Abstract
The mannan-binding lectin (MBL) pathway of complement activation is important in host defence against pathogens and possibly against cancer. We investigated the effect of major surgery on two central components of the MBL pathway; MBL and the MBL-associated serine protease MASP-2, and for comparison also measured the interleukin (IL)-6 and C-reactive protein (CRP) levels. Serial blood samples were obtained from patients belonging to two different cohorts. Cohort 1 comprised 60 patients undergoing open or laparoscopic colectomy for benign disease (n = 12) or colon cancer (n = 48). Cohort 2 comprised 27 patients undergoing elective, open surgery for colorectal cancer, and was included in order to cover blood sampling between days 2 and 6. As expected, the surgical stress induced a marked acute phase response, as evidenced by a large increase in IL-6 (18-fold) and CRP (13-fold) levels with maximum at 12 h and 2 days, respectively. However, in both cohorts the levels of MBL and MBL-associated serine protease 2 (MASP-2) were largely unaffected, except for a minor but significant increase around day 8 in cohort 1. The preoperative levels of IL-6 and CRP were correlated significantly in both cohorts (r= 0·71, P < 0·0001 and r = 0·65, P = 0·005, respectively). Preoperative MASP-2 correlated with preoperative CRP (r= 0·59, P = 0·001) and IL-6 (r= 0·55, P = 0·02) in cohort 2 only. In contrast to the marked effects on the levels of IL-6 and CRP, the surgery influenced only marginally the two proteins of the MBL pathway.
Keywords: acute phase reactants, complement, human, inflammation
Introduction
Surgical trauma leads to transient impairment of the function of the immune system [1–4]. The effect is proportional to the extent of the trauma [5–8], and renders the patient more susceptible to infectious complications. In cancer patients, the inflammatory response, postoperative immune suppression and infectious complications may influence the prognosis negatively [9].
The mannan-binding lectin (MBL) pathway of innate immunity is part of the first line of defence against microorganisms [10–13]. MBL recognizes and binds to carbohydrate patterns on the surface of microorganisms leading to complement activation by the MBL-associated serine protease 2 (MASP-2). Accordingly, MBL deficiency implies an increased susceptibility to infections, mainly in otherwise immune-compromised individuals [10,12]. Little is known about the influence of major surgery on the MBL pathway. If surgical stress compromises the function of the MBL pathway, this might play a role in postoperative infections.
The observation of an increase in MBL mRNA in the liver of a traffic casualty [14] led to the classification of MBL as an acute phase protein [15], and an early study on patients with malaria and patients undergoing surgery showed a late, relatively small increase in MBL levels [16].
Recently we found that preoperative serum levels of MBL were increased in patients with colorectal cancer. This could be due to an ongoing acute phase reaction, as evidenced by an increase in C-reactive protein (CRP) [17]. However, there was no correlation between preoperative CRP and MBL levels. Low preoperative MBL levels were associated with an increased risk of postoperative pneumonia [18]. A study of patients operated for abdominal malignancy (n = 172) found no change in MBL levels during the first 3 days postoperatively [19].
Major surgery is a well-described clinical model to evaluate the parameters of the inflammatory response [4]. The purpose of the present study was to evaluate the impact of major surgery on the MBL pathway compared to the well-established effects on interleukin (IL)-6 and CRP levels during the early and late postoperative period.
Materials and methods
Two cohorts were investigated. In cohort 1, patients scheduled to undergo elective colon resection were included consecutively and randomized to either laparoscopic-assisted or open colectomy. The patients followed a postoperative accelerated rehabilitation programme [20] and were discharged 2 days after surgery, visiting the out-patient clinic on postoperative day 8 for control. No blood samples were taken between postoperative days 2 and 8, and we therefore enrolled blood samples from a retrospective study of patients who stayed at the hospital for 8 days or more after elective colectomy.
Cohort 1 comprised 60 patients with either primary colon cancer (n = 48) or sigmoid obstruction due to benign disease (diverticulosis, adenomas, volvolus; n = 12). Patients less than 56 years of age or having received preoperative chemotherapy or patients with tumours distal to the anterior fold were not included. Fifty-seven patients were colectomized, and three patients with Dukes’ stage D colon cancer had debulking surgery only. No patients received chemotherapy within 30 days after the operation. The anaesthetic, analgesic and surgical procedures were standardized and are described in detail elsewhere [21–23].
Cohort 2 comprised 27 patients operated for primary colorectal cancer (n = 25) or sigmoid obstruction due to benign diseases (n = 2). Further patient characteristics are shown in Table 1.
Table 1.
Basic patient characteristics.
| Cohort 1 | Cohort 2 | |
|---|---|---|
| Total number of patients | 60 | 27 |
| Age in years, median and (range) | 76 (56–90) | 71(35–86) |
| Gender male/female | 26/34 | 16/11 |
| Operation type; number of patients | ||
| Open/laparoscopic operation* | 34/26 | 27/0 |
| Duration of operation in minutes; mean and (range)* * | ||
| Open operation | 131 (80–361) | Not recorded |
| Laparoscopic operation | 202 (79–335) | |
| Disease classification | ||
| Benign colon disease | 12 | 2 |
| Colon/rectum adenocarcinoma | 48/0 | 13/12 |
| Dukes’ stages; no. of patients | ||
| A | 5 | 6 |
| B | 19 | 8 |
| C | 15 | 7 |
| D | 9 | 4 |
Three patients with malignant disease were converted intraoperatively from laparoscopic to open surgery and are considered as treated with open surgery in the statistical analysis.
Duration of the operation was significantly longer for laparoscopic assisted surgery compared to open surgery (P< 0·0001).
In accordance with the Helsinki II declaration, written informed consent was obtained from all patients. The Regional Ethics Committees approved the studies.
Blood samples
Peripheral blood was collected in endotoxin-free silicone-coated tubes without anticoagulation additives (Becton-Dickinson, Mountain View, CA, USA). The samples were allowed to clot in refrigerator for 1 h, followed by centrifugation at 2500 g for 10 min at +4°C. The serum was removed and stored at −80°C until analysed. The samples from cohort 1 were obtained just before and at 1, 2, 6, 24 and 48 h after skin incision, and at days 8 and 30 after the operation. The samples from cohort 2 were obtained just before, and at 12 h, daily for 8 days, and at day 30 postoperatively.
The MBL concentration was determined by a time-resolved immunofluorometric assay (TRIFMA), described in detail in previous studies [24]. In brief, diluted serum is incubated in mannan-coated microtitre wells in the presence of Ca2+, which allows for the binding of MBL to mannan. Subsequently, bound MBL is detected with europium-labelled antibodies detect MBL and quantified by fluorometry. The serum dilution, applied routinely (1/200), can detect MBL levels between 10 ng and 5 µg MBL/ml serum. For samples exceeding 5 µg/ml, assays were repeated at higher serum dilutions.
A sandwich-type TRIFMA using a combination of two monoclonal anti-MASP-2 antibodies was used for the determination of the MASP-2 concentration. The assay has been described and validated in detail elsewhere [25]. Serum is incubated in microtitre wells coated with anti-MASP-2 antibody. Subsequently, the wells are incubated with biotin-labelled MASP-2 antibody, followed by europium-labelled streptavidin, and the bound europium is quantified by fluorometry. The serum dilution applied routinely (1/75) allows for the detection of between 10 ng and 10 µg MASP-2/ml serum.
The IL-6 concentration in plasma was determined by an enzyme-linked immunosorbent assay (ELISA) method (Quantikine®, human IL-6 immunoassay, R&D Systems, Inc., Minneapolis, MN, USA), with a detection limit of 0·7 pg/ml. All concentrations were analysed in duplicate and the mean concentration was used for further analysis.
The plasma CRP levels were determined using a routine nephelometric method (Dade Behring Inc., Deerfield, IL, USA), and the concentrations of CRP were expressed as mg/l with 2·6 mg/l as the lower detection limit. The upper normal limit of CRP was 10 mg/l.
Statistical analysis
The SAS® software package (version 8·2; SAS Institute, Cary, NC, USA) was used to manage patient data and for statistical analysis. Analysis of MBL, MASP-2, CRP and IL-6 fluctuations over time was performed using a generalized linear model assuming a normal distribution on the log scale and taking repeated measures into account. Estimates were obtained by generalized estimating equations. The model was adjusted for age, gender and benign/malignant disease as well as surgical technique. Descriptive statistics are given by geometric means. Spearman's rank correlation coefficients were used as measure for association. P-values less than 5% were considered significant.
Results
MBL
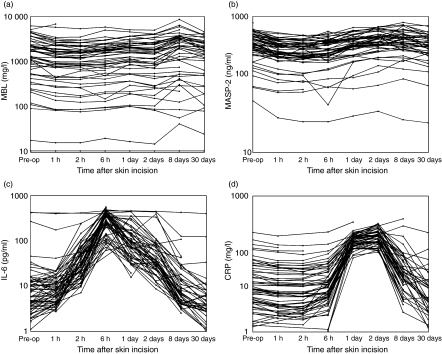
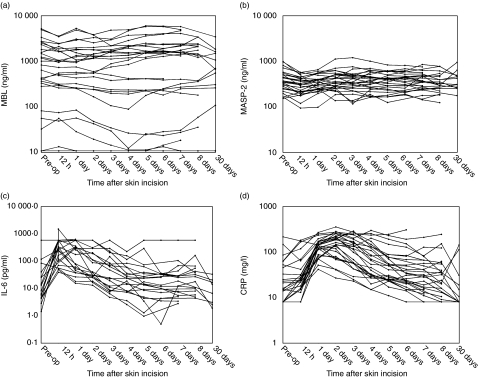
The MBL levels of each patient and at each time-point for cohort 1 are shown in Fig. 1a and for cohort 2 in Fig. 2a and the mean levels (geometric) are presented in Table 2. Patients with MBL levels below the detection limit of 10 ng/ml at all sample times (n = 5 in cohort 1 and n = 2 in cohort 2) were excluded from the statistical analysis of fluctuations in serum MBL levels over time. All other analyses are based on all patients.
Fig. 1.
Serum concentrations of mannan-binding lectin (MBL), MBL-associated serine protease (MASP-2), interleukin (IL)-6 and C-reactive protein (CRP) in cohort 1. (a) The serum levels of MBL (ng/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. In five patients, the MBL levels were below the detection limit for all samples, and the values have been set to 10 ng/ml. (b) The serum levels of MASP-2 (ng/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. (c) The serum levels of IL-6 (pg/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. (d) The serum levels of CRP (mg/l) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown.
Fig. 2.
Serum concentrations of mannan-binding lectin (MBL), MBL-associated serine protease (MASP-2), interleukin (IL)-6 and C-reactive protein (CRP) in cohort 2. (a) The serum levels of MBL (ng/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. In two patients, the MBL levels were below the detection limit for all samples, and the values have been set to 10 ng/ml. (b) The serum levels of MASP-2 (ng/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. (c) The serum levels of IL-6 (pg/ml) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown. (d) The serum levels of CRP (mg/l) for each patient sampled just prior to skin incision (designated ‘Pre-op’) and postoperatively at the time-points shown.
Table 2.
Mean (geometric) serum concentrations of mannan-binding lectin (MBL), MBL-associated serine protease (MASP-2), interleukin (IL-6) and C-reactive protein (CRP) at the different sample times preoperatively and hours/days postoperatively, cohort 1 (a) and cohort 2 (b).
| (a) | 0 h | 1 h | 2 h | 6 h | day 1 | day 2 | day 8 | day 30 |
|---|---|---|---|---|---|---|---|---|
| MBL (ng/ml)* | 1202 | 961 | 874 | 935 | 1101 | 980 | 1386 | 1100 |
| MASP-2 (ng/ml) | 368 | 285 | 278 | 293 | 358 | 404 | 413 | 358 |
| IL-6 (pg/ml) | 7·73 | 8·94 | 34·4 | 273 | 93·3 | 42·7 | 9·20 | 4·29 |
| CRP (mg/l) | 8·50 | 7·41 | 6·39 | 9·67 | 103 | 123 | 19·7 | 4·48 |
| (b) | 0 h | 12 h | day 1 | day 2 | day 3 | day 4 | day 5 | day 6 | day 7 | day 8 (n = 19) | day 30 (n = 16) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MBL* (ng/ml) | 802 | 576 | 716 | 654 | 621 | 627 | 721 | 590 | 754 | 865 | 425 |
| MASP-2 (ng/ml) | 375 | 285 | 326 | 366 | 346 | 361 | 354 | 367 | 351 | 344 | 406 |
| IL-6 (pg/ml) | 13·7 | 266 | 144 | 68·3 | 38·3 | 23·7 | 17·4 | 13·9 | 19·3 | 25·1 | 8·9 |
| CRP (mg/l) | 19·5 | 27·4 | 127 | 142 | 101 | 64·9 | 47·8 | 38·0 | 31·0 | 33·4 | 19·9 |
Only MBL measurements over the detection limit are included.
Cohort 1
A significant decrease in MBL level during the first 48 h (P< 0·0001) was found, followed by a significant increase at day 8 (P= 0·03) compared to the preoperative levels. The mean level at day 30 was not significantly different from the preoperative level (P= 0·47). Although significant, the actual changes in mean levels were modest with a mean decrease at 48 h of 17% (95% CI: 8–25%) and a mean increase at day 8 of 16% (95% CI: 1%–34%) compared to the preoperative level. There was no difference in fluctuations for gender (P= 0·55), age (P= 0·97), type of operation (P= 0·18) or benign and malignant disease (P= 0·76).
Cohort 2
A modest decrease of MBL up to day 4 compared to preoperative level (P= 0·01) was found, with a mean decrease of 19% on day 3; 95% CI: 5–32% on day 3. After day 4 no statistical differences were seen when comparing to the preoperative level. The median MBL level was lower on postoperative day 30 compared to day 3, as seen from Table 2b. The lack of statistical significance of this can reflect the lower number of patients at day 30.
No differences between the fluctuations for gender (P= 0·67) or age (P= 0·60) were found.
MASP-2
The MASP-2 levels of each patient and at each time-point are shown in Fig. 1b (cohort 1) and 2b (cohort 2) and the mean levels are presented in Table 2.
Cohort 1
A significant decrease of MASP-2 level was found up to 6 h after incision (P< 0·0001) followed by a significant increase at day 8 (P= 0·05) compared to the preoperative levels. The mean level at day 30 was not different from the preoperative level (P= 0·34). Although significant, the changes in mean levels compared to preoperative levels were insubstantial, with a mean decrease at 6 h of 21% (95% CI: 14–27%) and a mean increase at day 8 of 10% (95% CI: 0–21%) compared to the preoperative level. There were no significant differences between the MASP-2 levels for gender (P= 0·82), age (P= 0·09) type of operation (P= 0·07) or benign and malignant disease (P= 0·86).
Cohort 2
A significant decrease of the MASP-2 levels was found at 12 h postoperatively compared to preoperative levels (mean decrease 24%, P < 0·0001, 95% CI: 16–32%). No other significant differences were detected. There were no significant differences between the MASP-2 levels for gender (P= 0·90) or age (P= 0·24).
IL-6
The IL-6 levels of each patient and at each time-point are shown in Figs 1c and 2c and the mean levels are presented in Table 2.
Cohort 1
The IL-6 level showed a more than 10-fold mean increase 6 h after incision (P< 0·0001). The mean level on day 8 was not significantly different from the preoperative level (P= 0·19), whereas the level on day 30 was significantly lower (31%, P = 0·002). There was no significant difference between the IL-6 levels for gender (P= 0·92), age (P= 0·16) or type of operation (P= 0·84). A significant difference between benign and malignant disease at all sample times was found (P= 0·02).
Cohort 2
A highly significant increase in IL-6 levels with a maximum at 12 h postoperatively was found with a mean increase of more than 18-fold (P< 0·0001); returning to the preoperative level after day 3. The level on day 30 in this cohort was not significantly lower than the preoperative level. A statistically significant increasing trend for age (P= 0·0004) was found, and males had higher levels than females (P= 0·02)
CRP
The CRP levels of each patient and at each time-point are shown in Figs 1d and 2d and the mean levels are presented in Table 2.
Cohort 1
A slight, but significant decrease (19%, 95% CI: 6–31%) up to 2 h after incision was found (P= 0·004) followed by a significant increase with maximum on day 2 (13-fold increase, P < 0·0001) compared to the preoperative levels. The mean CRP level at day 30 was not significantly different from the preoperative level (P= 0·10). There was no difference of CRP levels for gender (P= 0·15), age (P= 0·55) or type of operation (P= 0·15). A highly significant difference was found between benign and malignant disease at all sample times (P< 0·0001).
Cohort 2
As for cohort 1, a highly significant increase with a maximum on day 2 (7·3-fold increase, P< 0·0001) was found. The preoperative CRP level was not significantly different from the level at day 30 (P= 0·11). CRP levels were significantly higher for males (P= 0·02) and increased significantly with age (P= 0·02).
Association between MBL, MASP-2, IL-6 and CRP
Cohort 1
The correlation coefficients between preoperative levels of MBL, MASP-2, CRP and IL-6 showed that only IL-6 and CRP were associated significantly (r= 0·71, P < 0·0001). The correlation between IL-6 concentration at 6 h (peak IL-6) and CRP at day 2 (peak CRP) was 0·26 (P= 0·04). All other correlations between peak values were lower and non-significant.
Cohort 2
The correlation coefficient between preoperative CRP and IL-6 was 0·65 (P= 0·005), and IL-6 at 12 h postoperatively correlated to CRP at day 2 (r= 0·70, P < 0·0001). Preoperative MASP-2 correlated to preoperative CRP (r= 0·59, P = 0·001) and IL-6 (r= 0·55, P = 0·02). MASP-2 and MBL were not correlated significantly (r= 0·21, P = 0·29).
Testing whether the early decrease of MBL and MASP-2 levels observed in cohort 1 might be linked to fluid administration showed no significant association to the serum concentrations of MBL, MASP-1, CRP or IL-6.
Discussion
The present study confirms the established knowledge that surgical trauma induces major changes in serum levels of the inflammatory markers IL-6 and CRP (Figs 1c,d and 2c,d) [26–28]. Hence, the early decrease of serum IL-6 observed, followed by a maximal increase at 6–12 h postoperatively and CRP increasing to a maximum 2 days after surgery, are in accordance with previous studies [5,29–31]. The finding of a significantly lower mean IL-6 level in cohort 1 30 days after surgery compared to the preoperative level should be validated, as comparable data are not available. IL-6 has been reported to be a prognostic factor in colorectal cancer with higher levels associated with poorer prognosis [31,32], and studies have indicated that IL-6 is produced by colorectal carcinoma cells [33]. Thus, hypothetically, removal of the tumour may lead to a postoperative decrease in serum IL-6. However, the present study is not powered to study in detail the relation between IL-6 levels, surgery and cancer.
The preoperative serum levels of IL-6 and CRP were correlated in both cohorts. In cohort 1, the levels were increased significantly at all sample times in patients with malignant disease compared to patients with benign disease and studies report a prognostic role of CRP in colorectal cancer, with higher levels associated with a poorer prognosis [34,35].
The findings support the well-established biology of IL-6 as an early inflammatory cytokine initiating the acute hepatic response, with CRP as one of the important acute phase proteins.
In cohort 2, but not in cohort 1, the influence of age and gender on the serum levels of both molecules reached significance. Surgical technique had no impact on the levels. Some studies comparing surgical techniques report higher IL-6 and CRP responses following open surgery compared to laparoscopy [5,36–38], whereas others find no difference [39–41].
The serum levels of MBL and MASP-2 were affected only slightly by surgery, and no dependence of age, gender or surgical technique on the serum concentrations of MBL or MASP-2 was detected The early decrease in serum levels observed is marginal but significant and could be the result of high requirements of the molecules due to bacterial contamination in bowel surgery, latency of synthesis of the molecules, trauma-induced impaired synthesis and release, as well as dilution due to fluid administration. However, the latter mechanism could not be verified by analysis correlating fluid volumes and MBL and MASP-2, respectively. The initial decrease of serum MBL and MASP-2 was followed by a return to preoperative levels in cohort 2, and in cohort 1 a small increase in serum levels to a peak on day 8. The levels of MBL and MASP-2 on day 30 were similar to the preoperative levels. The impact of surgery on serum MBL has been investigated in only a few studies. In a study of patients undergoing major orthopaedic surgery, a 1·5–3-fold increase of serum MBL between postoperative day 1 and 9 was found [16]. In another study of patients undergoing major surgery for abdominal malignancy, no changes in the MBL levels were observed during the first 3 days following operation [19].
The increase of MBL concentration seen in cohort 1 showed no correlation with individual increases in IL-6 or CRP levels, suggesting that MBL is not part of the hepatic acute phase response. Preoperative MASP-2 was correlated moderately with CRP and IL-6 in cohort 2 but not in cohort 1.
MASP-2 levels at an acute phase reaction, for example as a response to surgery, has not been studied previously. The lack of correlation between the peak levels of MBL and MASP-2 diverge from what might be expected, considering the fact that the molecules form a complex and, as such, activate the complement system. However, MASP-2 also forms functional complexes with two other proteins, l-ficolin and h-ficolin, for which the biological role remains largely unresolved. Both are found as liver-produced serum proteins and, furthermore, h-ficolin is synthesized in lung cells ([42] added after review). The assay for MASP-2 is not influenced by the concentrations of MBL, l-ficolin or h-ficolin in the sample, as MASP-2 is dissociated from all complexes by the high salt concentration and ethylenediamine tetraacetic acid (EDTA) in the sample dilution buffer. At present the potential functions of free forms of MBL and MASP-2 have not been described.
Trauma-induced impairment of the adaptive immune response is associated with risk of development of infectious complications [1–3,5,7,8,43,44]. The innate immune response is considered to be important for initiation of the adaptive responses; thus impairment of the MBL pathway might involve the insufficient function of adaptive immunity. In a recent study [18], we investigated the role of preoperative serum levels of MBL for postoperative infection in 611 patients with colorectal cancer, and found a significantly increased risk of pneumonia at low MBL levels. Another study comprising 172 patients found a similar association [19]. This is in accordance with the suggested function of MBL as a prominent molecule of the innate immune response in the recognition and eradication of pathogens. In summary, the present study suggests that the MBL complement-activating pathway of the innate immune system may be resistant to major surgical trauma. This may implicate an important role of MBL in perioperative immune competence when the adaptive immune system is temporarily impaired. This study does not support a role for MBL as an acute phase protein, and measurements of MBL pathway components are not useful in monitoring the acute phase response to surgery.
Acknowledgments
The authors thank Ms Birgitte Sander Nielsen, Lisbeth Jensen and Annette G. Hansen for expert execution of the analyses. The staff of the Departments of Surgical Gastroenterology and Anaesthesiology, Hvidovre University Hospital are thanked for collaboration in running the study. The study received financial support from the Kornerup Fund, the Aage and Johanne Louis-Hansen Fund, the Walter and O. Kristiane Christensen Fund, the Aase and Einar Danielsen Fund, the Arvid Nilsson Fund, the Leo Foundation, the Henrik Henriksen Fund, the Krista and Viggo Petersen Fund, the Sven and Ina Hansen Fund, Den Midtjyske Bladfond, the Else and Mogens Wedell-Wedellsborg Fund, the Sophus and Astrid Jacobsen Fund, the Einar Willumsen Fund, the Katrine and Vigo Skovgaard Fund, the Ulla and Mogens Folmer Andersen Fund, the IMK Fund, the Danish Pharmacy Foundation of 1991, the Danish Cancer Society (grant no. DP 02012) (H. J. N. is Danish Cancer Society Professor of Surgical Oncology) and the Danish Medical Research Council.
References
- 1.Haupt W, Riese J, Mehler C, Weber K, Zowe M, Hohenberger W. Monocyte function before and after surgical trauma. Dig Surg. 1998;15:102–4. doi: 10.1159/000018601. [DOI] [PubMed] [Google Scholar]
- 2.Herroeder S, Durieux ME, Hollmann MW. Inflammatory responses after surgery. Hosp Med. 2002;63:99–103. doi: 10.12968/hosp.2002.63.2.2088. [DOI] [PubMed] [Google Scholar]
- 3.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–44. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HJ. The effect of histamine type-2 receptor antagonists on posttraumatic immune competence. Dan Med. 1995;42:162–74. [PubMed] [Google Scholar]
- 5.Delgado S, Lacy AM, Filella X, et al. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum. 2001;44:638–46. doi: 10.1007/BF02234558. [DOI] [PubMed] [Google Scholar]
- 6.Faist E, Storck M, Hultner L, et al. Functional analysis of monocyte activity through synthesis patterns of proinflammatory cytokines and neopterin in patients in surgical intensive care. Surgery. 1992;112:562–72. [PubMed] [Google Scholar]
- 7.Kehlet H, Nielsen HJ. Impact of laparoscopic surgery on stress responses, immunofunction, and risk of infectious complications. New Horiz. 1998;6:S80–8. [PubMed] [Google Scholar]
- 8.Shirakawa T, Tokunaga A, Onda M. Release of immunosuppressive substances after gastric resection is more prolonged than after mastectomy in humans. Int Surg. 1998;83:210–4. [PubMed] [Google Scholar]
- 9.Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ Danish Ranx05 Colorectal Cancer Study Group. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Br J Surg. 2000;87:1553–62. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 10.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–8. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 11.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–49. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 12.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 13.Turner MW, Hamvas RM. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–22. [PubMed] [Google Scholar]
- 14.Ezekowitz RA, Day LE, Herman GA. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988;167:1034–46. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeway CA, Travers P, Walport M, Schlomchik M. Immunobiology. 5. New York: Garland Publishing; 2001. [Google Scholar]
- 16.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen HJ, Christensen IJ, Sorensen S, Moesgaard F, Brunner N The Ranx05 Colorectal Cancer Study Group. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. Ann Surg Oncol. 2000;7:617–23. doi: 10.1007/BF02725342. [DOI] [PubMed] [Google Scholar]
- 18.Ytting H, Christensen IJ, Jensenius JC, Thiel S, Nielsen HJ. Preoperative mannan-binding lectin pathway and prognosis in colorectal cancer. Cancer Immunol Immunother. 2005;54:265–72. doi: 10.1007/s00262-004-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siassi M, Riese J, Steffensen R, et al. Mannan-binding lectin and procalcitonin measurement for prediction of postoperative infection. Crit Care. 2005;9:483–9. doi: 10.1186/cc3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basse L, Hjort D, Bardram L, et al. Functional recovery after open vs. laparoscopic colonic resection. 2005. A randomised, blinded study. Ann Surg. [DOI] [PMC free article] [PubMed]
- 21.Bardram L, Funch-Jensen P, Jensen P, Crawford ME, Kehlet H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet. 1995;345:763–4. doi: 10.1016/s0140-6736(95)90643-6. [DOI] [PubMed] [Google Scholar]
- 22.Bardram L, Funch-Jensen P, Kehlet H. Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg. 2000;87:1540–5. doi: 10.1046/j.1365-2168.2000.01559.x. [DOI] [PubMed] [Google Scholar]
- 23.Basse L, Hjort JD, Billesbolle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51–7. doi: 10.1097/00000658-200007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiel S, Moller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology. 2002;205:446–54. doi: 10.1078/0171-2985-00145. [DOI] [PubMed] [Google Scholar]
- 25.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Meth. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–9. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Nakagoe T, Tsuji T, Sawai T, et al. Minilaparotomy may be independently associated with reduction in inflammatory responses after resection for colorectal cancer. Eur Surg Res. 2003;35:477–85. doi: 10.1159/000073386. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen LA, Nielsen HJ, Sorensen S, et al. Ranitidine reduces postoperative interleukin-6 induced C-reactive protein synthesis. J Am Coll Surg. 1995;181:138–44. [PubMed] [Google Scholar]
- 29.Berger D, Bolke E, Seidelmann M, Beger HG. Time-scale of interleukin-6, myeloid related proteins (MRP), C reactive protein (CRP), and endotoxin plasma levels during the postoperative acute phase reaction. Shock. 1997;7:422–6. doi: 10.1097/00024382-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ikushima H, Nishida T, Takeda K, et al. Expression of Toll-like receptors 2 and 4 is downregulated after operation. Surgery. 2004;135:376–85. doi: 10.1016/j.surg.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Salgado R, Junius S, Benoy I, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 32.Belluco C, Nitti D, Frantz M, et al. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol. 2000;7:133–8. doi: 10.1007/s10434-000-0133-7. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–9. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 35.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrandt U, Kessler K, Plusczyk T, Pistorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17:242–6. doi: 10.1007/s00464-001-9148-9. [DOI] [PubMed] [Google Scholar]
- 37.Nishiguchi K, Okuda J, Toyoda M, Tanaka K, Tanigawa N. Comparative evaluation of surgical stress of laparoscopic and open surgeries for colorectal carcinoma. Dis Colon Rectum. 2001;44:223–30. doi: 10.1007/BF02234297. [DOI] [PubMed] [Google Scholar]
- 38.Schwenk W, Jacobi C, Mansmann U, Bohm B, Muller JM. Inflammatory response after laparoscopic and conventional colorectal resections − results of a prospective randomized trial. Langenbecks Arch Surg. 2000;385:2–9. doi: 10.1007/s004230050002. [DOI] [PubMed] [Google Scholar]
- 39.Dunker MSHT, Bemelman WA, Slors JF, Gouma DJ, Van Deventer SJ. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum. 2003;46:1238–44. doi: 10.1007/s10350-004-6721-z. [DOI] [PubMed] [Google Scholar]
- 40.Mehigan BJ, Hartley JE, Drew PJ, et al. Changes in T cell subsets, interleukin-6 and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg Endosc. 2001;15:1289–93. doi: 10.1007/s004640020021. [DOI] [PubMed] [Google Scholar]
- 41.Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow-Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg. 2001;88:801–7. doi: 10.1046/j.1365-2168.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita M, Fujita T. Ficolins and the lectin complement pathway. Immunol Rev. 2001;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 43.Faist E. The mechanisms of host defense dysfunction following shock and trauma. Curr Top Microbiol Immunol. 1996;216:259–74. doi: 10.1007/978-3-642-80186-0_12. [DOI] [PubMed] [Google Scholar]
- 44.Kressner U, Graf W, Mahteme H, Pahlman L, Glimelius B. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316–21. doi: 10.1007/s10350-004-6174-4. [DOI] [PubMed] [Google Scholar]