Abstract
Recently, sera from children with active Henoch-Schönlein purpura (HSP) have been found to enhance interleukin (IL)-8 production by human umbilical venous endothelial cells (HUVEC). To further determine the possible factor with the ability to enhance endothelial IL-8 production in sera from acute stage of HSP, 10 children with HSP at the acute stage and 10 healthy controls were enrolled. IgA antiendothelial cell antibodies (AECA) were detected by cell-based ELISA. Active sera with or without pretreatment with anti-human IgA antibody, sera of controls, and immunoglobulin A (IgA) derived from sera were used to stimulate the HUVEC. The ability of these factors to enhance endothelial IL-8 production was evaluated. Furthermore, signalling pathways were also assayed by different inhibitors, and confirmed by immunoblotting. Serum levels of IgA AECA in HPS patients at the acute stage were significantly higher than in controls (P < 0.001). The active sera could enhance endothelial IL-8 production (P = 0.004, compared with control sera), and the ability of these sera was mostly abolished when pretreated with fixed anti-human IgA antibody. The supernatant IL-8 levels of endothelial cells stimulated by IgA derived from acute stage of HSP were statistically higher than controls (P< 0·001). PD98059, an inhibitor of ERK phosphorylation, significantly reduced IgA AECA-stimulated endothelial IL-8. IgA AECA also enhanced the phosphorylation of ERK1 with a time-dependent manner. Together with these findings, it is concluded that IgA AECA derived from acute stage of HSP may bind to endothelial and enhance endothelial cells to produce IL-8 via MEK/REK signalling pathway.
Keywords: Henoch-Schönlein purpura, IgA AECA, IL-8, MEK/ERK
Introduction
Henoch-Schönlein purpura (HSP), with an annual incidence of 13–20 per 100 000 < 17 years old children [1,2], is a kind of systemic small vessel vasculitis that occurs commonly in children. The disease course is usually benign and self-limited; however, internal organs such as gastrointestinal tract, kidney, and central nervous system involvement are rare but major morbidity of HSP [3–6]. Aggressive therapies with steroid or immunosuppressants are indicated in these conditions.
Although this disease is not uncommon in children, the aetiology and pathogenesis are still unknown. According to the pathological and laboratory findings of immunoglobulin A (IgA) deposits on small vessel wall, polymorphonuclear neutrophils (PMNs) infiltration around the vessel, and increased serum levels of IgA and proinflammatory cytokines during the acute stage, it is speculated that HSP is an immune-mediated vasculitis [3,7,8]. In the previous studies, we have revealed the development of peripheral transforming growth factor-β (TGF-β) secreting T cells during the acute stage, which may act as a switch factor for IgA production [9]. Some circulating IgA from acute but not convalescent sera would bind to endothelial cells and the binding activity of these IgA anti-endothelial cell antibodies (AECA) could be enhanced by tumour necrosis factor (TNF)-α [10]. IgG and IgM AECA found in other systemic vasculitis such as Kawasaki disease, Wegener's granulomatosis, and Takayasu's arteritis were undetectable in HSP. Some of these IgG and IgM antibodies are pathogenic; they may either activate endothelial cells or damage endothelial cells by complement-mediated or antibody-dependent cellular cytotoxic mechanisms [11–13]. Whether the development of IgA AECA during the acute stage of childhood HSP is just an epiphenomenon of vascular damage or has some pathogenic effects should be further evaluated. Another study revealed that sera derived from acute stage of HSP had the ability to enhance interleukin (IL)-8 production by human umbilical venous endothelial cells (HUVEC) [14]. In this study, we aimed to determine the possible factor that exists in acute sera and can enhance endothelial IL-8 production. The signalling pathways between IgA AECA of HSP and human endothelial cells were also clarified.
Materials and methods
Patients
Ten children with typical nonthrombocytopenic purpura were diagnosed with HSP according to the criteria defined by the American College of Rheumatology (ACR) 1990 [15]. Active sera were isolated from whole blood at the acute stage and before steroid or other immunosuppressants treatment. Ten age-matched healthy children were also enrolled as controls. The serum levels of IgA and C-reactive protein (CRP) were analysed by nephelometry, IL-8 was detected by DuoSet human IL-8 ELISA kit (R & D Systems, Inc., Minneapolis, MN, USA). Informed consents were obtained, and the study was approved by hospital institutional review boards.
Human umbilical vein endothelial cells (HUVEC) culture and cell-based ELISA
Human endothelial cells were separated from human umbilical vein by collagenase (Gibco BRL Life Technologies, Rockville, MD, USA), and cultured in complete medium 199 (Gibco BRL, Life Technologies). Cell-based ELISA was performed as previously described [10]. Briefly, HUVEC were seeded on gelatin-coated 96-well microtitre plates (NuncTM, Denmark). When the cellular growth became confluent 3–4 days later, cells were fixed with 0·2% glutaraldehyde. The serum samples, diluted at 1 : 50 for IgA detection, were incubated for 2 h at 37 °C. Peroxidase-conjugated rabbit anti-human IgA immunoglobulins were added to each well for further 2 h. After adding tetramethyl benzidine (TMB) (KPL, Gaithersburg, MD, USA) solution and stop solution, the optical density of each well was read at 450 nm by an ELISA reader. The results were expressed as ELISA ratio (ER)
where S is absorbance of sample, A is absorbance of negative control, and B is positive control.
Sera and IL-8 production by HUVEC
Because the study population was not totally the same as our previous study [14], in order to investigate whether the active sera of these patients had the same ability to enhance endothelial IL-8 production, we repeated the previous protocol [14]: HUVEC were stimulated by either sera from children with active HSP or sera from healthy controls, the concentrations of IL-8 of the supernatants were determined by commercial IL-8 ELISA kit.
TNF-α and endothelial IL-8 production
The serum levels of TNF-α were analysed by commercial ELISA kits (BD PharMingen, San Diego, MN, USA) according to the protocols of the manufactures. HUVEC were seeded on gelatin-coated 96-well microtitre plates at a concentration of 1 × 104 cells/well. When the cellular growth became confluent 3–4 days later, the supernatants were removed. Each well was then washed by PBS for two times, and the recombinant human TNF-α (Gibco BRL, Life Technologies) of different concentrations (0 pg/ml, 100 pg/ml, 1 ng/ml, 10 ng/ml, 50 ng/ml) was added to wells containing HUVEC and serum-free medium 199 alone. Twenty-four hours later, the supernatants were collected and the levels of IL-8 were assayed.
Pre-treated sera and endothelial IL-8 production
In this experiment, sera from children with active HSP were pretreated with neutralizing anti-human TNF-α antibody (R & D system) at a final optimal concentration of 1 µg/ml, or mouse monoclonal anti-human IgA antibody (Sigma, St Louis, MO, USA) that was precoated on a 24-well plate for 2 h. These pretreated sera (50 µl) were cocultured with the HUVEC and 150 µl serum-free medium 199 for 2 h at 37 °C in 5% CO2. After the procedure of washing, the cells were incubated with serum-free medium 199 at 37 °C in 5% CO2 for further 24 h, and the supernatants were then collected. Supernatant IL-8 levels were determined.
Circulating IgA and endothelial IL-8 production
Circulating IgA was isolated from sera by Immobilized Jacalin (Pierce, Rockford, IL, USA), a commercial kit that can isolate serum-circulating IgA1 by galactose-binding lectin. The levels of IgG, IgA, and IgM of eluent were assayed by laser nephelometry (Behring Diagnostics GmbH, Margurg, Germany). HUVEC were then incubated with these isolated IgA at 37 °C for 24 h, and the IL-8 levels of culture supernatants were evaluated.
Inhibition of IL-8 production
HUVEC were seeded and cultured in gelatin-coated 96-well plates and pretreated with 0·1% DMSO alone or four inhibitors (150 µl/well) which were initially dissolved in high concentration DMSO solution and diluted in serum-free medium 199 at a concentration of 0·1% DMSO: 1 µM Helenalin (BIOMOL Research Laboratories Inc., Plymouth Meeting, PA, USA) which inhibits nuclear factor (NF)-κB pathway, 20 µM SB203580 (Calbiochem, San Diego, CA, USA) which inhibits p38 kinase pathway, 25 µM PD98059 (Calbiochem) which inhibits mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway, and 5 µM JNK inhibitor II (Calbiochem) which inhibits Jun N-terminal protein kinase (JNK) pathway. After pretreatment with DMSO alone or inhibitors for 3 h, purified HSP IgA (50 µl/well) was added for another 24 h. Control group was incubated with serum-free medium 199 containing 0·1% DMSO without adding IgA. The supernatants were collected and the IL-8 levels were evaluated and compared.
ERK1/2 phosphorylation
HUVEC were seeded in gelatin-coated 6-well plates and cultured in (2 ml/well) complete medium 199 at 37 °C for 24 h, washed by PBS once, and incubated with serum-free medium 199 alone or 25 µM PD98059 (Calbiochem) (750 µl/well). After incubation for 3 h, cells were stimulated by HSP IgA (250 µl/well), and total cell lysate was extracted by Gold lysis buffer (137 mM NaCl, 20 mM Tris, 10 mM NaF, 5 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, 100 µM β–glycerophosphate, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin) in different time intervals (0 min, 10 min, 30min, 60 min). Samples were loaded (30 µg/well) into the wells of 10% polyacrylamide gel. Electrophoresis was performed at a fixed current for 90–120 min, and the separated gel was transferred to a PVDF membrane, washed with 0·05% TBST, and incubated with blocking buffer (5% milk in TBST) at 25 °C for one hour. The membrane was further incubated either with antiphospho-p44/p42 mitogen-activated protein kinase (MAPK) (ERK1/2) antibody (1: 1000) (phospho-MAPK family antibody sampler kit, Cell Signalling Technology) at 4°C overnight or with anti-p44/p42 MAPK antibody (1: 2000) (Cell Signalling Technology, Danvers, MA, USA) at room temperature for one hour. HRP-conjugated anti-rabbit IgG antibody (Cell Signalling Technology) was then added for another one hour. Finally, ECL plus was added onto the membrane and exposed the signal to the Hyperfilm.
Statistical analysis
The values of serum IgA, IL-8, TNF-α, AECA ELISA ratio, and supernatant IL-8 were expressed as mean ± SEM. The comparisons between groups were conducted using the Mann–Whitney U-test and one-way anova. A two-tailed P-value of <0·05 was considered statistically significant.
Results
Characteristics of patients
All 10 patients had typical skin purpura, 7 patients had abdominal angina and arthritis, and 2 patients suffered from glomerulonephritis. Serum levels of IgA and IL-8 in patients with active HSP were significantly higher than those in normal healthy controls (IgA: 249·5 ± 19·6 mg/dl versus 173·1 ± 2·3 mg/dl, P = 0·01; IL-8: 271·7 ± 88·0 pg/ml versus 6·5 ± 4·4 pg/ml, P = 0·014). Half of 10 patients had elevated CRP (normal value <0·9 mg/dl).
IgA AECA detection
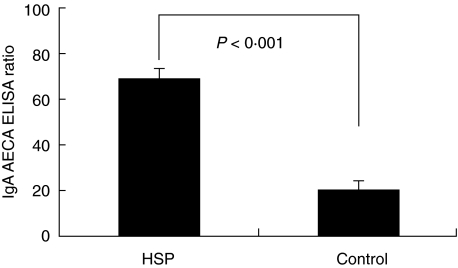
Antibodies of IgA isotype in 10 children with HSP at the acute stage were detected to bind to HUVEC. The serum levels of IgA AECA of these patients were significantly higher than healthy controls (Fig. 1).
Fig. 1.
Cell-based ELISA assay revealed the values of serum IgA AECA (expressed as ELISA ratio (ER)) of 10 HSP patients at the acute stage and 10 healthy controls.
Active sera enhance endothelial IL-8 production
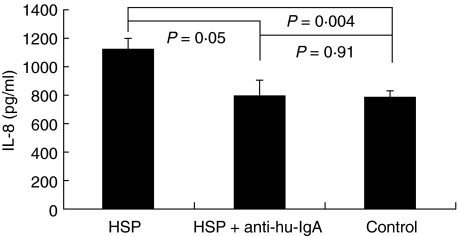
The supernatant IL-8 levels of HUVEC incubated with active sera were statistically higher than those of HUVEC incubated with controls’ sera (1124·3 ± 73·2 pg/ml versus 778·4 ± 51·3 pg/ml, P = 0·004) (Fig. 2).
Fig. 2.
The supernatant IL-8 levels between HUVEC cocultured with active sera, active sera pretreated by anti-human IgA antibody, and sera from healthy controls.
The effects of TNF-α and anti-human TNF-α antibody on endothelial IL-8 release
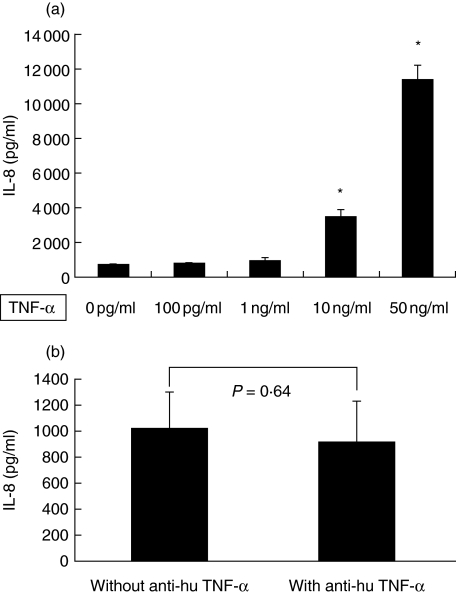
TNF-α was increased during the acute stage of HSP; the serum levels were significantly higher than those in healthy controls (65·3 ± 9·3 pg/ml versus 18·9 ± 4·8 pg/ml, P = 0·001). TNF-α of different concentrations could also enhance the production of IL-8 by HUVEC (0 ng/ml: 718·3 ± 30·2 pg/ml, 100 pg/ml: 781·1 ± 71·0 pg/ml, 1 ng/ml: 948·2 ± 178·9 pg/ml, 10 ng/ml: 3458·1 ± 433·5 pg/ml, 50 ng/ml: 11344·7 ± 873·8 pg/ml) and there was a dose-dependent relationship in this interaction (Fig. 3a). Because TNF-α had the effect of enhancing IL-8 release and the TNF-α serum levels were increased at the acute stage of childhood HSP, in order to determine whether TNF-α is the only factor in sera of patients to enhance endothelial IL-8 production, sera from patients with active HSP were pretreated by neutralizing anti-human TNF-α antibody before the stimulation test. The supernatant IL-8 levels of HUVEC incubated with sera pretreated by anti-human TNF-α antibody were lower than those of HUVEC cocultured with sera without antibody pretreatment, however, not statistically significant (915·6 ± 157·2 pg/ml versus 1021·4 ± 142·5 pg/ml, P = 0·64) (Fig. 3b).
Fig. 3.
(a) TNF-α of different concentrations (0 pg/ml, 100 pg/ml, 1 ng/ml, 10 ng/ml, 50 ng/ml) enhanced HUVEC to release IL-8 with a dose-dependent manner. *P < 0·001 versus 0 pg/ml. (b) The supernatant IL-8 levels between HUVEC cocultured with active sera pretreated by anti-human TNF-α antibody (1 µg/ml) and those cells incubated with sera without antibody pretreatment.
Depletion of circulating IgA by anti-human IgA antibody affects IL-8 release
Incubation with anti-human IgA antibody fixed on a 24-well plate was to remove circulating IgA from active sera. Although this procedure was not specific for IgA AECA depletion, and sera IgA were still detectable with relative low concentration (data not shown) after treatment by anti-human IgA antibody, the enhancement ability of these pretreated sera was mostly inhibited. Figure 2 showed a trend hat the IL-8 levels in supernatant from HUVEC incubated with pretreated sera were decreased when compared with active sera (1124·3 ± 73·2 pg/ml versus 793·1 ± 110·7 pg/ml, P = 0·05), and they were not significantly different from those in supernatant from HUVEC incubated with sera of controls (793·1 ± 110·7 pg/ml versus 778·4 ± 51·3 pg/ml, P = 0·91).
IgA AECA enhance endothelial IL-8 secretion
The eluent collected by Immobilized Jacalin contained only IgA (mean concentration: 200 mg/dl), IgG and IgM were undetectable. The purified IgA was added to stimulate HUVEC, and the IL-8 levels in supernatant from HUVEC incubated with IgA derived from active sera of HSP were significantly higher than controls (2329·6 ± 70·2 pg/ml versus 977·7 ± 32·5 pg/ml, P < 0·001).
PD98059 inhibit the endothelial IL-8 induced by IgA AECA
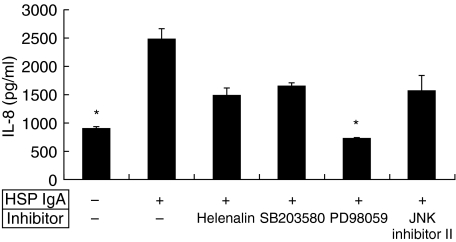
To clarify the possible signalling pathway, inhibitors of different pathways were tested to abolish the ability of IgA AECA to enhance the endothelial IL-8 production. Those inhibitors were finally dissolved in 0·01% DMSO, and the 0·01% DMSO did not influence the effect of IgA AECA on endothelial IL-8 production (2473·3 ± 189·2 pg/ml versus 898·7 ± 27·1 pg/ml, P = 0·001) (Fig. 4). Among these inhibitors, PD98059, an inhibitor of ERK phosphorylation, could significantly reduce the effect of IgA AECA. The IL-8 levels in supernatant of HUVEC pretreated with PD98059 and stimulated by HSP IgA AECA were statistically lower than those of HUVEC stimulated by IgA AECA alone (2473·3 ± 189·2 pg/ml versus 727·3 ± 14·9 pg/ml, P = 0·001) (Fig. 4).
Fig. 4.
HUVEC were stimulated by adding HSP IgA alone, or adding HSP IgA and 4 inhibitors: Helenalin (1 µM), SB203580 (20 µM), PD98059 (25 µM), and JNK inhibitor II (5 µM), respectively. PD98059 significantly inhibited the endothelial IL-8 releasing. *P = 0·001, compared with those HUVEC stimulated by HSP IgA only.
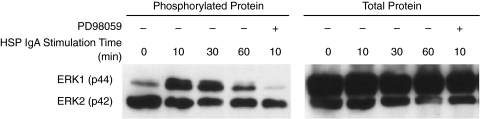
IgA AECA enhance ERK1/2 phosphrylation
Figure 5 revealed that ERK1 phosphorylation was enhanced by the stimulation of IgA AECA. The signal reached the highest 10 min after the stimulation, and then decreased gradually. The effect of IgA AECA on the phosphorylation of ERK1 was inhibited in those HUVEC pretreated by PD98059.
Fig. 5.
Immunoblotting for intracellular total protein of ERK1 and ERK2, and the phosphorylation of ERK1 after IgA AECA stimulation at different time intervals (0 min, 10 min, 30 min, 60 min), and after further treatment by PD98059.
Discussion
HSP is a common systemic childhood vasculitis with unknown pathogenesis. This vasculitis is initially classified as type III hypersensitivity immune-complex disease, however, IgA-containing circulating immune complexes are found only in part of patients [16,17]. According to the increased serum IgA levels, IgA deposition in inflamed blood vessels, we hypothesized that some immunoglobulins of IgA isotype during HSP acute stage may bind to endothelial cells. In 1998, Fujieda et al. [18] found that IgA antibodies against bovine glomerular endothelial cells could be detected in near one half of patients with HSP nephritis. Furthermore, our previous study also revealed that IgA, but not IgG or IgM AECA against human macro- and microvessels would develop at the acute stage of childhood HSP, and they were undetectable at the convalescent stage [10]. In this study, the enrolled patients were identified with IgA AECA by the same method of cell-based ELISA.
AECA represent an extremely heterogeneous family of autoantibodies in different diseases, not only because of the variety of their target endothelial antigens, but also the subsequent diversity of their effects [19]. IgG and/or IgM AECA derived from Kawasaki disease and Takayasu's arteritis have been shown to activate the classical complement pathway and induce lysis of endothelial cells [11,12,20]. In addition to the activation of complement, AECA in other diseases have also been found with different pathogenic roles. In dengue haemorrhagic fever, antibodies of IgG and IgM isotype against NS1 of dengue virus might cross-react with endothelial cells and induce endothelial cell apoptosis [21]. Del Papa et al. [22] revealed that AECA IgG from Wegener's granulomatosis patients did not display any significant cytotoxicity but were able to up-regulate the expression of E-selectin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) and to induce the secretion of cytokines such as IL-6 and IL-8. According to these reports, we desire to investigate whether IgA AECA in childhood HSP have any pathogenic effects.
Initially, we used IgA AECA-containing sera from acute stage of HSP to directly stimulate endothelial cells and the result was consistent with previous study [14], which showed that the IL-8 levels in supernatants of HUVEC incubated with sera from children with active HSP were statistically higher than the levels in supernatants of HUVEC incubated with sera from healthy controls. The presentation of adhesion molecules on endothelial surface was previously evaluated, but no enhancement was detected after stimulation by different sera [14]. These results indicated that sera of active HSP might contain some factors that could activate endothelial cells to release IL-8.
TNF-α is a cytokine with multiple functions in the immune responses. It has been well documented to play a major role in many systemic inflammatory diseases including systemic vasculitis [23]. We showed that the serum levels of TNF-α in acute stage of childhood HSP was significantly elevated and the mean value was around 65 pg/ml. Although TNF-α could enhance IL-8 secretion by HUVEC in a dose-dependent manner, TNF-α of a concentration 100 pg/ml, which was higher than the mean value of HSP patients, could not significantly enhance endothelial IL-8 production. Moreover, after blocking serum TNF-α by adding high dose neutralizing anti-human TNF-α antibody, the ability of these pretreated sera to enhance endothelial IL-8 production could not be significantly suppressed. According to these results, we could make a conclusion that in addition to TNF-α, there may be other major factors existing in the sera of active HSP that can activate endothelial cells to produce IL-8.
We designed two experiments in this study to determine whether IgA AECA in acute stage of HSP could activate endothelial cells to produce IL-8. In the first experiment, active sera that can enhance endothelial IL-8 secretion were incubated with mouse monoclonal anti-human IgA antibody. This antibody is specific for heavy chain of human IgA and does not crossreact with human IgG, IgM, IgD and IgE. The result indicated that these IgA-depleted sera could not significantly activate endothelial cells as they previously did. In the other experiment, circulating IgA1 in sera of children with active HSP separated and purified by Immobilized Jacalin could bind to endothelial cells and induce them to produce a large amount of IL-8. IL-8 is a powerful chemoattractant; it may recruit and activate PMNs to induce further endothelial cells damage [24]. The effect of IgA AECA on endothelial cells may contribute to the pathologic finding of perivascular PMNs infiltration in active HSP.
Many cells can produce IL-8 under a wide range of stimuli encompassing proinflammatory cytokines such as TNF-α or IL-1 [25], bacterial [26] or viral products [27,28], and cellular stress [29,30]. Recently, significant advances in the understanding of signalling pathways, which regulate IL-8 gene expression, have been made. As demonstrated in previous studies, IL-8 gene promoter element contains NF-κB and activating protein (AP)-1 binding sites, and factors that can induce IL-8 secretion have the capacity to modulate NF-κB and AP-1 activity. AP-1 is activated by MAPK. Three MAPK pathways contribute to IL-8 expression, the ERK, JNK and p38 kinase cascades [31]. The role of NF-κB is widely explored; those IL-8 serecting-cells stimulated by proinflammatory cytokines are mostly NF-κB dependent [25,32]. In the present study, specific inhibitors to these four pathways were tested to determine the possible signalling pathway. PD98059, the inhibitor of MEK/ERK, completely depressed the IgA AECA induced-endothelial IL-8 secretion. Phosphorylated ERK-1 could also be detected time-dependently under the stimulation of IgA AECA and inhibited after adding PD98059. From these findings, IgA AECA of HSP may bind to endothelial cells and augment the phosphorylation of ERK-1, which phosphorylates AP-1, and promote the IL-8 expression.
In conclusion, IgA AECA develop during the acute stage of childhood HSP, which may bind to a disease-specific endothelial antigenic peptide. Although the antigenic determinants of HSP and monoclonal antibody against endothelial cells are not yet identified, IgA AECA from acute stage of childhood HSP may have the possible pathogenic effect; these antibodies can directly activate endothelial cells to produce IL-8 through the MEK/ERK signalling pathway. These results establish the pathogenesis of HSP more thoroughly, and suggest that IgA AECA may be a target for new treatment strategies for HSP.
References
- 1.Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein Purpura in Taiwan. Rheumatology. 2005;44:618–22. doi: 10.1093/rheumatology/keh544. [DOI] [PubMed] [Google Scholar]
- 2.Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–202. doi: 10.1016/S0140-6736(02)11279-7. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger S. Henoch-Schonlein purpura. Curr Opin Rheumatol. 2003;15:591–4. doi: 10.1097/00002281-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bissonnette R, Dansereau A, D'Amico P, Pateneaude JV, Paradis J. Perforation of large and small bowel in Henoch-Schonlein purpura. Int J Dermatol. 1997;36:361–3. doi: 10.1111/j.1365-4362.1997.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 5.Coppo R, Mazzucco G, Cagnoli L, Lupo A, Schena FP. Long-term prognosis of Henoch-Schonlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch-Schonlein purpura. Nephrol Dial Transplant. 1997;12:2277–83. doi: 10.1093/ndt/12.11.2277. [DOI] [PubMed] [Google Scholar]
- 6.Belman AL, Leicher CR, Moshe SL, Mezey AP. Neurologic manifestations of Schoenlein-Henoch purpura: report of three cases and review of the literature. Pediatrics. 1985;75:687–92. [PubMed] [Google Scholar]
- 7.Besbas N, Saatci U, Ruacan S, Ozen S, Sungur A, Bakkaloglu A, Elnahas AM. The role of cytokines in Henoch Schonlein purpura. Scand J Rheumatol. 1997;26:456–60. doi: 10.3109/03009749709065719. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji Y, Abe Y, Hisano M, Sakai T. Urinary leukotriene E4 in Henoch-Schonlein purpura. Clin Exp Allergy. 2004;34:1259–61. doi: 10.1111/j.1365-2222.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang YH, Huang MT, Lin SC, Lin YT, Tsai MJ, Chiang BL. Increased TGF-β secreting T cells and IgA anticardiolipin antibodies levels during acute stage of childhood Henoch-Schönlein purpura. Clin Exp Immunol. 2000;122:285–90. doi: 10.1046/j.1365-2249.2000.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YH, Wang SJ, Chuang YH, Lin YT, Chiang BL. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-α treatment in children with Henoch-Schönlein purpura. Clin Exp Immunol. 2002;130:352–7. doi: 10.1046/j.1365-2249.2002.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung DYM, Collins T, Lapierre LA, Geha RS, Pober JS. Immunoglobulin M antibodies present in the acute phase Kawasaki syndrome lyse cultured vascular endothelial cells stimulated by gamma interferon. J Clin Invest. 1986;77:1428–35. doi: 10.1172/JCI112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathy NK, Upadhyaya S, Sinha N, Nityanand S. Complement and cell mediated cytotoxicity by antiendothelial cell antibodies in Takayasu's arteritis. J Rheumatol. 2001;28:805–8. [PubMed] [Google Scholar]
- 13.Savage COS, Pottinger BE, Gaskin G, Lockwood CM, Pusey CD, Pearson JD. Vascular damage in Wegener's granulomatosis and microscopic polyarteritis. presence of anti-endothelial cell antibodies and their relation to anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1991;85:14–9. doi: 10.1111/j.1365-2249.1991.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YH, Lai HJ, Huang CM, Wang LC, Lin YT, Chiang BL. Sera from children with active Henoch-Schönlein purpura can enhance the production of interleukin (IL)-8 by human umbilical venous endothelial cells. Ann Rheum Dis. 2004;63:1511–3. doi: 10.1136/ard.2003.016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Arthritis Rheum. 1990;33:1114–21. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 16.Wenner NP, Safai B. Circulating immune complexes in Henoch-Schonlein purpura. Int J Dermatol. 1983;22:383–5. doi: 10.1111/j.1365-4362.1983.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 17.Kauffmann RH, Herrmann WA, Meyer CJ, Daha MR, Van Es LA. Circulating IgA-immune complexes in Henoch-Schonlein purpura. A longitudinal study of their relationship to disease activity and vascular deposition of IgA. Am J Med. 1980;69:859–66. doi: 10.1016/s0002-9343(80)80011-8. [DOI] [PubMed] [Google Scholar]
- 18.Fujieda M, Oishi N, Naruse K, Hashizume M, Nishiya K, Kurashige T, Ito K. Soluble thrombomodulin and antibodies to bovine glomerular endothelial cells in patients with Henoch-Schönlein purpura. Arch Dis Child. 1998;78:240–4. doi: 10.1136/adc.78.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordron A, Revelen R, D’Arbonneau F, Dueymes M, Renaudineau Y, Jamin C, Youinou P. Functional heterogeneity of anti-endothelial cell antibodies. Clin Exp Immunol. 2001;124:492–501. doi: 10.1046/j.1365-2249.2001.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko K, Savage COS, Pottinger BE, Shah V, Pearson JD, Dillon M. antiendothelial cell antibodies can be cytotoxic to endothelial cells without cytokine pre-treatment and correlate with ELISA antibody measurement in Kawasaki disease. Clin Exp Immunol. 1994;98:264–9. doi: 10.1111/j.1365-2249.1994.tb06136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM, Chen SH, Lin YS. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol. 2003;69:82–90. doi: 10.1002/jmv.10261. [DOI] [PubMed] [Google Scholar]
- 22.Del Papa N, Guidali L, Sironi M, et al. Anti-endothelial cell IgG antibodies from patients with Wegener's granulomatosis bind to human endothelial cells in vitro and induce adhesion molecule expression and cytokine secretion. Arthritis Rheum. 1996;39:758–66. doi: 10.1002/art.1780390507. [DOI] [PubMed] [Google Scholar]
- 23.Nalbant S, Koc B, Top C, Kucukardali Y, Baykal Y, Danaci M, Kocer H. Hypersensitivity vasculitis and cytokines. Rheumatol Int. 2002;22:244–8. doi: 10.1007/s00296-002-0235-6. [DOI] [PubMed] [Google Scholar]
- 24.Baggiolini M, Walz A, Kunkel SL. Neutrophil activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasier AR, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo RP. A promoter recruitment mechanism for tumor necrosis factor-α-induced interleukin-8 transcription in type II pulmonary epithelial cells. J Biol Chem. 1998;273:3351–61. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 26.Aihara M, Tsuchimoto D, Takizawa H, et al. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infec Immun. 1997;65:3218–24. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastronarde JG, Monick MM, Mukaida N, Matsushima K, Hunninghake GW. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J Infect Dis. 1998;177:1275–81. doi: 10.1086/515279. [DOI] [PubMed] [Google Scholar]
- 28.Murayama T, Ohara Y, Obuchi M, Khabar KS, Higashi H, Mukaida N, Matsushima K. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol. 1997;71:5692–5. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee LF, Haskill JS, Mukaida N, Matsushima K, Ting JP. Identification of tumor-specific paclitaxel (Taxol) -responsive regulatory elements in the interleukin-8 promoter. Mol Cell Biol. 1997;17:5097–105. doi: 10.1128/mcb.17.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA. 1995;92:12230–4. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann E, Dittrich-Breiholz O, Hoffmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 32.Kuldo JM, Westra J, Asgeirsdottir SA, et al. Differential effects of NF-(kappa)B and p38 MAPK inhibitors and combinations thereof on TNF(alpha) and IL-1(beta) induced pro-inflammatory status of endothelial cells in vitro. Am J Physiol Cell Physiol. 2005;289:1229–39. doi: 10.1152/ajpcell.00620.2004. [DOI] [PubMed] [Google Scholar]