j.1365-3083.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 21.Anderson R, Degano P, Godkin A, Jewell D, Hill A. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 23.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 24.Makita S, Kanai T, Oshima S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–30. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 25.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–40. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 28.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 29.Iellem A, Colantonio L, D’Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur J Immunol. 2003;33:1488–96. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadakis KA, Landers C, Prehn J, Kouroumalis EA, Moreno ST, Gutierrez-Ramos JC, Hodge MR, Targan SR. CC chemokine receptor 9 expression defines a subset of peripheral blood lymphocytes with mucosal T cell phenotype and Th1 or T-regulatory 1 cytokine profile. J Immunol. 2003;171:159–65. doi: 10.4049/jimmunol.171.1.159. [DOI] [PubMed] [Google Scholar]
- 32.Walker-Smith J, Guandalini S, Schmitz J, Shmerling D, Visakorpi J. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition (ESPGAN) Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grubin C, Daniela T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–450. doi: 10.1007/BF00408469. [DOI] [PubMed] [Google Scholar]
- 34.Falorni A, Ortqvist E, Persson B, Lernmark Å. Radioimmunoassys for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Meth. 1995;86:89–99. doi: 10.1016/0022-1759(95)00139-2. [DOI] [PubMed] [Google Scholar]
- 35.Chorzelski TP, Sulej J, Tchorzewska H, Jablonska S, Beutner EH, Kumar V. IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease. Ann NY Acad Sci. 1983;420:325–34. doi: 10.1111/j.1749-6632.1983.tb22220.x. [DOI] [PubMed] [Google Scholar]
- 36.Sjöroos M, Iitiä A, Ilonen J, Reijonen H, Lövgren T. Triple-Label Hybridization Assay for Type-1 Diabetes-Related HLA Alleles. Biotechniques. 1995;18:870–7. [PubMed] [Google Scholar]
- 37.Larsson HE, Lynch K, Lernmark B, Nilsson A, Hansson G, Almgren P, Lernmark A, Ivarsson SA. Diabetes-associated HLA genotypes affect birthweight in the general population. Diabetologia. 2005;48:1484–91. doi: 10.1007/s00125-005-1813-4. [DOI] [PubMed] [Google Scholar]
- 38.Hansson T, Dahlbom I, Rogberg S, Dannaeus A, Hopfl P, Gut H, Kraaz W, Klareskog L. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr Res. 2002;51:700–5. doi: 10.1203/00006450-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Agardh D, Dahlbom I, Daniels T, Lorinc E, Ivarsson SA, Lernmark A, Hansson T. Autoantibodies Against Soluble and Immobilized Human Recombinant Tissue Transglutaminase in Children with Celiac Disease. J Pediatr Gastroenterol Nutr. 2005;41:322–7. doi: 10.1097/01.mpg.0000174845.90668.fa. [DOI] [PubMed] [Google Scholar]
- 40.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25 (high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Katou F, Ohtani H, Nakayama T, et al. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am J Pathol. 2001;158:1263–70. doi: 10.1016/S0002-9440(10)64077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–56. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology. 2001;121:246–54. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]
- 45.Anderson RP, van Heel DA, Tye-Din JA, Barnardo M, Salio M, Jewell DP, Hill AV. T cells in peripheral blood after gluten challenge in coeliac disease. Gut. 2005;54:1217–23. doi: 10.1136/gut.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]