Abstract
Multiple myeloma (MM) is a B cell cancer characterized by clonal proliferation in the bone marrow and impaired immunity. Because MM is an incurable malignancy, efficient consolidation is needed urgently. Targeting clonotypic B cells by idiotype vaccination has proved the principle to be effective and indicated that future strategies, including dendritic cell-based vaccination, could be a suitable approach. However, as MM patients suffer from a general impaired immunity, which may include dendritic cells (DCs), a careful evaluation of phenotypic traits and functionality of DCs from MM patients is necessary before an efficient vaccine can be developed. This study determined the number, phenotypic profile and functionality of myeloid and plasmacytoid DCs purified directly from blood from MM patients at diagnosis. A reduced number and lower expression of human leucocyte antigen (HLA) molecules was observed on both myeloid and plasmacytoid DCs in MM patients compared to healthy controls. Also, the expression of CCR5, CCR7 and DEC205 was lower in MM patients compared to normal donors. In addition, the capacity to stimulate allogeneic T cell proliferation and to stimulate cytokine production was decreased, suggesting that DCs from these patients are functionally impaired. Finally, the analysis of samples following chemotherapy and transplantation demonstrated an increased expression of HLA molecules, suggesting that this time-point is optimal for harvest and use in vaccination.
Keywords: functionality, multiple myeloma, myeloid dendritic cells, phenotypic profile, plasmacytoid dendritic cells
Introduction
Multiple myeloma (MM) is a B cell cancer characterized by clonal proliferation of plasma cells in the bone marrow. The incidence is 6–9 per 100 000 per year with 30–40% of patients below 65 years of age. The prognosis has improved recently, with a median survival time of 5–6 years following autologous stem cell transplantation. Because MM is an incurable malignancy the need for efficient consolidation strategies is urgent. One of the most effective ways of controlling the malignant B cells in MM seems to be allogeneic transplantation [1,2], demonstrating that the immune system could be a very important player in controlling this cancer. However, allogeneic transplantation also leads to serious side effects due to graft-versus-host disease [3]. To avoid such complications, initiation of a specific immune response targeting the malignant B cell clone is essential.
The cells responsible for initiating a specific T cell response are dendritic cells (DC) [4,5]. These cells have been studied intensively for the last decade; not only do they seem to be involved in initiating immune responses, they are also capable of tolerizing T cells, thereby preventing autoreactivity. In cancer treatment, DCs have been used as a cellular vaccine aimed at initiating anti-tumour responses in patients. The results of these studies vary and interpretations are difficult due to limited study size and differences in protocols [6]. So far, the DC subset and the particular maturation stimuli required for optimal anti-tumour immunity is not known, but it seems very important not to use an immature subset of DC for immunization, as this could lead ultimately to tolerance instead of immunity [7–9].
The two most well-characterized subsets of DCs are the myeloid DCs (mDC) and plasmacytoid DCs (pDC) [10]. mDCs are generally considered as the DC subset responsible for initiating immune responses, while the role of pDCs is less clear. pDCs were described originally in humans as producers of type I interferon during virus infection [11], but were demonstrated later to function as antigen-presenting cells (APCs) involved in both Th1 and Th2 immunity, although less efficiently than mDCs (for review see [12]). In addition, they were suggested to be capable of inducing tolerance [13].
In order to evaluate the potential use of DCs from MM patients, the DCs must be evaluated carefully as consolidation. Contradictory results have been obtained regarding the number, phenotypic status and functionality of DCs from MM patients. Some studies demonstrate that patients with MM have a reduced number of DCs compared to control donors [14–16], while other studies show that the level of DCs in these patients is normal [17,18]. Also, conflicting results exist regarding the expression of DC maturation markers and the ability to stimulate allogeneic proliferation in these patients [14–18]. Therefore, additional experiments are needed in order to clarify the status of DCs in MM patients. In the present study, the expression of various DC maturation markers on mDCs and pDCs enriched from peripheral blood was investigated and their capacity to induce proliferation and cytokine production of allogeneic T cells was evaluated. Contrary to most other studies, DCs were enriched directly from peripheral blood in order to minimize manipulation of the cells prior to analysis. In addition, the phenotypic status of DCs following autologous stem cell transplantation was evaluated in order to identify the optimal time-point for DC vaccination.
Materials and methods
Patients and healthy controls
Included in this study were 19 MM patients at diagnosis and eight MM patients who had received chemotherapy and autologous stem cell transplantation (referred to as MM patients after treatment). Samples from this latest patient group were taken 2–3 months after autologous stem cell transplantation. The study protocol was approved by the Institutional Ethical Committees, Copenhagen County, and written informed consent was obtained from all patients at study entry. Blood donors were used as healthy controls.
Dendritic cell enrichment procedure
Mononuclear cells (MNC) were obtained from peripheral blood by gradient centrifugation (Lymphoprep, 1·077 g/ml, Nycomed, Pharma AS, Oslo, Norway) from (1) MM patients at diagnosis, (2) MM patients after treatment and (3) healthy donors. Red blood cells were lysed and MNCs were cryopreserved in fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, CA, USA) containing 5% dimethylsulphoxide (DMSO) until use. For analysis of phenotypic markers MNCs were thawed in MACS buffer containing 0025 mg/ml pulmozyme (Roche, Basel, Switzerland) to prevent clotting of columns. Monocytes and B cells were depleted using the non-DC-depleting cocktail contained in the blood dendritic cell isolation kit II and LD depletion columns in a VarioMACSTM separator according to the manufacturer's recommendations (all from Miltenyi Biotec, Bergisch Gladbach, Germany). Following enrichment the cells were stained for analysis of different phenotypic markers (see below). For analysis of DC functionality (ability to stimulate proliferation and cytokine production) MNCs were thawed in MACS buffer containing pulmozyme and DCs were enriched using the full blood DC isolation kit II (Miltenyi Biotec) according to the manufacturer's recommendations. For all functional analyses the DC pool contained myeloid DC type 1 (mDC1), myeloid DC type 2 (mDC2) and plasmacytoid DCs (pDC), but for analysis of the phenotypic profile and cell number only mDC1s and pDCs were evaluated, as it was not possible to detect a sufficient number of mDC2 to perform this analysis. Henceforth mDC1 are referred to as mDC.
Flow cytometry analysis
Directly conjugated monoclonal antibodies were purchased from Becton Dickinson (San Jose, CA, USA): anti-CD80 fluorescein isothiocyanate (FITC), anti-CD86 FITC, anti-CD40 FITC, anti-CD83 FITC, anti-CCR5 FITC, anti-human leucocyte antigen D-related (HLA-DR) FITC, anti-HLA-A,B,C FITC, anti-CD4 FITC, anti-CD3 phycoerythrin (PE), anti-CD14 PE, anti-CD19 PE, anti-CD8 peridinin chlorophyll (PerCP) and anti-interferon (IFN)-γ APC. From R&D Systems (Abingdon, UK) were purchased anti-CCR7 FITC and anti-intercellular adhesion molecule (ICAM-1) FITC; from eBioscience (San Diego, CA, USA): anti-DEC-205 FITC; and from Miltenyi Biotec: blood dendritic cell antigen (BDCA)-1 APC and anti-BDCA-2 APC. Isotype controls of irrelevant specificity were used for setting limits of non-specific immunoglobulin cell binding. Flow cytometry was performed on a FACScalibur (Becton Dickinson) and the data were analysed using CellQuest software (Becton Dickinson).
Phenotypic analysis and quantification of DCs
The cells were stained with a panel of relevant antibodies for 20 min at 4°C and washed in phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA). mDCs and pDCs were identified as lineage-negative (Lin–) cells (CD3–CD14–CD19–) and BDCA-1+ or BDCA-2+ cells, respectively. Fifty thousand events were collected in R1 excluding dead cells. The percentage of mDC and pDCs were determined as Lin–, BDCA-1+ and Lin–, BDCA-2+ cells, respectively. The mean fluorescence intensity of various DC maturation markers was analysed in the BDCA-1 and BDCA-2 positive cells fractions.
PKH proliferation assay
For analysis of T cell proliferation induced by allogeneic DCs, DCs from MM patients at diagnosis or healthy donors were enriched as described above. These cells were cultured in round-bottomed 96-well microtitre plates at various concentrations in RPMI-1640 + GlutaMAXTM +25 mm HEPES (Gibco), supplemented with 10% FBS (Gibco) + penicillium-streptomycin (100 µg/ml) with 200 000 MNCs from buffy coats from normal blood donors. Cells from the same buffy coat were used in all experiments. Prior to culture, MNCs were stained with PKH26 (Sigma, St Louis, MO, USA) following the manufacturer's protocol. Around 20 × 106 cells were stained in a 15 × 10−6 M PKH26. After 7 days in culture at 37°C in a humidified 5% CO2 atmosphere the cells were stained with anti-CD4 FITC and anti-CD8 PerCP and analysed for proliferation (dilution of the PKH26 dye) on a FACScalibur. Thirty thousand events were obtained in R1 excluding dead cells. PHK26-labelled MNC cultured without DCs were included as negative controls.
Intracellular cytokine staining
For analysis of production of IFN-γ induced by allogeneic DCs, DCs from MM patients at diagnosis or healthy donors were enriched as described above. These cells were cultured in round-bottomed 96-well microtitre plates at various concentrations in RPMI-1640 + GlutaMAXTM +25 mM HEPES (Gibco) supplemented with 10% FBS (Gibco) + penicillium-streptomycin (100 µg/ml), with 200·000 MNCs from buffy coats. As maturation stimuli, CD40L (Amgen, Seattle, WA, USA) was added to the cultures (0·5 µg/ml final concentration). After 7 days in culture at 37°C in a humidified 5% CO2 atmosphere, anti-CD28 and anti-CD2 antibodies were added to the positive control wells. After 1 h Brefeldin A (Sigma) was added to the cultures (at a final concentration of 10 µg/ml) and the cells were incubated for 5 h at 37°C. After incubation the cells were harvested and fluorescence activated cell sorter (FACSTM) lysing solution (Becton Dickinson) was added for 10 min at room temperature (RT). The cells were centrifuged and FACSTM permeabilizing solution (Becton Dickinson) was added to the cells and incubated for 10 min at RT. After washing in PBS + 0·5% BSA, antibodies to CD4, CD8 and IFN-γ were added and the cells were incubated for 20 min at 4°C. The cells were analysed on a FACScalibur. Thirty thousand events were obtained in R1, excluding dead cells. For analysis the cells were gated on lymphocytes, then the proportion of CD4+ IFN-γ+ or CD8+ IFN-γ+ cells were identified.
Production of cytokines in co-culture of DCs and allogeneic MNCs
To analyse a broader spectrum of cytokines we used the human Th1/Th2 cytokine cytometric bead array kit (CBA) from BD Bioscience. The supernatants from the co-cultures used for measuring intracellular IFN-γ were collected and kept frozen until use. The assay was performed as recommended by the manufacturer.
Statistics
The data were analysed using a Mann–Whitney U-test for non-parametric data.
Results
Reduced number and low expression of maturation markers on myeloid and plasmacytoid DCs from MM patients at diagnosis
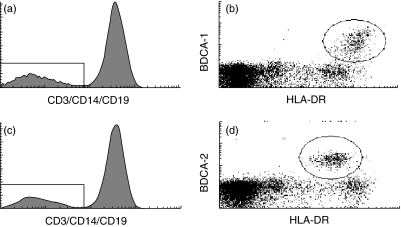
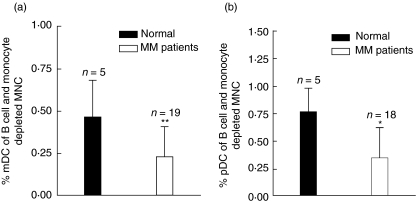
First, the percentage of mDCs and pDCs was evaluated. MNCs purified from peripheral blood from MM patients at diagnosis or healthy blood donors were depleted for B cells and monocytes and labelled with a lineage cocktail (CD3, CD14, CD19) and either BDCA-1 (mDCs) or BDCA-2 (pDCs). mDCs were identified as lineage-negative, BDCA-1-positive cells (Fig. 1a,b) and pDCs were identified as lineage-negative, BDCA-2-positive cells (Fig. 1c,d). It was found that MM patients have approximately half as many of both DC subsets compared to normal donors (Fig. 2a,b).
Fig. 1.
Defining the population of myeloid dendritic cells (mDC) and plasmacytoid DCs (pDC). (a, b) Mononuclear cells (MNCs) purified from peripheral blood from multiple myeloma (MM) patients at diagnosis or blood donors were depleted for B cells and monocytes and labelled with lineage cocktail (CD3, CD14, CD19) and blood dendritic cell antigen (BDCA-1) to identify the fraction of mDC. The histogram shows the definition of lineage negative cells, while the dot-plot shows the population of BDCA-1 positive cells [shown here together with expression of anti-human leucocyte antigen D-related (HLA-DR)]. The population of BDCA-1, HLA-DR-positive cells was used for evaluating the percentage of mDC. (c, d) MNCs purified from peripheral blood from MM patients at diagnosis or blood donors were depleted for B cells and monocytes and labelled with lineage cocktail (CD3, CD14, CD19) and BDCA-2 to identify the fraction of pDCs. The histogram shows the definition of lineage negative cells, while the dot-plot shows the population of BDCA-2 positive cells (shown here together with expression of HLA-DR). The population of BDCA-2, HLA-DR-positive cells was used for evaluating the percentage of pDC.
Fig. 2.
Percentage of myeloid dendritic cells (mDC) and plasmacytoid DCs (pDC) in multiple myeloma (MM) patients and healthy controls. Mononuclear cells (MNCs) purified from peripheral blood from MM patients at diagnosis or blood donors were depleted for B cells and monocytes and labelled with lineage cocktail (CD3, CD14, CD19) and either blood dendritic cell antigen (BDCA-1)+anti-human leucocyte antigen D-related (HLA-DR) or BDCA-2+HLA-DR to identify the fraction of mDC or pDC. (a) Percentage of mDC in MM patients and healthy controls after depletion of B cells and monocytes. (b) Percentage of pDC in MM patients and healthy controls after depletion of B cells and monocytes. *P < 0·05, **P < 0·01.
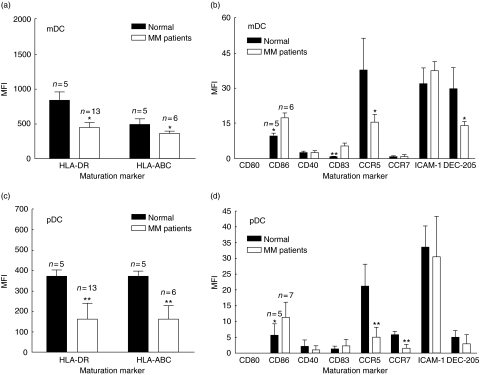
Next, the expression level (mean fluorescence intensity) of various maturation markers was evaluated for both DC subsets. As shown in Fig. 3a, the expression of HLA-DR on mDC was approximately two times lower in MM patients than in normal donors. In addition, there was a significant tendency towards a lower expression of HLA-A,B,C on mDC from MM patients compared to normal donors (Fig. 3a). As seen in Fig. 3b, the expression of CCR5 and DEC-205 was also approximately two times lower on mDC from MM patients compared to normal donors, while the expression of CD86 and CD83 was higher on mDC from MM patients compared to normal controls (1·8× and 6·6×, respectively). Figure 3c shows the expression of HLA-DR and HLA-A,B,C on pDCs. As shown in Fig. 3, both the expression of HLA-DR and HLA-A,B,C was approximately two times lower on pDCs from MM patients than on normal donors. Furthermore, the expression of CCR5 and CCR7 was approximately four times lower on pDCs from MM patients compared to normal control donors (Fig. 3d). Conversely, the expression of CD86 was two times higher on pDCs from MM patients (Fig. 3d) compared to pDCs from normal donors.
Fig. 3.
Expression of phenotypic markers in myeloid dendritic cells (mDC) and plasmacytoid DCs (pDC) in DCs from multiple myeloma (MM) patients and healthy controls. Mononuclear cells (MNCs) purified from peripheral blood from MM patients at diagnosis, or blood donors were depleted for B cells and monocytes and labelled with lineage cocktail (CD3, CD14, CD19) and either blood dendritic cell antigen (BDCA-1) or BDCA-2 to identify the fraction of mDC or pDC. (a) Expression of human leucocyte antigen (HLA) molecules in mDC. (b) Expression of various phenotypic markers in mDC. (c) Expression of HLA-molecules in pDC. (d) Expression of various phenotypic markers in pDC. *P < 0·05, **P < 0·01.
In summary, DCs from MM patients had a lower expression of various maturation markers compared to healthy control donors, except for CD86 and CD83, which were expressed at higher levels in MM patients. Regarding the two different DC subsets, both mDCs and pDCs from MM patients demonstrated this reduced expression of maturation markers. Finally, MM patients showed reduced levels of both mDCs and pDCs compared to normal donors.
DCs from MM patients at diagnosis are less efficient at stimulating allogeneic T cell proliferation compared to healthy donors
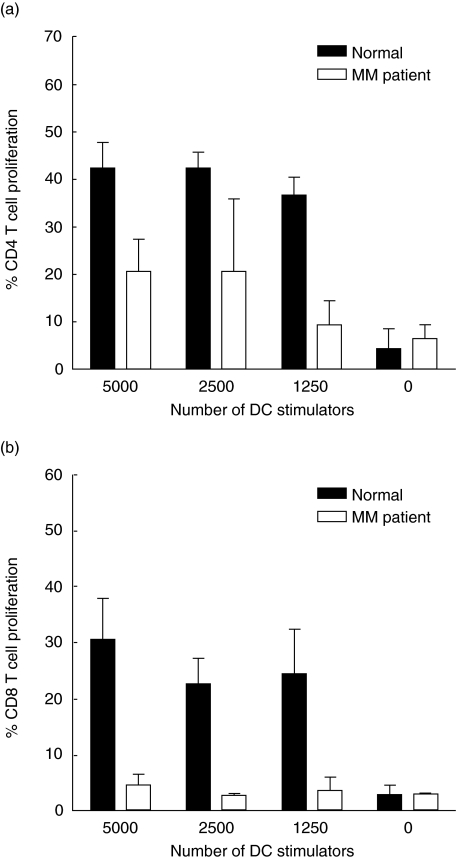
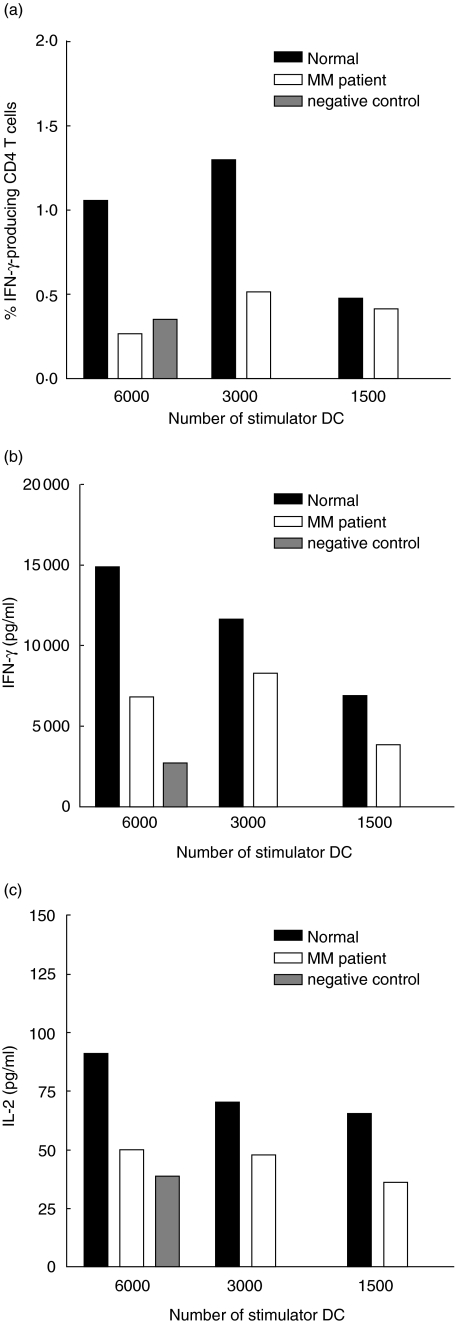
In order to examine the functionality of DCs from MM patients, mixed lymphocyte cultures were performed using DCs enriched from peripheral blood as stimulator cells and MNCs from buffy coats as responder cells. Figure 4a shows one representative experiment, showing that DCs from the MM patient induced weaker CD4+ T cell proliferation than DCs from the healthy control donor. When evaluating the CD8+ T cell proliferation it was also found that DCs from MM patients were less efficient at stimulating these cells compared to DCs from healthy donors (Fig. 4b shows one representative experiment). This was the case in four of five experiments, demonstrating that DCs from MM patients are impaired in their ability to stimulate CD4+ as well as CD8+ T cell proliferation.
Fig. 4.
Proliferation of allogeneic CD4+ and CD8+ T cells stimulated by dendritic cells (DCs) from multiple myeloma (MM) patients and healthy controls. DCs were enriched from peripheral blood and were cultured at various ratios together with PKH26-labelled mononuclear cells (MNCs). After 7 days of co-culture the cells were harvested and the proliferation was measured. (a) Proliferation of allogeneic CD4+ T cells in one MM patient and one control. (b) Proliferation of allogeneic CD8+ T cells in one MM patient and one control. The negative control contains only PKH26-labelled MNCs to obtain the background proliferation. One representative experiment of five is shown in each case.
Low production of cytokines in allogeneic MNCs stimulated by DCs from MM patients
To investigate the production of cytokines from allogeneic MNCs stimulated by DCs from either MM patients or healthy donors, intracellular cytokine staining for IFN-γ was performed after 7 days of co-culture. Compared to healthy donors, a lower percentage of allogeneic CD4+ T cells produced IFN-γ when stimulated by DCs from MM patients (Fig. 5a). This was the case in four of five experiments. The production of intracellular IFN-γ in CD8+ T cells was undetectable (data not shown). To examine a broader spectrum of cytokines the human Th1/Th2 cytokine cytometric bead array kit (CBA), which measures the level of six cytokines in the co-culture supernatants, was used. Figure 5b shows one representative experiment of five of IFN-γ production in one co-culture. As seen from Fig. 5, less IFN-γ is produced in co-cultures of allogeneic MNCs and DCs from MM patients than in co-cultures with healthy DCs. This was the case in five of five experiments. Also, when measuring interleukin (IL)-2 production, in five of five experiments it was found that this was lowest when using DCs from MM patients (Fig. 5c). When analysing the levels of IL-4, IL-5 and tumour necrosis factor (TNF)-α no differences were found between the two groups (data not shown). There was a tendency towards a lower expression of IL-10 in co-culture with DCs from MM patients compared to healthy DCs, but the levels were very low (data not shown).
Fig. 5.
Production of interferon (IFN)-γ and interleukin (IL)-2 in allogeneic T cells stimulated by dendritic cells (DCs) from multiple myeloma (MM) patients and healthy controls. DCs were enriched from peripheral blood and were cultured at various ratios together with allogeneic mononuclear cells (MNCs) and CD40L. After 7 days of co-culture the supernatants were harvested for analysis of cytokine production and intracellular cytokine production was also measured. (a) Production of intracellular IFN-γ in allogeneic CD4+ T cells. The allogeneic T cells were stimulated with DCs from one MM patient and one healthy control donor. One representative experiment of five is shown. (b) Production of IFN-γ from allogeneic MNCs. The allogeneic MNCs were stimulated with DCs from one MM patient and one healthy control donor. One representative experiment of five is shown. (c) Production of IL-2 from allogeneic MNCs. The allogeneic MNCs were stimulated with DCs from one MM patient and one healthy control donor. One representative experiment of five is shown. The negative control contains only MNCs and CD40L to obtain the background production of cytokines.
Expression of HLA molecules and percentage of DCs after chemotherapy and autologous stem cell transplantation
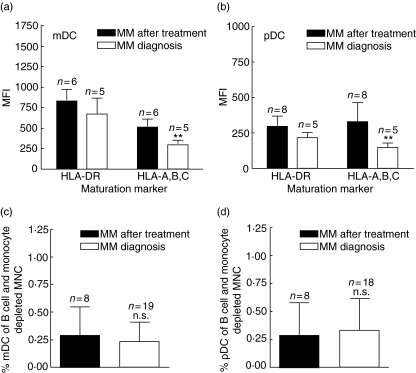
To investigate the effect of myeloma cell load on the DC compartment in MM patients we evaluated the expression of HLA molecules in patients who had received chemotherapy followed by autologous stem cell transplantation (referred to as MM after treatment) and compared these to MM patients at diagnosis. As seen in Fig. 6a and b, there was a tendency (not significant) towards higher expression of HLA-DR in MM patients after treatment compared to MM patients at diagnosis in both mDCs and pDCs. The expression of HLA-A,B,C was also higher after treatment than before treatment in both cell types (mDC 1·7× and pDC 2·3×). Conversely, the percentage of both mDC and pDCs after treatment was as low as in MM patients at diagnosis (Fig. 6c,d).
Fig. 6.
Expression of phenotypic markers and percentage of myeloid dendritic cells (mDC) and plasmacytoid DCs (pDC) in DCs from multiple myeloma (MM) patients at diagnosis and MM patients after treatment. Mononuclear cells (MNCs) purified from peripheral blood from MM patients at diagnosis or MM patients after treatment was depleted for B cells and monocytes and labelled with lineage cocktail (CD3, CD14, CD19) and either blood dendritic cell antigen (BDCA-1) or BDCA-2 to identify the fraction of mDC or pDC. (a) Expression of human leucocyte antigen (HLA) molecules in mDC. (b) Expression of HLA-molecules in pDC. (c) Percentage of mDC. (d) Percentage of pDC. **P < 0·01.
Discussion
The results of this study are in line with other studies also demonstrating lower levels of DCs in MM patients [14–16], together with decreased expression of maturation markers [14–17]. However, in contrast to most other studies the expression of phenotypic markers was evaluated on DCs purified directly from blood in order to minimize manipulation of the cells prior to use. It was found that HLA molecules were expressed at lower levels on MM patients’ DCs compared to healthy controls, probably making DCs from MM patients inferior at antigen presentation. Other markers such as CCR5, CCR7 and DEC-205 were also expressed at lower levels on DCs from MM patients compared to healthy controls. CCR5 directs the migration of immature DCs into the tissue during inflammation, while CCR7 directs mature DCs to the draining lymph nodes [19]. Therefore, low expression of these molecules indicates that the migration capacity of these cells is impaired. In general, the expression of CCR7 was low compared to CCR5, indicating that the DCs had an immature phenotype. DEC-205 is a multi-lectin receptor for endocytosis expressed on DCs [20,21]. This receptor was expressed at lower levels on mDCs from MM, indicating that myeloid DCs from MM patients are less efficient at sampling antigens, at least by DEC-205. In contrast, we found that the expression of CD86 was higher on both DC subsets from MM patients compared to healthy controls. Because CD86 is a co-stimulatory molecule it is considered generally to be an important factor in the induction of immune responses. Therefore, high expression of this molecule could indicate a better capacity of inducing immune responses, which would be favourable when attempting to induce anti-tumour immunity. The observed expression level, however, is low and the fact that DCs from MM patients demonstrated an inferior ability to stimulate both proliferation and cytokine production (see below) indicates that the higher expression of CD86 is functionally insignificant.
It was also demonstrated that DCs from MM patients stimulated allogeneic T cell proliferation less efficiently than DCs from healthy donors, indicating that the functionality of these cells was impaired. This is in line with an earlier study, demonstrating that DCs from MM patients did not stimulate proliferation of allogeneic T cells as well as DCs from healthy donors [14]. Furthermore, to stress the fact that DCs from MM patients are functionally impaired, the ability of these cells to induce production of IFN-γ in alloreactive T cells was investigated. Production of IFN-γ is a good supplementary indicator of a functional immune response as T cell proliferation alone could indicate an early event of tolerance induction [7,9]. It was found that DCs from MM patients did not stimulate production of IFN-γ as efficiently as DCs from normal donors, demonstrating that the DC system in MM patients does not function optimally. To our knowledge, no other studies have demonstrated a reduced ability of inducing IFN-γ production in MM patients. It was also found that DCs from MM patients did not induce production of IL-2 in allogeneic T cells as efficiently as normal DCs. With regard to the production of other cytokines the expression of IL-4, IL-5, IL-10 and TNF-α was also investigated, but no clear tendency was observed. Furthermore, there was no production of cytokines in cultures containing only DCs. Taken together, the results presented here demonstrate a general impairment of DCs from MM patients.
In other forms of cancer the DC compartment also seems to be influenced by the presence of cancer cells. In patients with chronic lymphocytic leukaemia the DCs were impaired [22,23], and in other haematological malignancies the level of DCs was also lower than normal [24,25] or the DCs had an impaired maturation capacity and allostimulatory activity [26]. In patients with breast, head, neck and lung cancer the number of DCs in peripheral blood was reduced significantly [27,28], and other studies involving breast cancer patients demonstrates that DCs do not function in an optimal way [29,30]. These studies indicate that there is a general tendency towards a dysfunctional DC compartment in cancer patients. However, other studies exist involving haematological malignancies, demonstrating no differences in cell number, functionality and expression of maturation markers [17,18,31,32]. In contrast to most of the above-mentioned studies, DCs from these studies were grown from peripheral blood mononuclear cells (PBMC) and not used directly from the blood; therefore, the environment in which the DCs were propagated was distinct from the environment found in cancer patients. This could explain why DCs in these studies contain a normal phenotype and functionality.
In order to investigate the impact of myeloma cell load on development and maturation status of DCs in MM patients, the expression of HLA class I and II and the number of cells in patients who received chemotherapy along with autologous stem cell transplantation was evaluated. These patients are in remission and therefore fewer tumour cells are present than at diagnosis. It was found that there was a tendency towards a higher expression of HLA class II after treatment and also a higher expression of HLA class I compared to newly diagnosed MM patients. This might indicate that DCs from MM patients in remission are in a better condition. This is relevant information for development of a DC-based vaccine, because a logical time-point to administer this supplementary treatment would be after the conventional treatment with chemotherapy. In contrast, no differences in the number of DCs in the two patient groups was observed. In terms of vaccination strategies this could have an impact on the outcome of the vaccination, but it is still difficult to predict the effect of this low number of DCs.
In conclusion, this study demonstrates that DCs from MM patients are phenotypically and functionally impaired and found in lower numbers than in healthy donors. Our results also indicate that the best strategy for DC vaccination in MM patients may be as post-transplant consolidation.
Acknowledgments
This work was supported by the Simon Fougner Hartmanns Familiefond, the Købmand Sven Hansen og Hustru Ina Hansens Fond, the Fabrikant Einar Willumsens Mindelegat and the Familien Hede Nielsens Fond.
References
- 1.Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18:3031–7. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 2.Cavo M, Terragna C, Martinelli G, et al. Molecular monitoring of minimal residual disease in patients in long-term complete remission after allogeneic stem cell transplantation for multiple myeloma. Blood. 2000;96:355–7. [PubMed] [Google Scholar]
- 3.Socie G. Current issues in allogeneic stem cell transplantation. Hematology. 2005;10(Suppl. 1):63. doi: 10.1080/10245330512331389971. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–86. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 7.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 9.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–7. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 15.Do TH, Johnsen HE, Kjaersgaard E, Taaning E, Svane IM. Impaired circulating myeloid DCs from myeloma patients. Cytotherapy. 2004;6:196–203. doi: 10.1080/14653240410006004. [DOI] [PubMed] [Google Scholar]
- 16.Brown R, Murray A, Pope B, et al. Either interleukin-12 or interferon-gamma can correct the dendritic cell defect induced by transforming growth factor beta in patients with myeloma. Br J Haematol. 2004;125:743–8. doi: 10.1111/j.1365-2141.2004.04984.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–8. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 18.Raje N, Gong J, Chauhan D, et al. Bone marrow and peripheral blood dendritic cells from patients with multiple myeloma are phenotypically and functionally normal despite the detection of Kaposi's sarcoma herpesvirus gene sequences. Blood. 1999;93:1487–95. [PubMed] [Google Scholar]
- 19.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Swiggard WJ, Heufler C, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 21.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orsini E, Guarini A, Chiaretti S, Mauro FR, Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63:4497–506. [PubMed] [Google Scholar]
- 23.Orsini E, Pasquale A, Maggio R, et al. Phenotypic and functional characterization of monocyte-derived dendritic cells in chronic lymphocytic leukaemia patients: influence of neoplastic CD19 cells in vivo and in vitro. Br J Haematol. 2004;125:720–8. doi: 10.1111/j.1365-2141.2004.04971.x. [DOI] [PubMed] [Google Scholar]
- 24.Boissel N, Rousselot P, Raffoux E, et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia. 2004;18:1656–61. doi: 10.1038/sj.leu.2403474. [DOI] [PubMed] [Google Scholar]
- 25.Bourguin-Plonquet A, Rouard H, Roudot-Thoraval F, et al. Severe decrease in peripheral blood dendritic cells in hairy cell leukaemia. Br J Haematol. 2002;116:595–7. doi: 10.1046/j.0007-1048.2001.03318.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohty M, Jarrossay D, Lafage-Pochitaloff M, et al. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–6. doi: 10.1182/blood.v98.13.3750. [DOI] [PubMed] [Google Scholar]
- 27.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 28.Della BS, Gennaro M, Vaccari M, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–72. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–90. [PubMed] [Google Scholar]
- 30.Satthaporn S, Robins A, Vassanasiri W, et al. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53:510–8. doi: 10.1007/s00262-003-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuillier F, Maloum K, Thomas EK, Jouanne C, Dighiero G, Scott-Algara D. Functional monocyte-derived dendritic cells can be generated in chronic lymphocytic leukaemia. Br J Haematol. 2001;115:831–44. doi: 10.1046/j.1365-2141.2001.03223.x. [DOI] [PubMed] [Google Scholar]
- 32.Rezvany MR, Jeddi-Tehrani M, Biberfeld P, et al. Dendritic cells in patients with non-progressive B-chronic lymphocytic leukaemia have a normal functional capability but abnormal cytokine pattern. Br J Haematol. 2001;115:263–71. doi: 10.1046/j.1365-2141.2001.03117.x. [DOI] [PubMed] [Google Scholar]